Although cases of Guillain–Barré syndrome (GBS) ensuing from COVID‐19 vaccination have been recently reported,1, 2, 3, 4, 5 the development of GBS within 6 weeks of vaccination does not necessarily infer causation. This association may, however, be supported if postvaccination GBS displays a specific phenotype. Interestingly, 3 case series1, 2, 3 have reported an associated facial paralysis (FP) in all cases of GBS developing after administration of the Oxford–AstraZeneca vaccine, a much higher frequency than expected. All cases but one of GBS reported after adenovirus‐vectored vaccine administration were associated with FP.1, 2, 3, 4, 5 To confirm this phenotype and substantiate the possible causal relationship, we compared herein the frequency of FP in cases of GBS (FP‐GBS) occurring after adenovirus‐vectored vaccines to that occurring after mRNA‐based vaccines using pharmacovigilance data.
We first analyzed all cases reported to the World Health Organization pharmacovigilance database, VigiBase. As of June 29, 2021, among the 1,257,497 cases reported with COVID‐19 vaccines, 1,256 (0.1%) “acute polyneuropathies” (all cases corresponding to GBS and GBS variants) were reported (422 USA, 387 UK, 328 Europe [40 France], and 119 elsewhere). Among these, patients with FP‐GBS were identified using the MedDRA Preferred Terms: “Bell's palsy”, “facial paralysis”, “facial nerve disorder”, “facial paresis”, and “oculofacial paralysis”. A total of 142 of 1,256 GBS patients experienced FP (11.3%). This included 26 of 488 (5.3%) GBS patients who received mRNA vaccines (12/328 [3.7%] Pfizer–BioNTech, 14/160 [8.8%] Moderna), 114 of 744 (15.3%) who received adenovirus‐vectored vaccines (86/630 [13.7%] Oxford–AstraZeneca, 28/114 [24.6%] Johnson & Johnson), and 2 of 24 (8.3%) who received other vaccines. FP‐GBS was significantly more frequent after adenovirus‐vectored vaccines (χ2: p = 6.44 × 10−8; Fig 1).
FIGURE 1.
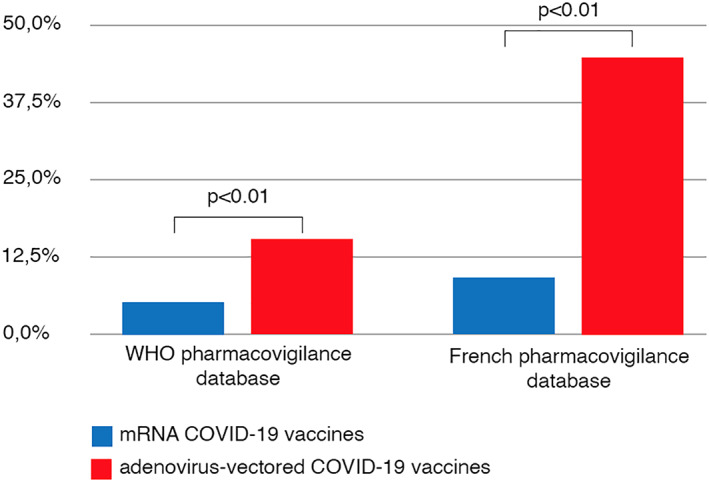
Frequency of facial paralysis associated with Guillain–Barré syndrome after COVID‐19 vaccine administration. WHO = World Health Organization. [Color figure can be viewed at www.annalsofneurology.org]
We then extracted all cases reported in the French pharmacovigilance database (June 29, 2021), which is more detailed and more up to date than the VigiBase. Among the 48,907 cases reported with COVID‐19 vaccines, there were 69 (0.1%) cases of GBS, of which 23 involved FP (33.3%). This included 2 of 22 (9.1%) GBS patients who received mRNA vaccines (Pfizer–BioNTech) and 21 of 47 (44.7%) who received adenovirus‐vectored vaccines (20/44 [45.5%] Oxford–AstraZeneca, 1/3 [33.3%] Johnson & Johnson), also indicating a higher frequency of FP‐GBS occurring after adenovirus‐vectored vaccines (Fisher exact test: p = 0.0053; see Fig 1).
These results indicate that cases of GBS occurring after administration of adenovirus‐vectored vaccines present a specific phenotype, which supports a causal relationship between such exposure and this syndrome. Although it is likely that underreporting of FP exists in these databases, there is no reason for differential reporting of FP‐GBS between vaccines. Future prospective studies are needed to elucidate the specific immunopathological mechanism underlying this possible complication.
Author Contributions
A.P., A.V., and E.B. contributed to the conception and design of the study. All authors contributed to the acquisition and analysis of data. A.P. and A.V. contributed to drafting the manuscript. A.P. contributed to drafting the figure.
Potential Conflicts of Interest
Nothing to report.
Acknowledgments
We thank VigiBase and the French network of pharmacovigilance centers for giving us access to the data. The data supplied to VigiBase come from a variety of sources, and the likelihood of a causal relationship is not the same in all reports. The information does not represent the opinions of the Uppsala Monitoring Centre or the World Health Organization. We thank Philip Robinson (DRS, Lyon Civil Hospices) for help in manuscript preparation.
References
- 1. Maramattom BV, Krishnan P, Paul R, et al. Guillain‐Barre syndrome following chadox1‐s/ncov ‐19 vaccine. Ann Neurol 2021;90:312–314. [DOI] [PubMed] [Google Scholar]
- 2. Allen CM, Ramsamy S, Tarr AW, et al. Guillain‐Barre syndrome variant occurring after sars‐cov‐2 vaccination. Ann Neurol 2021;90:315–318. [DOI] [PubMed] [Google Scholar]
- 3. Bonifacio GB, Patel D, Cook S, et al. Bilateral facial weakness with paraesthesia variant of Guillain‐Barré syndrome following Vaxzevria COVID‐19 vaccine. J Neurol Neurosurg Psychiatry (in press). 10.1136/jnnp-2021-327027 [DOI] [PubMed] [Google Scholar]
- 4. Patel SU, Khurram R, Lakhani A, Quirk B. Guillain‐Barre syndrome following the first dose of the chimpanzee adenovirus‐vectored COVID‐19 vaccine, ChAdOx1. BMJ Case Rep 2021;14:e242956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Márquez Loza AM, Holroyd KB, Johnson SA, et al. Guillain‐Barré syndrome in the placebo and active arms of a COVID‐19 vaccine clinical trial: temporal associations do not imply causality. Neurology 2021;96:1052–1054. [DOI] [PubMed] [Google Scholar]


