Abstract
Background
Optimal timing for tracheotomy for critically ill COVID‐19 patients requiring invasive mechanical ventilation (IMV) is not established.
Methods
Multicenter prospective cohort including all COVID‐19 patients admitted to intensive care units (ICUs) in 36 hospitals who required tracheotomy during first pandemic wave. With a target emulation trial framework, we studied the causal effects of early (7–10 days) versus late (>10 days) tracheotomy (LT) on time from tracheotomy to weaning, postoperative mortality, and tracheotomy complications.
Results
Of 696 patients, 20.4% received early tracheotomy (ET). ET was associated with faster weaning (hazard ratio [HR] [95% confidence interval, CI]: 1.25 [1.00–1.56]) without differences in mortality (HR [95% CI]: 0.85 [0.60–1.21]) or complications (adjusted rate ratio [95% CI]: 0.56 [0.23–1.33]).
Conclusions
ET had a similar or lower post‐tracheotomy weaning time than LT, potentially shortening IMV and ICU stays, without changing complication or mortality rates in COVID‐19 patients.
Keywords: complications, intensive care, respiratory failure, SARS‐CoV‐2, weaning
1. INTRODUCTION
Tracheotomy is the most common procedure performed for patients in the intensive care unit (ICU), required by 10%–24% of patients under invasive mechanical ventilation (IMV) for prolonged respiratory support or weaning. 1 Although substantial variation in the type and timing of this procedure has been reported, 2 some studies have suggested that performing an early tracheotomy (ET) may reduce the lengths of IMV and ICU care required. 3 , 4
About 3% of patients hospitalized with COVID‐19 5 , 6 suffer from respiratory failure and require IMV. Tracheotomy is therefore the most frequent surgical procedure performed during lockdowns for the SARS‐CoV‐2 pandemic. 7 The main indications for a tracheotomy are long‐term intubation, management of secretions, sedation reduction needs, progression to weaning, and prevention of laryngeal edema. Tracheotomy in these patients minimizes the long‐term risk of laryngotracheal stenosis and reduces the lengths of mechanical ventilation and ICU stay. 8 This last aspect is crucial when ICU space is under strain.
Early in the SARS‐CoV‐2 pandemic, there were concerns that performing a tracheotomy could put the surgical team at risk of infection. Some centers were unable to perform the procedure as they lacked adequate personal protective equipment (PPE). 9 Published high mortality rates and the difficulties and risks of transferring patients from the ICU to the operation room were also drawbacks. 6 Many scientific societies issued guidelines and recommendations so that the procedure could be performed safely for both patient and surgeon. 10 , 11 , 12 Many of the recommendations were based on the experience gained from SARS‐CoV‐1 and Middle East respiratory syndrome and drew on the opinions of expert surgeons and epidemiologists. 13 As it was believed that it would be safer to perform the procedure when the patient's viral load was lower, many guidelines recommended late or very late tracheotomy (LT). 14 However, guidance disagreed on which type of tracheotomy was safest (open surgical vs. percutaneous) or where it should be performed. 15 All guidelines agreed that maneuvers generating aerosols should be minimized at the time of tracheal entrance to protect surgical teams. 9 , 16 , 17
Preliminary data from different centers during the pandemic showed that a tracheotomy could be performed safely, even at the patient's bedside, if the standard PPE recommendations were followed. 18 The complication rates under this scenario seemed to be similar to those reported before the pandemic. 18 Some data suggested that ET might reduce time to weaning and ICU length of stay. 4 , 18
We therefore evaluated the effect of disease‐ and tracheotomy‐related variables on the weaning and mortality rates in a large multicenter cohort of COVID‐19 ICU patients that required a tracheotomy during IMV. The study was performed in Spain during the first wave of the COVID‐19 pandemic and focused on determining the optimal time to perform a tracheotomy for these patients.
2. METHODS
2.1. Study design and setting
We used a prospective cohort study design. All patients receiving a tracheotomy between March 11, 2020 and July 20, 2020 in 36 hospitals who met the inclusion criteria were included. Fifty patients of the series have been reported elsewhere. 18
A data collection proposal was sent to all members of the Spanish Society of Otorhinolaryngology and Head and Neck Surgery by the senior author (F. X. A.‐J.). Thirty‐six hospitals showed interest. Researchers from each hospital collected the data from ICU admission to weaning/death/end of study and filled in on an MS Excel sheet (MS Excel for mac v16.16.27. Microsoft 2018) sent to each center at the beginning of study. Once the recruitment period was over, each hospital sent the database anonymously to the coordinator (F. X. A.‐J.) via a secure server. Treatment, weaning criteria, and sedation agents were not standardized among centers.
2.2. Inclusion and exclusion criteria
Patients suffering from respiratory failure caused by SARS CoV‐2 infection, confirmed by polymerase chain reaction, requiring IMV and subsequent tracheotomy, performed before July 20, 2020 were included.
Patients with a missing tracheotomy, orotracheal intubation, or outcome date or missing age or sex were excluded. Following our target trial framework, we excluded patients with a tracheotomy performed in the first 7 days after orotracheal intubation.
2.3. Target trial and follow‐up
We used a trial emulation framework to minimize confounding and bias. Our exposure was ET or LT. Our decision time (D7) was 7 days after the initiation of IMV (D0), when we expect a decision of whether a patient should have a tracheotomy to be made and when we would expect to randomize in a randomized trial. All baseline characteristics were considered before or on this date. As all of the study participants received a tracheotomy, an intention‐to‐treat analysis was impossible, and we instead used a per‐protocol analysis. We followed up participants from the day of the tracheotomy (T0) until death, weaning, or the end of July 20, 2020, whichever was sooner. Participants who had not died or weaned by this date were then censored. Figure 1 describes these timings.
FIGURE 1.
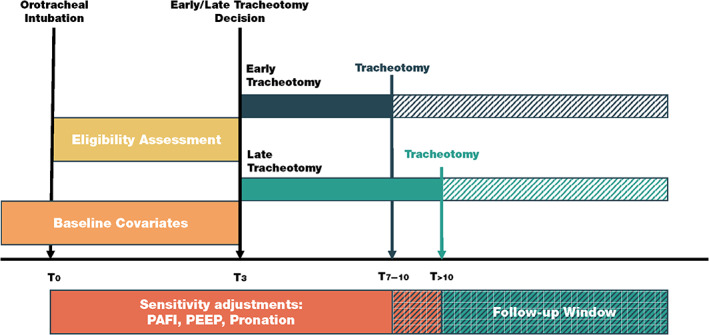
Target trial description. OTI, orotracheal intubation; PAFI, PaO2/FiO2 ratio; PEEP, positive end‐expiratory pressure [Color figure can be viewed at wileyonlinelibrary.com]
2.4. Study outcome
The main outcome was time to weaning, defined as days from tracheotomy to weaning from IMV. Secondary outcomes included death, defined as days from tracheotomy to death, and rates of intraoperative bleeding (excessive bleeding that difficult standard tracheotomy or requiring additional hemostatic measures), postoperative bleeding (bleeding that required revision of stoma) and ventilatory complications (air leak).
2.5. Exposures and measurements
The main exposure variable was ET versus LT. “Early” was defined as occurring on Day 7–10 after orotracheal intubation, and “late” as on Day 11 or later. Sex and year of birth were acquired at hospital admission. We selected the comorbidities that were most likely to be risk factors for COVID‐19 based on previous literature. We included hypertensive disease, immunosuppression, heart failure, autoimmune disease, chronic obstructive pulmonary disease, pregnancy, diabetes mellitus, neuromuscular disease, and ischemic heart disease. We registered the start and end days of pronation cycles. We obtained measures of the PaO2/FiO2 ratio (PAFI) and positive end‐expiratory pressure (PEEP) at intubation (D0), 7 days after intubation (decision date, D7), and at tracheotomy (T0). APACHE II and SOFA scores were obtained at ICU admission. We collected the international normalized ratio (INR), use of anticoagulants, use of vasoactive drugs, presence of secretion problems, and indication at surgery. We also collected total lymphocyte and leukocyte count, INR, D‐dimer, ferritin, lactate dehydrogenase, and C‐reactive protein at admission. These variables are obtained from the electronic medical records, which included analytical parameters, dates of procedures, and ventilatory parameters.
2.6. Ethical approval and informed consent
The local ethics committee approved the study protocol and waved informed consent given the observational nature of the study.
2.7. Statistical analysis
We calculated the proportion or mean and SD of each variable for the population as a whole and stratified by exposure and included/excluded status. We computed weekly and total incidence rates (events per 100 person‐day) of weaning and death overall and stratified by ET versus LT. We plotted cumulative incidence curves of weaning and death by exposure. We fitted a multivariable Cox model to estimate cause‐specific hazard ratios (csHRs) of weaning and death for ET versus LT.
We fitted multivariable Poisson models to estimate the relative risk of intraoperative and postoperative bleeding and ventilatory complications. All models were repeat‐adjusted for age and sex. All models were further adjusted for age, sex, PAFI, PEEP, anticoagulant use, and pronation days.
Missing PAFI, PEEP, APACHE II, anticoagulant use, and comorbidity data were imputed using multiple imputation with chained equations. We used predictive mean matching with 5 k nearest neighbors for continuous variables and logistic models dichotomic variables, generating 100 imputed data sets. 19 We pooled estimators using Rubin's rules. 20
We tested for interactions between tracheotomy timing and age, sex, APACHE II, SOFA, PEEP, PAFI, and days of pronation. We compared the participants in the ET group who did and did not wean within 14 days of intubation.
We performed data management in SPSS 27 (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp). We performed all analyses in STATA version 16.0 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC).
2.8. Study report
We followed the reporting guidelines of the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement for cohort studies. 21
3. RESULTS
Of 794 possible participants, 98 were excluded. Figure 2 shows the flow of patients and numbers included and excluded for each criterion. Table S1 compares the characteristics of the included and excluded patients.
FIGURE 2.
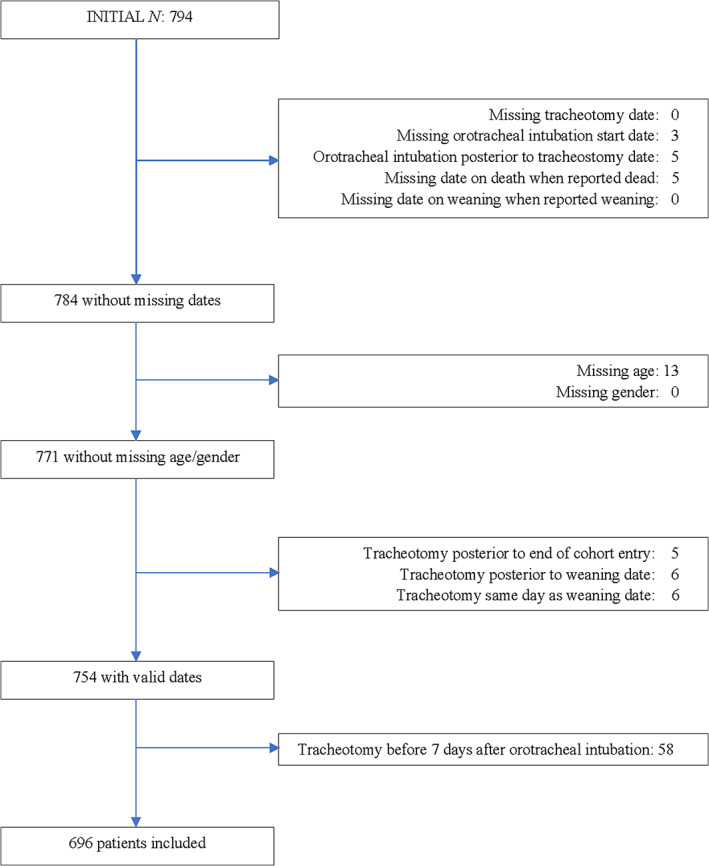
Inclusion and exclusion of study participants [Color figure can be viewed at wileyonlinelibrary.com]
Table 1 shows the baseline characteristics of the study participants, overall and stratified by tracheotomy timing. Of 696 participants receiving a tracheotomy, 215 (30.9%) were women and 142 (20.4%) received an ET. The participants had a mean age of 63 years old. ET and LT recipients did not differ on any collected variables except for PAFI at ICU admission, PEEP on tracheotomy day, use of anticoagulant drugs, and days of pronation before tracheotomy.
TABLE 1.
Baseline characteristics in tracheotomized patients stratified by tracheotomy timing after imputation
| Total | Late (>10 days after OTI) | Early (≤10 days after OTI) | p‐value | |
|---|---|---|---|---|
| N = 696 | N = 554 | N = 142 | ||
| Sex, female | 30.9% | 29.4% | 36.6% | 0.10 |
| Age (years) | 63.0 (10.2) | 63.0 (10.4) | 63.2 (9.2) | 0.86 |
| Tobacco consumption | 0.44 | |||
| Never | 74.6% | 74.2% | 76.1% | |
| Smoker | 16.1% | 15.3% | 19.0% | |
| Missing | 9.3% | 10.5% | 4.9% | |
| Smoking index (pack/year) | 3.4 (12.6) | 3.3 (12.6) | 3.4 (12.6) | 0.96 |
| Missing | 16.8% | 17.1% | 15.5% | 0.64 |
| Weight (kg) | 83.1 (15.5) | 83.0 (15.1) | 83.2 (17.3) | 0.92 |
| Missing | 19.7% | 19.7% | 19.7% | 0.99 |
| Height | 168.6 (9.1) | 168.8 (9.0) | 167.9 (9.3) | 0.35 |
| Missing | 21.8% | 22.0% | 21.1% | 0.82 |
| BMI | 29.3 (5.4) | 29.2 (5.2) | 29.8 (6.3) | 0.31 |
| Missing | 23.7% | 23.5% | 24.6% | 0.77 |
| Comorbidities | ||||
| HBP | 46.6% | 44.6% | 54.2% | 0.04 |
| Immunosuppression | 7.0% | 7.6% | 4.9% | 0.27 |
| Heart failure | 3.4% | 3.4% | 3.5% | 0.96 |
| Autoimmune disease | 5.7% | 6.1% | 4.2% | 0.38 |
| COPD | 7.2% | 7.0% | 7.7% | 0.77 |
| Pregnancy | 0.4% | 0.5% | 0.0% | 0.38 |
| DM | 21.6% | 20.8% | 24.6% | 0.31 |
| Neuromuscular disease | 1.4% | 1.4% | 1.4% | 0.97 |
| Ischemic cardiopathy | 9.3% | 8.8% | 11.3% | 0.38 |
| APACHE II | 15.1 (6.6) | 15.3 (6.7) | 11.2 (6.1) | 0.12 |
| Missing | 18.2% | 16.8% | 23.9% | 0.05 |
| SOFA | 6.1 (3.6) | 6.0 (3.4) | 6.7 (4.4) | 0.09 |
| Missing | 21.8% | 22.6% | 19.0% | 0.36 |
| INR at tracheotomy | 1.6 (2.1) | 1.5 (1.9) | 1.8 (2.7) | 0.15 |
| Missing | 17.8% | 18.6% | 14.8% | 0.29 |
| PAFI at intubation | 142.1 (70.0) | 139.2 (69.2) | 153.8 (72.1) | 0.03 |
| Missing | 0.0% | 0.0% | 0.0% | |
| PAFI at Day 7 | 182.9 (73.6) | 182.6 (74.7) | 183.9 (69.8) | 0.86 |
| Missing | 13.2% | 14.6% | 7.7% | 0.03 |
| PAFI at tracheotomy | 192.7 (69.3) | 195.0 (69.7) | 184.1 (67.4) | 0.10 |
| Missing | 7.6% | 8.7% | 3.5% | 0.04 |
| PEEP at intubation | 12.6 (5.1) | 12.6 (5.6) | 12.5 (3.2) | 0.99 |
| Missing | 10.8% | 12.3% | 4.9% | 0.01 |
| PEEP at Day 7 | 11.1 (7.8) | 11.3 (8.6) | 10.6 (3.3) | 0.41 |
| Missing | 13.9% | 15.2% | 9.2% | 0.07 |
| PEEP at tracheotomy | 9.7 (3.0) | 9.5 (2.9) | 10.6 (3.4) | <0.001 |
| Missing | 0.0% | 0.0% | 0.0% | |
| Pronation days at Day 7 | 4.9 (2.9) | 5.0 (2.5) | 4.6 (4.1) | 0.19 |
| Complications | 0.41 | |||
| Ventilator problems | 13.9% | 14.4% | 12.0% | |
| Missing | 1.1% | 1.4% | 0.0% | |
| Anticoagulant treatment | 56.2% | 60.1% | 40.8% |
<0.001 |
| Vasoactive drugs at tracheotomy | 40.4% | 40.8% | 38.7% | |
| Missing | 12.8% | 13.9% | 8.5% | |
| Vasoactive drugs at OTI | 52.3% | 52.7% | 50.7% | 0.67 |
| Missing | 4.9% | 4.9% | 4.9% | |
| Secretions problems | 0.33 | |||
| No | 73.7% | 72.0% | 80.3% | |
| Increase pressure | 12.6% | 13.2% | 10.6% | |
| Obstruction | 3.9% | 3.4% | 5.6% | |
| Missing | 9.8% | 11.4% | 3.5% | |
| Indication tracheotomy | 0.07 | |||
| Prolonged mechanical ventilation | 81.6% | 83.2% | 75.4% | |
| Secretions management | 10.2% | 9.6% | 12.7% | |
| Other | 8.0% | 7.0% | 12.0% | |
| Missing | 0.1% | 0.2% | 0.0% | |
| Lymphocyte count | 5304.9 (26 886.6) | 6167.0 (29 741.4) | 1961.1 (9140.1) | 0.10 |
| Missing | 1.9% | 2.0% | 1.4% | 0.65 |
| INR | 1.6 (2.3) | 1.5 (2.0) | 2.1 (3.0) | 0.02 |
| Missing | 8.5% | 8.8% | 7.0% | 0.49 |
| D‐dimer | 1515.0 (1734.6) | 1511.5 (1733.5) | 1528.3 (1746.4) | 0.93 |
| Missing | 22.1% | 22.6% | 20.4% | 0.58 |
| Ferritin | 1367.7 (1312.6) | 1385.0 (1298.7) | 1300.2 (1369.6) | 0.55 |
| Missing | 24.9% | 24.9% | 24.6% | 0.95 |
| LDH | 599.0 (705.3) | 571.5 (564.8) | 70.4 (1081.7) | 0.06 |
| Missing | 13.8% | 14.3% | 12.0% | 0.48 |
| Leukocyte count | 4538.0 (9721.8) | 4497.1 (10 380.7) | 4697.2 (6581.9) | 0.83 |
| Missing | 1.6% | 1.6% | 1.4% | 0.85 |
| Lymphocytes | 62.0 (180.1) | 64.5 (186.3) | 52.6 (154.2) | 0.48 |
| Missing | 1.4% | 1.8% | 0.0% | 0.11 |
| CRP | 20.9 (22.7) | 21.2 (22.5) | 20.0 (23.7) | 0.65 |
| Missing | 44.1% | 47.3% | 31.7% | <0.001 |
Note: Categorical variables are expressed in count (%) and continuous variables are expressed in mean (SD).
Abbreviations: APACHE, Acute Physiology and Chronic Health disease Classification System; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; DM, diabetes mellitus; HBP, high blood pressure; INR; international normalized ratio; LDH, lactate dehydrogenase; OTI, orotracheal intubation; PAFI, PaO2/FiO2 ratio; PEEP, positive end‐expiratory pressure; SOFA, Sequential Organ Failure Assessment score.
Table S2 compares the frequency of pronation, days of pronation, and whether pronation finished after or before tracheotomy in the two groups. Participants with LTs had more total days of pronation (9.5 days for late vs. 6.8 for early) and more often had their last pronation cycle before tracheotomy (50% for late and 33% for early). The proportion of participants pronated in the first 7 days after orotracheal intubation and how many days these participants were pronated were similar in the LT and ET groups.
The ET group weaned more quickly than the late group (Table 2). The median follow‐up time from tracheotomy to weaning or death was 13 days for LT and 12 days for ET. Among those who were successfully weaned, participants with an ET was seen 11 days of weaning since tracheotomy and 19 days since orotracheal intubation, whereas LT group was seen 12 days and 29 days, respectively.
TABLE 2.
Days to weaning by tracheotomy timing, since orotracheal intubation and since tracheotomy
| Early tracheotomy | Late tracheotomy | |||||
|---|---|---|---|---|---|---|
| Median | p25 | p75 | Median | p25 | p75 | |
| Days to weaning or death or censoring | ||||||
| Since tracheotomy (T0) | 12 | 6 | 22 | 13 | 7 | 26 |
| Since decision (D7) | 13 | 8 | 24 | 23 | 16 | 37 |
| Since orotracheal intubation (D0) | 20 | 15 | 31 | 30 | 23 | 44 |
| Days to weaning (those who wean) | ||||||
| Since tracheotomy (T0) | 11 | 6 | 17 | 12 | 7 | 21 |
| Since decision (D7) | 12 | 8 | 20 | 22 | 16 | 33 |
| Since orotracheal intubation (D0) | 19 | 15 | 27 | 29 | 23 | 40 |
| Days to death (those who die) | ||||||
| Since tracheotomy (T0) | 13 | 7 | 23 | 14 | 7 | 25 |
| Since decision (D7) | 16 | 9 | 26 | 24 | 17 | 35 |
| Since orotracheal intubation (D0) | 23 | 16 | 33 | 31 | 24 | 42 |
The LT group had a lower rate of successful weaning: 360 of the 554 participants in the LT group were weaned before the end of follow‐up (3.1 patients weaning per 100 patient‐days [2.8–3.4]), whereas 102 out of 142 were weaned in the ET group (3.9 patients weaning per 100 patient‐days [3.1–4.7]). The ET group therefore had a crude csHR of 1.25 (1.00–1.56) and 1.18 (0.93–1.51) after adjusting of weaning post‐tracheotomy (Table 3).
TABLE 3.
Associations of tracheotomy timing with time to weaning and time to death
| Weaning | Death | |
|---|---|---|
| csHR (95% CI) | csHR (95% CI) | |
| Early vs. late tracheotomy | ||
| Cox | 1.25 (1.00–1.56) | 0.85 (0.60–1.21) |
| Cox age and gender adjusted | 1.25 (1.00–1.56) | 0.87 (0.61–1.25) |
| Cox fully adjusted | 1.18 (0.93–1.51) | 0.90 (0.62–1.30) |
Note: Fully adjusted model included PAFI, PEEP, anticoagulant treatment, and pronation days as covariates.
Abbreviations: CI, confidence interval; csHR, cause‐specific hazard ratio; PAFI, PaO2/FiO2 ratio; PEEP, positive end‐expiratory pressure; RR, relative risk; sdHR, subdistribution hazard ratio.
Both groups had a mortality rate of 0.32 deaths per 100 patient‐days (0.23–0.44), with 170/554 participant deaths in the late group and 38/142 in the early group (0.32 deaths per 100 patient‐days [0.23–0.44]), giving an unadjusted csHR of 0.85 (95% confidence interval [CI] = 0.60–1.21) and a fully adjusted csHR of 0.90 (0.62–1.30). Figures 3 and 4 show cumulative incidence function plots for weaning and death and Table 3 reports csHRs for weaning and death in unadjusted, age‐ and sex‐adjusted, and fully adjusted multivariable models.
FIGURE 3.
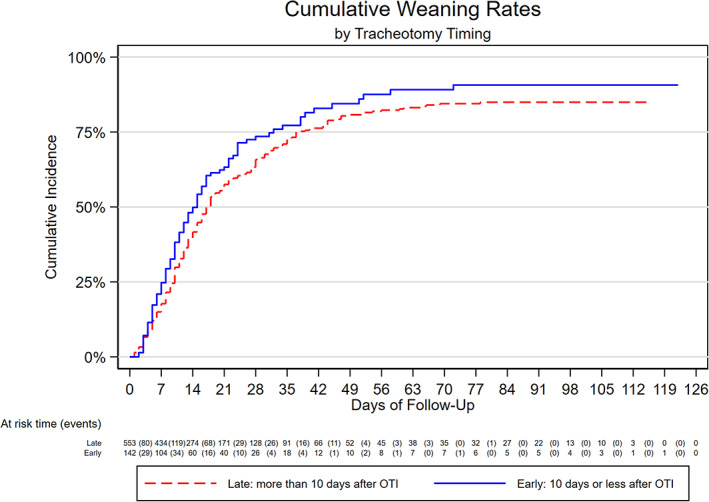
Cumulative incidence of weaning by tracheotomy timing [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.
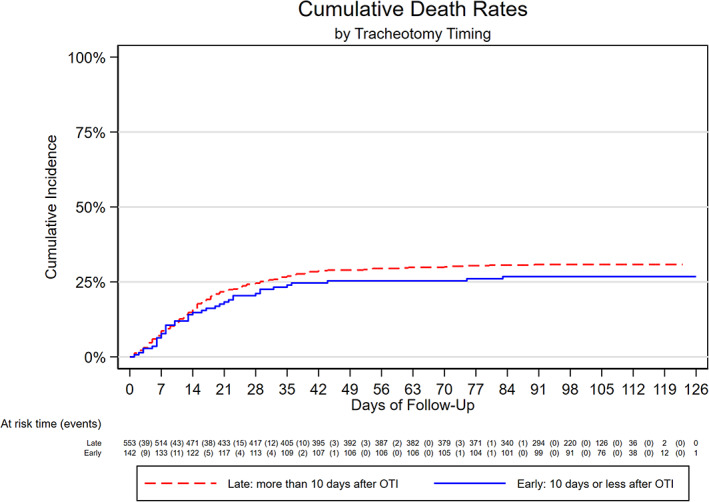
Cumulative incidence of death by tracheotomy timing [Color figure can be viewed at wileyonlinelibrary.com]
ET was associated with a risk ratio for intraoperative complications of 0.57 (95% CI = 0.24–1.34) in the crude analyses and fully adjusted of 0.56 (0.23–1.33) in the adjusted analyses. We did not observe any differences in the risk of any studied intraoperative or postoperative complications between the two groups (Table 4).
TABLE 4.
Associations of tracheotomy timing with intraoperative and postoperative complications incidence
| N | Crude | Fully adjusted | ||
|---|---|---|---|---|
| Early | Late | RR (95% CI) | RR (95% CI) | |
| Early vs. late tracheotomy | ||||
| Intraoperative | 6 | 41 | 0.57 (0.24–1.34) | 0.56 (0.23–1.33) |
| Bleeding | 4 | 20 | 0.78 (0.27–2.28) | 0.80 (0.27–2.37) |
| Ventilatory problems | 4 | 22 | 0.71 (0.24–2.05) | 0.69 (0.23–2.05) |
| Postoperative | 41 | 136 | 1.17 (0.83–1.66) | 1.10 (0.77–1.59) |
| Bleeding | 33 | 102 | 1.26 (0.85–1.87) | 1.19 (0.79–1.79) |
| Ventilatory problems | 8 | 34 | 0.92 (0.42–1.98) | 0.85 (0.39–1.85) |
Note: Fully adjusted model included PaO2/FiO2 (PAFI), positive end‐expiratory pressure (PEEP), anticoagulant treatment, and pronation days as covariates.
Abbreviations: CI, confidence interval; RR, relative risk.
Participants with ET who were weaned in less than 14 days after orotracheal intubation were younger (63.8 vs. 60.2 years) and had a higher PAFI at the time of tracheotomy (216.6 vs. 177.6) than those who took more than 2 weeks to be weaned (Table S4).
4. DISCUSSION
To the best of our knowledge, this is the largest study evaluating the optimal timing of tracheotomy in a multicenter prospective cohort of ICU‐admitted patients with COVID‐19 who required IMV. Our results suggest that ET leads to similar or faster weaning without increased complications or mortality. When clinically appropriate, the ET strategy might therefore be preferable, as it can release ICU space.
The optimal timing of tracheotomy may be influenced by many factors, such as the initial cause of IMV, the severity of the disease, neurological status, complications, and the possibility of recovery. In the COVID‐19 scenario, the initial cause of IMV is always severe respiratory failure, which may have high pronation requirements and be complicated by systemic failure, thrombosis, or other COVID‐19‐related conditions.
Many consensus documents were published in 2020 on best practices for tracheotomy in critically ill COVID‐19 patients, generally aiming to prevent surgeon infection. Most recommended delayed tracheotomy, 14 while others focused on the type of tracheotomy, 15 the necessary protective equipment, 16 , 17 or where best to perform a tracheotomy. 22 Almost no existing guidance evaluated which clinical parameters influence weaning outcomes, total days of IMV, or mortality.
Bier‐Laning et al. analyzed the tracheotomy protocols and practices put in place by 29 institutions around the world in response to the COVID‐19 pandemic. They found insufficient evidence for recommending a specific timing for tracheotomy in COVID‐19‐related respiratory failure. 23 Tornari et al. conducted an observational cohort study to understand the factors that influenced the trajectory from tracheotomy to decannulation to facilitate ICU capacity planning and improve outcomes. Higher FiO2 at tracheotomy time and higher pretracheotomy peak cough flow were associated with longer delays in decannulation of COVID‐19 tracheotomy patients. 24
Recent publications have suggested that earlier tracheotomy might facilitate the weaning process and reduce the length of mechanical ventilation required. Avilés‐Jurado et al. 18 evaluated 50 consecutive patients that required tracheotomy in the first wave of the pandemic in Spain in one single center. ET reduced the duration of IMV by reducing the days between orotracheal intubation and the tracheotomy procedure, releasing ICU beds for other patients. Our multicenter study of 696 tracheotomized patients from the first wave of COVID‐19 confirms their preliminary findings. We found a 31% (2%–81%) reduction in the time from orotracheal intubation to weaning when tracheotomy was performed early (fully adjusted HR [95% CI]: 1.31 [1.02–1.81]), without increasing complications or mortality rates.
The ideal timing for a tracheotomy in patients receiving IMV also remains controversial in other disease scenarios, despite decades of experience of using this technique. Two large randomized prospective studies addressing this topic have been published so far. Terragni et al. randomized 419 patients to ET (6–9 days of intubation) or LT (13–15 days of intubation). 25 They found no differences in complications, pneumonia associated with mechanical ventilation or mortality at 28 days of intubation. Young et al. randomized 909 patients to early (within 10 days of intubation) or LT (after 10 days of intubation) and also found no difference in mortality. 26 Neither of these randomized controlled trials examined the days of mechanical ventilation as an outcome. Our results agree with the trials' finding of no difference in mortality between ET and LT, but we also found a 31% reduction in IMV duration in the ET group.
A Cochrane review published in 2015 found that patients who underwent early (≤10 days) tracheotomy had a higher probability of discharge from the ICU at Day 28 than those who underwent later tracheotomy. 3 A recent meta‐analysis found that ET was associated with shorter mechanical ventilation and hospital stays, without differences in mortality. 25 Our findings agree with these synthesized results. Reducing IMV and ICU stays is extremely important given the current shortage of ICU beds.
In daily practice, the decision about when to perform a tracheotomy is based on clinical and ventilatory criteria, previous institutional experience, and staff availability to do the procedure. To overcome the lack of a prospective randomized trial, we emulated a trial framework randomizing at Day 7, which is approximately when the necessity of doing a tracheotomy arises. We included demographic and objective parameters of severity respiratory failure in the analysis. We also included parameters known to influence the intensivist decision, such as pronation requirements, PAFI, PEEP levels, age, comorbidities, complications, difficulties in secretions management, and poor prognosis. In our study, treatment, sedation agents, and weaning criteria were not standardized among centers. Although it may be seen as a drawback, it represents real practice in the present pandemic scenario, and may give external validity to the cohort.
McGrath et al. 14 published a consensus document suggesting that tracheotomy be delayed until at least Day 10 of mechanical ventilation in COVID‐19 patients and only be considered if patients showed signs of clinical improvement. They also advised against tracheotomy when patients needed high fractions of inspired oxygen (FiO2), the prone position, or high ventilator requirements. Our participants in the ET and LT groups had similar characteristics at admission. Although the early group had higher PEEP at tracheotomy day (10.6 vs. 9.5, p < 0.001), this difference has limited clinical value.
The prone position is often used to improve the ventilation/perfusion quotient in patients with COVID‐19. The presence of a tracheotomy can make pronation maneuvers more difficult, and cannula displacement may also be favored. Some guidelines suggest tracheotomy be delayed if prone maneuvers are still required or even discourage tracheotomy altogether. 9 , 14 However, pronation is not a formal contraindication for ET. As expected, our LT group had greater pronation requirements than our early group. Nonetheless, 63.4% of the ET patients were proned at some point, mostly (60.6%) before tracheotomy. Almost 25% continued pronation after tracheotomy without major incidences. Our results therefore suggest that pronation should not rule out ET and that patients should be considered on a case‐by‐case basis.
We found that the shorter IMV duration seen in the ET group was independent of ventilatory parameters at admission or tracheotomy. Previous reports based on smaller series have described similar, albeit not statistically significant, findings. 18
One of the criticisms of doing an ET is that we may perform more procedures than is strictly necessary. To test this hypothesis, we compared our ET participants who were weaned within 2 weeks of intubation (early) with those who were weaned after 2 weeks. Participants who weaned earlier were younger and had higher PAFI at tracheotomy, suggesting that the tracheotomy decision could be delayed in younger patients with clear signs of improvement. One can argue that perhaps some of these patients could have been extubated skipping a tracheotomy, or at least a trial of extubation being performed first. However, in the early days of the pandemic, lack of knowledge about the behavior of the disease, the risk of infection of staff, and the possibility of reintubation led to a conservative approach to risky maneuvers.
Another criticism of ET is the higher risk of complications in critically ill patients. The high mortality rate initially reported for COVID‐19 patients favored avoiding invasive maneuvers, including surgery. Many of these patients also required intensive pronation, and some received anticoagulant drugs due to concomitant thrombosis, which may increase tracheotomy complications. Relevant postoperative complications after tracheotomy in non‐COVID‐19 patients range between 5.6% and 27.2%. 27 , 28 , 29 In contrast, Long and colleagues found that up to 55.3% of tracheotomized COVID‐19 patients were seen postoperative complications within the first 30 days, with no differences between percutaneous and open procedures. 15 The main complications were local infection (36%), hemorrhage (19%), and subcutaneous emphysema (8.5%). Other studies have shown that minor bleeding from the stoma, usually managed with local measures, is the most common complication in tracheotomized COVID‐19 patients and that few patients need revision surgery. We found a complication rate of 4.2% for ET and 11.2% for LT across our 696 participants from different institutions, tracheotomy types, and timelines. This difference was not statistically significant.
Our study has limitations as well as strengths. The study design and inclusion criteria prevented us from analyzing the causal effects of tracheotomy timing on total days of IMV. Although the observed differences in total IMV duration post‐tracheotomy described here are attributable to the tracheotomy timing, the observed differences in total duration of IMV were artificially inflated by immortal time bias 30 and should not be interpreted as causal estimates. A randomized controlled trial with an intention‐to‐treat analysis would be preferable to establish the effect of ET on the total duration of IMV for patients admitted to ICU with COVID‐19, although it is unlikely that it can be performed in the pandemic scenario.
As we used observational data, one can argue that residual confounding could be at least partially responsible for the observed findings. The most important bias could have been confounding by indication, where doctors may have decided to postpone tracheotomy in patients that were critically ill on the decision day. However, although the groups were not totally comparable, we did not find any evidence of confounding by indication, with baseline characteristics well balanced between the ET and LT groups. Moreover, multivariable adjustment, including APACHE II, SOFA, PAFI, PEEP, anticoagulant use, and pronation, did not attenuate the observed effects, with a good statistical power.
Among the strengths of the present study are the large number of participants and the prospective cohort design. We designed the analyses following a trial emulation framework 31 with randomization at Day 7 of intubation for robustness. Using multiple centers, each with their own local protocols, no standardized weaning criteria or sedation agents, and variations in the type of tracheotomy, we ensured that this study has external validity. In addition, by collecting multiple objective clinical and ventilatory variables, we were able to gather wide, robust prognosis information. These aspects could have influenced the observed weaning and mortality rates. However, we expect any potential differences to be hospital‐specific and therefore uninformative.
In conclusion, our prospective cohort study suggests that ET, when appropriate, may provide quicker weaning and ICU discharge for COVID‐19 patients without added complications or increased mortality. These findings may help to release ICU beds, which is particularly necessary during the pandemic outbreak.
CONFLICT OF INTEREST
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: Daniel Prieto‐Alhambra reports grants and other from AMGEN; grants, nonfinancial support, and other from UCB Biopharma; grants from Les Laboratoires Servier, outside the submitted work; and Janssen, on behalf of IMI‐funded EHDEN and EMIF consortiums, and Synapse Management Partners have supported training programs organized by Daniel Prieto‐Alhambra's department and open for external participants. Albert Prats‐Uribe reports grants from Fundación Alfonso Martin Escudero and the Medical Research Council. Pedro Castro reports honoraria received for talks on behalf of Merck Sharp and Dohme, Pfizer, Gilead, and Alexion. Isabel Vilaseca reports honoraria received for talks on behalf of Merck Sharp and Dohme and Lumenis outside the submitted work. The authors confirm that there are no other relationships or activities that could appear to have influenced the submitted work. The authors declare no conflict of interest related to the study.
AUTHOR CONTRIBUTIONS
All authors contributed to the design of the study, interpretation of the results, and manuscript review. Albert Prats‐Uribe, Daniel Prieto‐Alhambra, and Francesc Xavier Avilés‐Jurado had access to the data, performed the statistical analysis, and acted as guarantor. Albert Prats‐Uribe wrote the first draft of the manuscript. Francesc Xavier Avilés‐Jurado and Isabel Vilaseca are the senior authors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
TRANSPARENCY DECLARATION
Francesc‐Xavier Avilés‐Jurado affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
The authors acknowledge English language editing by Dr Jennifer A. de Beyer of the Centre for Statistics in Medicine, University of Oxford. We thank the Spanish Society of Otorhinolaryngology‐Head Neck Surgery (SEORL‐CCC) for facilitating communication between centers.
TraqueoCOVID SEORL Group: Ana García Miguélez (Hospital Marqués de Valdecilla. Santander. Spain); Antonio Belinchon (Hospital General de Albacete. Albacete. Spain); Yolanda Escamilla Carpintero (Hospital Parc Tauli. Sabadell. Spain Barcelona); Jesús Martínez Salazar (Hospital Universitario del Sureste. Arganda del Rey. Spain); Fabián Alzate (Hospital Santa Tecla, Tarragona. Spain); Zenaida Piñeiro (Hospital de Mar. Barcelona. Spain); Alfonso Bonilla (Hospital Son Llàtzer. Mallorca. Spain) José Manuel Morales Puebla (Hospital Quironsalud de Ciudad Real. Ciudad Real. Spain); José Ignacio Benito Orejas (Hospital Clínico de Valladolid. Valladolid. Spain); Antonio Miguel Moreno Rueda (Hospital Universitario de Móstoles. Móstoles. Spain); Silvia Verónica Domínguez Ovejas (Hospital Universitario Severo Ochoa. Leganés. Spain); Mariana Maldonado (Hospital del Vinalopó. Elche. Spain); Kiara Tudela Cabello (Hospital Universitario Basurto. Bilbao. Spain); Enrique Coscarón (Complejo Asistencial de Zamora. Zamora. Spain); Carlos Calvo (Hospital Plató. Barcelona. Spain); Jorge Prada Pendolero (Hospital Universitario de La Princesa. Madrid. Spain); Jorge Ignacio de Abajo Larriba (CUN Madrid. Madrid. Spain); Alfonso Campos González (HU Fundación Jiménez Díaz. Madrid. Spain); Juan Carlos Villatoro (Hospital General de Catalunya. Sant Cugat. Spain); Claudia Vera Ching (Hospital Universitari Josep Trueta. Girona. Spain).
Prats‐Uribe A, Tobed M, Villacampa JM, et al. Timing of elective tracheotomy and duration of mechanical ventilation among patients admitted to intensive care with severe COVID‐19: A multicenter prospective cohort study. Head & Neck. 2021;43(12):3743‐3756. doi: 10.1002/hed.26863
Isabel Vilaseca and Francesc Xavier Avilés‐Jurado contributed equally to this work.
Funding informationThe research was partially supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). D. P.‐A. is funded through an NIHR Senior Research Fellowship (Grant number SRF‐2018‐11‐ST2‐004). A. P.‐U. is supported by Fundación Alfonso Martín Escudero and the Medical Research Council (grant numbers MR/K501256/1, MR/N013468/1). I. V. and F. X. A.‐J. are supported by Agència de Gestió d'Ajuts Universitaris i de Recerca AGAUR (Grant 2017‐SGR‐01581).
The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Esteban A, Anzueto A, Alia I, et al. How is mechanical ventilation employed in the intensive care unit: an international utilization review. Am J Respir Crit Care Med. 2000;161:1450‐1458. [DOI] [PubMed] [Google Scholar]
- 2. Mehta AB, Cooke CR, Wiener RS, et al. Hospital variation in early tracheotomy in the United States: a population‐based study. Crit Care Med. 2016;44:1506‐1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andriolo BN, Andriolo RB, Saconato H, Atallah AN, Valente O. Early versus late tracheotomy for critically ill patients. Cochrane Database Syst Rev. 2015;1:CD007271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang H, Li Y, Ariani F, Chen X, et al. Timing of tracheotomy in critically ill patients: a meta‐analysis. PLoS One. 2014;9:e92981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID‐19 outbreak: Wuhan's experience. Anesthesiology. 2020;132:1317‐1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan W‐j, Ni Z‐y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burn E, Sena AG, Prats‐Uribe A, et al. Use of dialysis, tracheostomy, and extracorporeal membrane oxygenation among 240,392 patients hospitalized with COVID‐19 in the United States. February 12, 2021. Accessed February 15, 2021. https://www.medrxiv.org/content/10.1101/2020.11.25.20229088v2
- 8. Piazza C, Filauro M, Dikkers FG, et al. Long‐term intubation and high rate of tracheostomy in COVID‐19 patients might determine an unprecedented increase of airway stenoses: a call to action from the European Laryngological Society. Eur Arch Otorhinolaryngol. 2020;6:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith D, Montagne J, Raices M, et al. Tracheostomy in the intensive care unit: guidelines during COVID‐19 worldwide pandemic. Am J Otolaryngol. 2020;41:102578. 10.1016/j.amjoto.2020.102578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shiba T, Ghazizadeh S, Chhetri D, St. John M, Long J. Tracheostomy considerations during the COVID‐19 pandemic. OTO Open. 2020;4:2473974X20922528. 10.1177/2473974X20922528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sommer DD, Engels PT, Weitzel EK, et al. Recommendations from the CSO‐HNS taskforce on performance of tracheotomy during the COVID‐19 pandemic. J Otolaryngol Head Neck Surg. 2020;49:23. 10.1186/s40463-020-00414-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chao TN, Braslow BM, Martin ND, et al. Tracheotomy in ventilated patients with COVID‐19. Ann Surg. 2020;272:e30‐e32. 10.1097/SLA.0000000000003956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tay JK, Khoo ML, Loh WS. Surgical considerations for tracheostomy during the COVID‐19 pandemic: lessons learned from the severe acute respiratory syndrome outbreak. JAMA Otolaryngol Head Neck Surg. 2020;146:517‐518. 10.1001/jamaoto.2020.0764 [DOI] [PubMed] [Google Scholar]
- 14. McGrath BA, Brenner MJ, Warrillow SJ, et al. Tracheostomy in the COVID‐19 era: global and multidisciplinary guidance. Lancet Respir Med. 2020;8:717‐725. 10.1016/S2213-2600(20)30230-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Long SM, Chern A, Feit NZ, et al. Percutaneous and open tracheostomy in patients with COVID‐19: comparison and outcomes of an institutional series in New York City. Ann Surg. 2021;273:403‐409. [DOI] [PubMed] [Google Scholar]
- 16. Takhar A, Walker A, Tricklebank S, et al. Recommendation of a practical guideline for safe tracheotomy during the COVID‐19 pandemic. Eur Arch Otorhinolaryngol. 2020;277:2173‐2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bernal‐Sprekelsen M, Avilés‐Jurado FX, Álvarez‐Escudero J, et al. Consensus document of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC), the Spanish Society of Otorhinolaryngology and Head and Neck Surgery (SEORL‐CCC) and the Spanish Society of Anesthesiology and Resuscitation (SEDAR) on tracheotomy in patients with COVID‐19 infection. Acta Otorrinolaringol Esp. 2020;71:386‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Avilés‐Jurado FX, Prieto‐Alhambra D, González‐Sánchez N, et al. Timing, complications, and safety of tracheotomy in critically ill patients with COVID‐19. JAMA Otolaryngol Head Neck Surg. 2020;8:e203641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morris TP, White IR, Royston P. Tuning multiple imputation by predictive mean matching and local residual draws. BMC Med Res Methodol. 2014;14:75. 10.1186/1471-2288-14-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley; 1987. [Google Scholar]
- 21. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344‐349. [DOI] [PubMed] [Google Scholar]
- 22. Picetti E, Fornaciari A, Taccone FS, et al. Safety of bedside surgical tracheostomy during COVID‐19 pandemic: a retrospective observational study. PLoS One. 2020;15:e0240014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bier‐Laning C, Cramer JD, Roy S, et al. Tracheostomy during the COVID‐19 pandemic: comparison of international perioperative care protocols and practices in 26 countries. Otolaryngol Head Neck Surg. 2021;164(6):1136‐1147. [DOI] [PubMed] [Google Scholar]
- 24. Tornari C, Surda P, Takhar A, et al. Tracheostomy, ventilatory wean, and decannulation in COVID‐19 patients. Eur Arch Otorhinolaryngol. 2020;1:1‐10. 10.1007/s00405-020-06187-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Terragni PP, Antonelli M, Fumagalli R, et al. Early vs late tracheostomy for prevention of pneumonia in mechanically ventilated adult ICU: a randomized controlled trial. JAMA. 2010;303:1483‐1489. 10.1001/jama.2010.447 [DOI] [PubMed] [Google Scholar]
- 26. Young D, Harrison D, Cuthbertson B, et al. Early vs late tracheostomy for prevention of pneumonia in mechanically ventilated adult ICU. The tracman randomized trial. JAMA. 2013;309:2121‐2129. 10.1001/jama.2013.5154 [DOI] [PubMed] [Google Scholar]
- 27. Oliver ER, Gist A, Gillespie MB. Percutaneous versus surgical tracheotomy: an updated meta‐analysis. Laryngoscope. 2007;117:1570‐1575. 10.1097/MLG.0b013e318093edae [DOI] [PubMed] [Google Scholar]
- 28. Halum SL, Ting JY, Plowman EK, et al. A multi‐institutional analysis of tracheotomy complications. Laryngoscope. 2012;122:38‐45. 10.1002/lary.22364 [DOI] [PubMed] [Google Scholar]
- 29. Ülkümen B, Eskiizmir G, Tok D, Çivi M, Çelik O. Our experience with percutaneous and surgical tracheotomy in intubated critically ill patients. Turk Arch Otorhinolaryngol. 2018;56:199‐205. 10.5152/tao.2018.3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suissa S. Immortal time bias in pharmaco‐epidemiology. Am J Epidemiol. 2008;167:492‐499. 10.1093/aje/kwm324 [DOI] [PubMed] [Google Scholar]
- 31. Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183:758‐764. 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


