1. INTRODUCTION
In an effort to fight the ongoing SARS‐CoV‐2 pandemic, the mRNA‐1273 SARS‐CoV‐2 vaccine manufactured by Moderna has been granted Emergency Use Authorization, because of its high efficacy and safety profile. 1 We report the first documented case of AHA after receiving the mRNA‐1273 SARS‐CoV‐2 vaccine.
2. CASE
This is a 70‐year‐old male patient with a past medical history of polymyalgia rheumatica and hepatitis C virus with spontaneous clearance, who presented with extensive right upper extremity bruising 8 days after receiving the first dose of the mRNA‐1273 SARS‐CoV‐2 vaccine. He was in his usual state of health when he received the vaccine. The vaccine was applied to his right upper extremity, and his immediate post‐vaccine monitoring period elapsed without complications. On the same day, he developed arm soreness at the injection site, and 2 days after, he noticed an ecchymosis on his right shoulder. The ecchymosis progressed distally over the following days reaching his elbow on day four and extending to his hand on day five, despite no additional trauma to the area. The expanding ecchymosis was associated with right arm swelling, soreness, tightness, and limited range of motion. By day seven, the swelling and range of motion in his right hand started to improve, but he developed a new ecchymosis on his left forearm.
On presentation to the hospital, he denied right upper extremity pain, pressure, numbness, tingling, and decreased range of motion. He denied any other bleeding symptoms. He did not have a prior history of bleeding. The patient denied any family history of bleeding, clotting, or autoimmune disorders. His home medications included prednisone 10 mg daily and aspirin/paracetamol/caffeine (Excedrin) 1500 mg daily. He was afebrile and hemodynamically stable on admission. Physical examination was notable for extensive ecchymosis from his right shoulder to right hand (Figure 1) with preserved range of motion and sensation. Distal pulses were present and symmetric in both extremities and swelling had markedly decreased from pictures taken earlier in the week. Ecchymoses were also noted on his left forearm (4 × 1 inches) and right lower extremity (3 × 3 inches). Laboratory testing demonstrated prolonged aPTT, 57.5 s, and normal prothrombin time (PT), 13.5 s. Further investigation demonstrated failure of prolonged aPTT to correct with PTT mix, 45.9 s. FVIII activity was decreased, .03 IU/ml, and a FVIII inhibitor was present, 39.9 Bethesda units (BU). Other pertinent coagulation laboratory tests are noted in Table 1. White blood cell count was 22.2 × 109 per L (79% neutrophils), haemoglobin was 9.5 g/dl (baseline 12 g/dl) and platelet count was 554 × 109 per L. Erythrocyte sedimentation rate and C‐reactive protein were elevated, 75 mm/h and 3.2 mg/dl, respectively. Upper extremity CT angiogram with and without intravenous contrast showed patent right upper extremity arterial vasculature without evidence of arterial injury.
FIGURE 1.
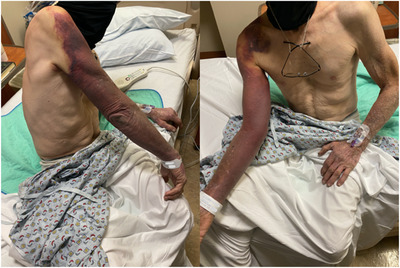
Ecchymosis on admission day
TABLE 1.
Admission laboratories
| Laboratories | Value | Reference range |
|---|---|---|
| aPTT | 57.5 s | 23–3.8 |
| PT | 13.5 s | 9.4–11.2 |
| PTT mix | 45.9 s | 25.6–40.8 |
| FVIII activity | .03 IU/ml | .60– 2.50 |
| Factor IX activity | 1.5 IU/ml | .60–1.50 |
| Factor XI activity | .85 IU/ml | .60–1.40 |
| Factor XII activity | .95 IU/ml | .40—1.40 |
| FVIII inhibitor | 39.9 BU | < = .4 |
Our patient was diagnosed with AHA given the findings of severe ecchymoses, prolonged aPTT that did not correct with mix, decreased FVIII activity, the presence of a FVIII inhibitor, and no prior history of bleeding. 2 , 3 Right upper extremity bleeding was initially treated with FEIBA (anti‐inhibitor coagulant complex) 5000 IU every 6 h alternating every 3 h with recombinant factor VIIa 90 mcg/kg every 6 h for the first 24 h then FEIBA alone thereafter. Immunosuppression was started with prednisone 1 mg/kg daily and cyclophosphamide 2 mg/kg daily along with trimethoprim sulfamethoxazole for Pneumocystis jirovecii prophylaxis. 4 FEIBA dosing was tapered to every 12 h after 3 days then stopped on day 6. At that time, FVIII activity was increased to .07 IU/ml and FVIII inhibitor was decreased to 11.4 BU. Right upper extremity bleeding had resolved and ecchymoses had improved. The patient was discharged home on prednisone and cyclophosphamide along with plan for outpatient haematology follow up.
3. DISCUSSION
AHA is a rare disorder caused by autoantibodies against endogenous FVIII that result in bleeding and affect between 1.2 and 1.5 persons per million per year. 5 Risk factors associated with the development of AHA include a history of autoimmune disease, age greater than 65 years, malignancy, and medications, such as interferon alpha or fludarabine; however, 50% of cases are idiopathic. 2 , 5 Most cases present spontaneously or after a surgical procedure. 6 There have been a few cases of AHA following administration of the influenza vaccine, 7 , 8 but to date no cases have been reported following COVID‐19 vaccine administration.
In the midst of an ongoing global pandemic, in December 2020, the Food and Drug Administration granted Emergency Use Authorization for the mRNA‐1273 SARS‐CoV‐2 vaccine manufactured by Moderna. In the phase 3 trial, the mRNA‐1273 SARS‐CoV‐2 vaccine, demonstrated 94.1% efficacy at preventing severe COVID‐19 infections. 1 Vaccine reactogenicity was related to local injection site reactions. Systemic adverse events included fever, chills, fatigue, myalgia, arthralgia, and headache that resolved within 72 h following vaccine administration. 1 One month after vaccine approval, some cases of allergic reactions including anaphylaxis had been reported to the Centres for Disease Control and Prevention. 9 Further, a possible association between the COVID‐19 RNA vaccine BNT162b1 manufactured by Pfizer and immune thrombocytopenia has been published. 10
In our patient, the temporal relationship of the development of AHA following mRNA‐1273 SARS‐CoV‐2 vaccine administration may suggest a possible etiologic contribution, especially in light of previous studies of AHA after receiving the influenza vaccine. 7 , 8 The exact mechanism by which this could occur is unknown. We hypothesized that this patient's pre‐existing autoimmune condition (polymyalgia rheumatica) increased his risk for the development of FVIII autoantibodies, which could have been triggered by mRNA‐1273 SARS‐CoV‐2 induced immune stimulation. Alternatively, AHA could have already been present prior to mRNA‐1273 SARS‐CoV‐2 vaccine administration, and the intramuscular injection precipitated bleeding, prompting evaluation and diagnosis. However, if AHA was already present, bleeding would have been expected to occur shortly after the injection rather than 48 h later.
4. CONCLUSION
Our patient, without a prior history of bleeding, presented with clinical and laboratory findings consistent with AHA 8 days after receiving the first dose of the mRNA‐1273 SARS‐CoV‐2 vaccine. To our knowledge, this is the first case report suggesting a possible etiologic contribution of mRNA 1273 SARS‐CoV‐2 vaccine to AHA. Continuing surveillance and timely reporting are needed to increase awareness about the possibility of this rare disease in those receiving the vaccine.
CONFLICT OF INTEREST
Craig Seaman has acted as a paid consultant for Bayer Pharmaceuticals, Genentech, HEMA Biologics, Sanofi Genzyme, Spark Therapeutics, and Takeda Pharmaceuticals. The remaining authors stated that they had no interests which might be perceived as posing a conflict or bias.
AUTHOR CONTRIBUTIONS
Lemoine and Giacobbe drafted the case report. Bonifacino, Karapetyan and Seaman offered corrections and medical expertise in the case. All authors reviewed final version and agreed on submission.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021; 384: 403‐‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Delgado J, Jimenez‐Yuste V, Hernandez‐Navarro F, Villar A. Acquired haemophilia: review and meta‐analysis focused on therapy and prognostic factors. Br J Haematol. 2003; 121: 21‐35. [DOI] [PubMed] [Google Scholar]
- 3. Franchini M, Lippi G. Acquired factor VIII inhibitors. Blood. 2008; 112: 250‐255. [DOI] [PubMed] [Google Scholar]
- 4. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020; 26 S6: 1‐158. [DOI] [PubMed] [Google Scholar]
- 5. Kruse‐Jarres R, Kempton CL, Baudo F, et al. Acquired hemophilia A: updated review of evidence and treatment guidance. Am J Hematol. 2017; 92: 695‐705. [DOI] [PubMed] [Google Scholar]
- 6. Knoebl P, Marco P, Baudo F, et al. Demographic and clinical data in acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). J Thromb Haemost. 2012; 10: 622‐631. [DOI] [PubMed] [Google Scholar]
- 7. Pirrotta MT, Bernardeschi P, Fiorentini G. A case of acquired haemophilia following H1N1 vaccination. Haemophilia. 2011; 17: 815. [DOI] [PubMed] [Google Scholar]
- 8. Moulis G, Pugnet G, Bagheri H, et al. Acquired factor VIII haemophilia following influenza vaccination. Eur J Clin Pharmacol. 2010; 66: 1069‐1170. [DOI] [PubMed] [Google Scholar]
- 9. CDC COVID‐19 Response Team, Food and Drug Administration . Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID‐19 vaccine ‐ United States, December 21, 2020‐January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021; 70:125‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tarawneh O, Tarawneh H. Immune thrombocytopenia in a 22‐year‐old post Covid‐19 vaccine. Am J Hematol. 2021; 96(5): E133‐E134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


