Abstract
Background and purpose
High mortality rates have been reported in patients with cerebral venous sinus thrombosis (CVST) due to vaccine‐induced immune thrombotic thrombocytopenia (VITT) after vaccination with adenoviral vector SARS‐CoV‐2 vaccines. The aim of this study was to evaluate whether the mortality of patients with CVST‐VITT has decreased over time.
Methods
The EudraVigilance database of the European Medicines Agency was used to identify cases of CVST with concomitant thrombocytopenia occurring within 28 days of SARS‐CoV‐2 vaccination. Vaccines were grouped based on vaccine type (adenoviral or mRNA). Cases with CVST onset until 28 March were compared to cases after 28 March 2021, which was the day when the first scientific paper on VITT was published.
Results
In total, 270 cases of CVST with thrombocytopenia were identified, of which 266 (99%) occurred after adenoviral vector SARS‐CoV‐2 vaccination (ChAdOx1 nCoV‐19, n = 243; Ad26.COV2.S, n = 23). The reported mortality amongst adenoviral cases with onset up to 28 March 2021 was 47/99 (47%, 95% confidence interval 37%–58%) compared to 36/167 (22%, 95% confidence interval 16%–29%) in cases with onset after 28 March (p < 0.001). None of the four cases of CVST with thrombocytopenia occurring after mRNA vaccination died.
Conclusion
The reported mortality of CVST with thrombocytopenia after vaccination with adenoviral vector‐based SARS‐CoV‐2 vaccines has significantly decreased over time, which may indicate a beneficial effect of earlier recognition and/or improved treatment on outcome after VITT.
Keywords: cerebral venous sinus thrombosis; COVID‐19 vaccines; mortality; sinus thrombosis, intracranial; thrombocytopenia
The EudraVigilance database of the European Medicines Agency was used to identify cases of cerebral venous sinus thrombosis (CVST) with concomitant thrombocytopenia occurring within 28 days of SARS‐CoV‐2 vaccination. The reported mortality of CVST with thrombocytopenia after vaccination with adenoviral vector‐based vaccines significantly decreased over time with a mortality rate of 47% (95% confidence interval 37%–58%) in cases with CVST onset prior to 28 March 2021 compared to 22% (95% confidence interval 16%–29%) in cases after 28 March (p < 0.001). This declining mortality may indicate a beneficial effect of earlier recognition and/or improved treatment on outcome after vaccine‐induced thrombotic thrombocytopenia.

INTRODUCTION
Since March 2021, cases of cerebral venous sinus thrombosis (CVST) with thrombocytopenia after vaccination with the adenovirus‐based SARS‐CoV‐2 vaccines ChAdOx1 nCoV‐19 (Vaxzevria, AstraZeneca/Oxford) and Ad26.COV2.S (Janssen/Johnson&Johnson) have been reported (vaccine‐induced immune thrombotic thrombocytopenia [VITT]) [1, 2, 3, 4, 5, 6. Early reported mortality rates amongst patients with VITT, especially those with CVST, were high, ranging between 30% and 60% [1, 2, 3, 4. After the discovery that the underlying pathophysiology of anti‐platelet factor 4 antibody‐induced platelet activation resembled an auto‐immune variant of heparin induced thrombocytopenia, specific treatment recommendations different from standard CVST care were proposed, based on the following three pillars: (i) use of non‐heparin based anticoagulants, (ii) use of immunomodulation, with intravenous immunoglobulin as first‐line treatment and (iii) avoidance of platelet transfusion [1, 7.
Now that VITT is a recognized side effect and treatment recommendations are in place which are widely endorsed by international organizations [8, 9, the question arises whether the outcome of patients with CVST‐VITT has improved over time. In an attempt to answer this question, acute mortality rates from the EudraVigilance database of the European Medicines Agency (EMA) were compared in two different time periods.
METHODS
Data selection
This is an extension of a previously published study which also used EudraVigilance data and where the methods are described in more detail [4]. Briefly, EudraVigilance is a passive pharmacovigilance system hosted and maintained by EMA in which suspected adverse drug reactions (ADRs) are collected from countries inside and outside the European Economic Area (EEA). Marketing authorization holders and national competent authorities are obliged to report any suspected ADRs occurring within the EEA as well as any suspected serious ADRs occurring within and outside the EEA [10]. The authors were granted level 2A access to the Medical Dictionary for Regulatory Activities High Level Group Term (MedDRA HLGT, version 24.0) ‘Central nervous system vascular disorders’ [11]. For the current study, data on all suspected adverse events reported to EudraVigilance until 13 June 2021 for the four available SARS‐CoV‐2 vaccines approved through EMA were extracted.
All cases of CVST with reported concomitant thrombocytopenia and symptom onset within 28 days after vaccination with one of the adenoviral vector‐based SARS‐CoV‐2 vaccines approved by EMA (ChAdOx1 nCov‐19 and Ad26.COV2.S) were identified. For comparison, cases after mRNA‐based SARS‐CoV‐2 vaccines (BNT162b2 and mRNA‐1273) were collected. CVST with reported concomitant thrombocytopenia was assumed to be CVST‐VITT, as thrombocytopenia has been found to be rare in CVST prior to the COVID‐19 pandemic [12]. Reported adverse reactions coded with ‘Preferred Terms’ (PTs) of the MedDRA were screened by two authors (AM and KK) for PTs corresponding to CVST and potential CVST (Table S1). Cases marked as ‘suspected CVST’ after screening were adjudicated by a senior vascular neurologist (JMC). Cases were marked as having concomitant thrombocytopenia if a PT related to thrombocytopenia was reported (Table S1), or if they had a reported platelet count of <150 × 109/L (Table S2). For the analysis, the adenoviral vector‐based vaccines were grouped together, as were the mRNA vaccines. Because of a potential delay between CVST onset and death, all cases with CVST onset after 30 May 2021 were excluded from the analysis.
As a baseline characteristic, the adenoviral vector vaccine group was screened for adverse reactions coded with PTs related to intracranial haemorrhage (Table S1). Only intracranial haemorrhages with a reported onset date prior to or equal to the CVST onset date were included. In addition, the adenoviral vector vaccine group was screened for cases with any confirmed COVID‐19 infection (Tables S1 and S2).
Data analysis
To evaluate a shift in mortality over time, cases with CVST onset up to and including 28 March were compared to those with onset after 28 March 2021. This cut‐off date was selected because on that day the first paper on VITT, which included treatment recommendations, was published on a preprint server, receiving worldwide attention both amongst physicians and in the media [1, 13. Medians and interquartile ranges were calculated for continuous variables, and counts and percentages for categorical variables. Ninety‐five percent confidence intervals (95% CI) for mortality rates were calculated using the Clopper–Pearson exact method. A chi‐square test was performed to compare the mortality of CVST between the two time periods. A sensitivity analysis was performed restricted to subjects within the EEA and UK because of a potential reporting bias by non‐EEA countries, which initiated reporting of cases at a later stage. Analyses were performed with IBM SPSS Statistics for Windows, version 26.0.0.1 (IBM Corp., Armonk, NY, USA).
RESULTS
Amongst 8537 individual cases with at least one reaction in the MedDRA HLGT ‘Central nervous system vascular disorders’, 270 cases of CVST with thrombocytopenia within 28 days of SARS‐CoV‐2 vaccination were identified (Figure 1). Of these, 266 (99%) occurred after vaccination with an adenoviral vector‐based vaccine (ChAdOx1 nCov‐19, n = 243; Ad26.COV2.S, n = 23). Only 3/243 cases occurred after a second vaccination with ChAdOx1 nCov‐19. Group characteristics are shown in Table 1.
FIGURE 1.
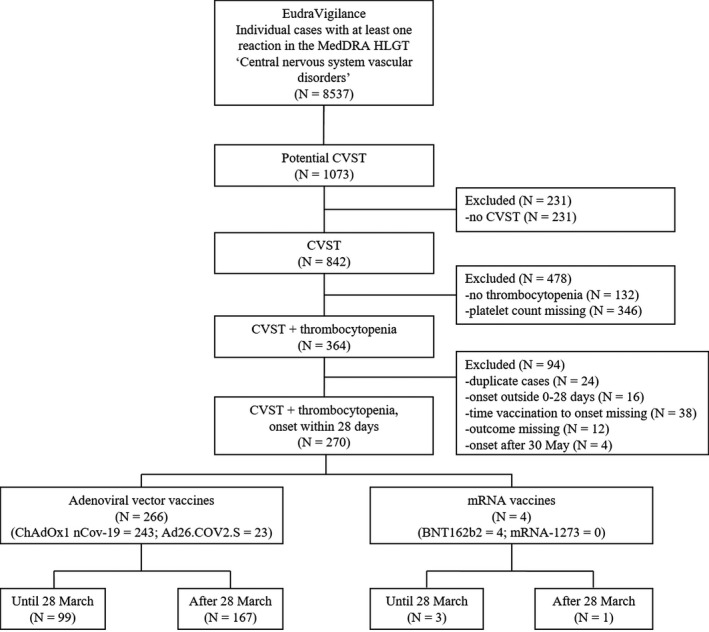
Flowchart of case selection. CVST, cerebral venous sinus thrombosis; MedDRA HLGT, Medical Dictionary for Regulatory Activities High Level Group Term
TABLE 1.
Characteristics of cerebral venous sinus thrombosis (CVST) cases with thrombocytopenia within 28 days after adenoviral vector SARS‐CoV‐2 vaccination
|
Patients with CVST after adenoviral vector vaccination N = 266 |
||
|---|---|---|
|
Until 28 March N = 99 |
After 28 March N = 167 |
|
| Age, median (IQR), years | 46 (33–57)† | 46 (37–55)‡ |
| Female sex, n/N (%) | 83/99 (84) | 108/167 (65) |
| Intracranial haemorrhage at baseline, n/N (%) a | 28/79 (35) | 43/144 (30) |
| Confirmed COVID‐19 infection, n/N (%) b | 1/99 (1) | 2/167 (1) |
| Lowest reported platelet count, median (IQR), ×109/L | 27 (14–60)†† | 42 (20–65)‡‡ |
| Mortality, n/N (%) | 47/99 (47) c | 36/167 (22) c |
Number of missing values: †18; ‡29; ††17; ‡‡46.
Abbreviations: COVID‐19, coronavirus disease 2019; CVST, cerebral venous sinus thrombosis; IQR, interquartile range.
Missing cases (N = 43) had an intracranial haemorrhage with an unknown onset date.
One patient had a COVID‐19 infection prior to CVST onset; the date of COVID‐19 infection onset was unknown in the other two patients.
p < 0.001.
Overall mortality was 83/270 (31%, 95% CI 25%–37%). In the adenoviral vector vaccine group, mortality was 83/266 (31%, 95% CI 26%–37%). Mortality after ChAdOx1 nCov‐19 vaccination was 79/243 (33%, 95% CI 27%–39%) and after Ad26.COV2.S vaccination 4/23 (17%, 95% CI 5%–39%). In the adenoviral vector vaccine group with onset until 28 March, mortality was 47/99 (47%, 95% CI 37%–58%) compared to 36/167 (22%, 95% CI 16%–29%) in cases with CVST onset after 28 March (p < 0.001). No fatalities were reported in the mRNA vaccine group (n = 4).
In the sensitivity analysis using only cases from the EEA and UK, mortality rates were comparable to the mortality rates when including all countries with 45/95 (47%, 95% CI 37%–58%) in cases with onset until 28 March compared to 34/147 (23%, 95% CI 17%–31%) in cases with CVST onset after 28 March.
DISCUSSION
It was found that the reported mortality of CVST with thrombocytopenia after vaccination with adenoviral vector SARS‐CoV‐2 vaccines has fallen substantially, from 47% to 22% in cases with symptom onset before and after 28 March 2021, respectively. This decrease in mortality could be the result of earlier diagnosis and/or improved treatment, but this cannot be ascertained with certainty from the EudraVigilance data. Although limited information on treatment effects in CVST‐VITT is available [8], intravenous immunoglobulins have been found to raise platelet counts and decrease hypercoagulability in patients with autoimmune heparin induced thrombocytopenia [1].
Other potential explanations for the decrease in mortality should be taken into account. First, increased awareness of VITT amongst physicians after 28 March may have resulted in the identification and subsequent reporting of less severe cases. Although sex, age and baseline intracranial haemorrhage were relatively similar in both time periods, no other detailed information, for instance on baseline characteristics, comorbidities and markers of CVST severity, is available with level 2A access to EudraVigilance. Secondly, the population that received vaccination may have shifted over time. For instance, most countries prioritized vaccination for those most at risk of severe disease [14], such as older people and people with underlying health conditions, and these people could have a higher risk of death after VITT. Thirdly, mortality can theoretically have been higher in the earlier cases because of a longer follow‐up time. Unfortunately, date of death is not systematically recorded in EudraVigilance. However, since the mortality rate in the earlier cases is similar to our previous analysis which contained EudraVigilance data until 8 April 2021 (mortality rate 49%) [4], the longer follow‐up duration is an unlikely explanation for the observed difference in mortality.
As previously reported [4], the most important limitation of the study is the quality and completeness of the EudraVigilance database. Because EudraVigilance is a passive pharmacovigilance system, a risk of underreporting is present, especially for less severe cases. Data were not centrally validated, and thus the accuracy and completeness of the reported information are unknown. Because marketing authorization holders and national competent authorities can only report ADRs that they are aware of, a risk of selective reporting remains present. In addition, some patients with CVST‐VITT prior to 28 March 2021 probably remained undiagnosed, because VITT was an unknown condition at that time. Acute death caused by CVST‐VITT could have been misclassified, potentially resulting in an underestimation of the reported mortality rate before 28 March. The numbers of CVST cases after Ad26.COV2.S vaccination were limited, making it difficult to draw any conclusions on trends in mortality for the adenoviral vector vaccines separately.
In summary, the reported mortality of CVST with thrombocytopenia after vaccination with adenoviral vector‐based SARS‐CoV‐2 vaccines decreased over time, which could indicate a beneficial effect of earlier diagnosis and/or treatment recommendations for VITT on patient outcome. Nevertheless, even after 28 March, reported mortality rates remained high, especially in comparison with mortality rates of CVST patients prior to the COVID‐19 pandemic [4, 15.
CONFLICT OF INTEREST
AM, KK, MSK, KJ, JAKH and ML have nothing to disclose. DAS reports travel support from Boehringer Ingelheim, advisory board participation for AstraZeneca, DSMB participation for the SECRET trial, and being a member of the ESO Executive Committee. MRH reports grants from the Swiss Heart Foundation and Bangerter Foundation, travel support from Bayer, and DSMB or advisory board participation for Amgen, and being a member of the ESO Board of Directors and of the ESO Education Committee. EL reports academic grants from the Swedish Neurological Society, Elsa and Gustav Lindh's Foundation, P‐O Ahl's Foundation and Rune and Ulla Amlöv's Foundation. TT has served/serves on scientific advisory boards for Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Inventiva, Portola Pharm and PHRI; has/has had research contracts with Bayer, Boehringer Ingelheim and Bristol Myers Squibb. JP reports grants paid to his institution from the Academy of Finland, Hospital District of Helsinki and Uusimaa, and Finnish Foundation for Cardiovascular Research, consulting fees from Boehringer‐Ingelheim, Bayer and Herantis Pharma, payment for honoraria, lectures, presentations, speakers bureaus, manuscript writing or educational events from Boehringer Ingelheim, Bayer and Abbot, and stock ownership in Vital Signum. SM reports grants from Bayer paid to her institution, personal fees from Bayer paid to her institution, grants from Pfizer paid to her institution, personal fees from BMS/Pfizer paid to her institution, grants from Boehringer Ingelheim paid to her institution, personal fees from Boehringer Ingelheim paid to her institution, personal fees from Abbvie paid to her institution, personal fees from Portola/Alexion paid to her institution, grants from Daiichi Sankyo paid to her institution, and personal fees from Daiichi Sankyo paid to her institution outside the submitted work. MA reports honoraria for lectures from Bayer, AstraZeneca, Covidien and Medtronic, and honoraria for scientific advisory board participation from Amgen, Bayer, BMS, Daiichi Sankyo, Medtronic and Novartis. JMF reports fees and DSMB or advisory board participation for Boehringer Ingelheim and consulting fees from Bayer. JMC reports grants, paid to his institution from Boehringer Ingelheim and Bayer, and payments, paid to his institution for DSMB participation by Bayer. There were no other relationships or activities that could appear to have influenced the submitted work.
AUTHOR CONTRIBUTIONS
Anita van de Munckhof: Data curation (equal); formal analysis (equal); investigation (equal); project administration (equal); validation (equal); visualization (equal); writing—original draft (equal); writing—review and editing (equal). Katarzyna Krzywicka: Data curation (equal); investigation (equal); project administration (equal); validation (equal); visualization (equal); writing—original draft (equal); writing—review and editing (equal). Diana Aguiar de Sousa: Investigation (equal); writing—original draft (equal); writing—review and editing (equal). Mayte Sanchez Van Kammen: Investigation (equal); writing—review and editing (equal). Mirjam Rachel Heldner: Investigation (equal); writing—review and editing (equal). Katarina Jood: Investigation (equal); writing—review and editing (equal). Erik Lindgren: Investigation (equal); writing—review and editing (equal). Turgut Tatlisumak: Investigation (equal); writing—review and editing (equal). Jukka Putaala: Investigation (equal); writing—review and editing (equal). Johanna A Kremer Hovinga: Investigation (equal); writing—review and editing (equal). Saskia Middeldorp: Investigation (equal); writing—review and editing (equal). Marcel Levi: Investigation (equal); writing—review and editing (equal). Marcel Arnold: Conceptualization (equal); investigation (equal); writing—review and editing (equal). José Manuel Morão Cabral Ferro: Conceptualization (equal); investigation (equal); resources (equal); supervision (equal); writing—original draft (equal); writing—review and editing (equal). Jonathan M Coutinho: Conceptualization (equal); investigation (equal); resources (equal); supervision (equal); writing—original draft (equal); writing—review and editing (equal).
STATEMENT OF ETHICS
The corresponding author affirms that this research complies with internationally accepted standards for research practice and reporting. This analysis is based on data collected for pharmacovigilance purposes. No patients were directly recruited or actively involved. Since no human participants were involved, ethical approval or patient informed consent was not required.
Supporting information
Tables S1‐S2
ACKNOWLEDGEMENTS
The authors greatly acknowledge the support of EMA staff with the analysis and interpretation of the data.
van de Munckhof A, Krzywicka K, Aguiar de Sousa D, et al. Declining mortality of cerebral venous sinus thrombosis with thrombocytopenia after SARS‐CoV‐2 vaccination. Eur J Neurol. 2022;29:339–344. doi: 10.1111/ene.15113
Anita van de Munckhof, Katarzyna Krzywicka, José M. Ferro and Jonathan M. Coutinho are Shared first and last co‐authorship.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors.
See commentary by B. Casolla and C. Cordonnier on page 1
DATA AVAILABILITY STATEMENT
De‐identified participant data from the EudraVigilance database are not publicly available but may be obtained from the European Medicines Agency upon official request [11].
REFERENCES
- 1. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med. 2021;384(22):2092‐2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schultz NH, Sorvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384(22):2124‐2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384(23):2202‐2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krzywicka K, Heldner MR, Sanchez van Kammen M, et al. Post‐SARS‐CoV‐2‐vaccination cerebral venous sinus thrombosis: an analysis of cases notified to the European Medicines Agency. Eur J Neurol. 2021;00:1‐7. 10.1111/ene.15029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448‐2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cines DB, Bussel JB. SARS‐CoV‐2 vaccine‐induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384(23):2254‐2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin‐induced thrombocytopenia. J Thromb Haemost. 2017;15(11):2099‐2114. [DOI] [PubMed] [Google Scholar]
- 8. Furie KL, Cushman M, Elkind MSV, Lyden PD, Saposnik G; American Heart Association/American Stroke Association Stroke Council Leadership . Diagnosis and management of cerebral venous sinus thrombosis with vaccine‐induced immune thrombotic thrombocytopenia. Stroke. 2021;52(7):2478‐2482. [DOI] [PubMed] [Google Scholar]
- 9. The International Society on Thrombosis and Haemostasis . ISTH Interim Guidance for the Diagnosis and Treatment on Vaccine‐Induced Immune Thrombotic Thrombocytopenia. [Internet]. Accessed July 5, 2021. https://www.isth.org/news/561406/The‐ISTH‐Releases‐Interim‐Guidance‐on‐Vaccine‐Induced‐Immune‐Thrombotic‐Thrombocytopenia‐VITT‐.htm
- 10. European Medicines Agency . EudraVigilance: Electronic reporting. [Internet]. Accessed July 20, 2021. https://www.ema.europa.eu/en/human‐regulatory/research‐development/pharmacovigilance/eudravigilance/eudravigilance‐electronic‐reporting
- 11. European Medicines Agency . Access to EudraVigilance Data. [Internet]. Accessed August 13, 2021. https://www.ema.europa.eu/en/human‐regulatory/research‐development/pharmacovigilance/eudravigilance/access‐eudravigilance‐data
- 12. Sanchez van Kammen M, Heldner MR, Brodard J, et al. Frequency of thrombocytopenia and platelet factor 4/heparin antibodies in patients with cerebral venous sinus thrombosis prior to the COVID‐19 pandemic. JAMA. 2021;326(4):332‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dewan A, Greene RA. Here's What to Know about the Risk of Blood Clots and the AstraZeneca Vaccine. [Internet]. Accessed August 7, 2021. https://edition.cnn.com/2021/04/02/health/astrazeneca‐blood‐clots‐explainer‐intl‐cmd‐gbr/index.html
- 14. European Centre for Disease Prevention and Control . COVID‐19 Vaccination—Prioritisation of Target Groups for Vaccination. [Internet]. Accessed August 3, 2021. https://www.ecdc.europa.eu/en/covid‐19/prevention‐and‐control/vaccines
- 15. Ferro JM, Canhao P, Stam J, Bousser MG, Barinagarrementeria F; ISCVT Investigators . Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664‐670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S2
Data Availability Statement
De‐identified participant data from the EudraVigilance database are not publicly available but may be obtained from the European Medicines Agency upon official request [11].


