Abstract
Background
Limited information exists on nursing home (NH) residents regarding BNT162b2 vaccine efficacy in preventing SARS‐CoV‐2 and severe COVID‐19, and its association with post‐vaccine humoral response.
Methods
396 residents from seven NHs suffering a SARS‐CoV‐2 B.1.1.7 (VOC‐α) outbreak at least 14 days after a vaccine campaign were repeatedly tested using SARS‐CoV‐2 real‐time reverse‐transcriptase polymerase chain reaction on nasopharyngeal swab test (RT‐qPCR). SARS‐CoV‐2 receptor‐binding domain (RBD) of the S1 subunit (RBD‐IgG) was measured in all residents. Nucleocapsid antigenemia (N‐Ag) was measured in RT‐qPCR‐positive residents and serum neutralizing antibodies in vaccinated residents from one NH.
Results
The incidence of positive RT‐qPCR was lower in residents vaccinated by two doses (72/317; 22.7%) vs one dose (10/31; 32.3%) or non‐vaccinated residents (21/48; 43.7%; p < .01). COVID‐19–induced deaths were observed in 5 of the 48 non‐vaccinated residents (10.4%), in 2 of the 31 who had received one dose (6.4%), and in 3 of the 317 (0.9%) who had received two doses (p = .0007). Severe symptoms were more common in infected non‐vaccinated residents (10/21; 47.6%) than in infected vaccinated residents (15/72; 21.0%; p = .002). Higher levels of RBD‐IgG (n = 325) were associated with a lower SARS‐CoV‐2 incidence. No in vitro serum neutralization activity was found for RBD‐IgG levels below 1050 AU/ml. RBD‐IgG levels were inversely associated with N‐Ag levels, found as a risk factor of severe COVID‐19.
Conclusions
Two BNT162b2 doses are associated with a 48% reduction of SARS‐CoV‐2 incidence and a 91.3% reduction of death risk in residents from NHs facing a VOC‐α outbreak. Post‐vaccine RBD‐IgG levels correlate with BNT162b2 protection against SARS‐CoV‐2 B.1.1.7.
Keywords: antibody response, BNT162b2 vaccine, COVID‐19, efficacy, neutralizing antibodies, nucleocapsid antigenemia, nursing homes, residents, SARS‐CoV‐2, symptoms
This study assesses the incidence of positive SARS‐CoV‐2 B.1.1.7 by RT‐qPCR results according to the vaccination status in nursing home residents facing a VOC‐α outbreak. Two BNT162b2 doses are associated with a major reduction of SARS‐CoV‐2 B.1.1.7 incidence and COVID‐19. Post‐vaccine RBD‐IgG levels correlate with protection in this population.Abbreviations: AU/ml, arbitrary units per milliliter; AU/ml, arbitrary units per milliliter; COVID‐19, coronavirus disease 2019; RBD, receptor‐binding domain; RT‐qPCR, real‐time reverse‐transcriptase polymerase chain reaction; SARS‐CoV‐2, severe respiratory syndrome coronavirus 2; VOC, variant of concern

Abbreviations
- AU/mL
arbitrary units per milliliter
- AU/mL
arbitrary units per milliliter
- COVID‐19
coronavirus disease 2019
- RBD
receptor‐binding domain
- RT‐qPCR
real‐time reverse‐transcriptase polymerase chain reaction
- SARS‐CoV‐2
severe respiratory syndrome coronavirus 2
- VOC
variant of concern
1. INTRODUCTION
Nursing home (NH) residents are at high risk of serious illness and death from coronavirus disease 2019 (COVID‐19). 1 Vaccination is safe and effective in adults, but less documented in NH residents. 2 , 3 , 4 Vaccine monitoring is partly based on post‐vaccine IgG response against SARS‐CoV‐2 receptor‐binding domain (RBD) of the S1 subunit (RBD‐IgG). 5 Based on RBD‐IgG levels, 6 , 7 , 8 it is recommended in France to administer one mRNA vaccine dose in adults with prior COVID‐19 and a third dose in immunocompromized patients. The interest of a third vaccine dose also arises in NH residents without prior COVID‐19 who may low post‐vaccine RBD‐IgG levels. 2 , 9 , 10 , 11 It remains, however, unclear as to whether RBD‐IgG levels are predictive of SARS‐CoV‐2 infection and COVID‐19 severe symptoms.
The recent emergence of SARS‐CoV‐2 “variants of concern” (VOC) has transformed the epidemic, 12 possibly because RBD‐IgG produced by vaccinated individuals is less effective in in vitro binding and neutralizing VOCs than the “wild type” SARS‐CoV‐2. 13 , 14 , 15 , 16 , 17
Between January and March 2021, all French NH residents, including those of the Occitanie region, were offered two BNT162b2 doses at a three‐week interval. Between March and April 2021, 7 NHs of the Montpellier area (Occitanie) faced a SARS‐CoV‐2 B.1.1.7 (VOC‐α) outbreak after the vaccine campaign.
The primary aim of this study was to assess the incidence of positive SARS‐CoV‐2 real‐time reverse‐transcriptase polymerase chain reaction (RT‐qPCR) results according to the vaccination status. Secondary aims were to compare COVID‐19–related deaths and RBD‐IgG levels according to the vaccination status. Other secondary aims were to assess the links in RT‐qPCR‐positive residents between symptom severity, blood RBD‐IgG levels, and SARS‐CoV‐2 nucleocapsid antigenemia (N‐Ag). Exploratory objectives were to analyze the link between RBD‐IgG levels and serum neutralization activity against VOC‐α and SARS‐CoV‐2 wild type in one NH, and the links between biological markers with COVID‐19 severity in RT‐qPCR‐positive residents (N‐Ag, C‐reactive protein, and glomerular filtration rate). 18 , 19
2. METHODS
2.1. Design of the study
Between March and April 2021, among the 122 NHs (6241 residents) followed by the Montpellier COVID‐19 support platform after vaccination, 20 13 had at least one RT‐qPCR‐positive resident. Among the 747 residents from these 13 NHs, 124 had a positive RT‐qPCR result. Among these 13 NHs, a prospective cohort study was carried out in seven, each with more than five RT‐qPCR‐positive residents tested at least 14 days after the end of the vaccination campaign. The exposure risk of these residents was considered sufficient to study the effect of the vaccination on COVID‐19 incidence. The clinical characteristics of all residents present at the time of the first positive RT‐qPCR were studied, regardless of the date of their arrival in the NH. As for other Occitanie NHs, when a first RT‐qPCR‐positive resident was diagnosed, the same infection prevention and control (IPC) measures were implemented, all residents and staff members were tested using real‐time reverse‐transcriptase polymerase chain reaction on nasopharyngeal swab test (RT‐qPCR), and all RT‐qPCR‐negative individuals were retested weekly until no new cases were diagnosed for at least 14 days. No new resident entry was permitted until the end of the outbreak. (Appendix S1: Online supplement 1). 21 , 22 Blood was drawn on the day of positive RT‐qPCR testing in the first infected residents, and within the 5 following days in all the others. Blood collection within this delay was possible in 6/7 NHs. The blood sample allowed the measurement of IgG‐RBD and nucleoprotein IgG levels and, in RT‐qPCR‐positive residents, N‐Ag. Serum neutralizing antibodies were assessed in a subsample of 36 vaccinated residents from the first NH facing a SARS‐CoV‐2 outbreak, chosen for its wide range of IgG‐RBD values.
2.2. Participants
When a first resident had a positive RT‐qPCR, residents and their family or legal representative were informed of the possibility to measure RBD‐IgG and nucleocapsid IgG in order to assess humoral immunity.
All residents of the 6 NHs in which the blood sample was offered were tested, except those in a palliative medical situation and those for whom informed consent for the blood collection and the use of anonymized data was not obtained. History of previous RT‐qPCR testing and the Charlson comorbidity index were determined based on the residents’ medical files. 23
The study was approved by the Montpellier University Hospital institutional review board (IRB‐MTP_2020_06_202000534 and IRB‐MTP _2021_04_202000534).
2.3. Outcomes
2.3.1. Main outcome: incidence of SARS‐CoV‐2 infection
The diagnosis of SARS‐CoV‐2 infection was performed using RT‐qPCR on nasopharyngeal swab (viral transport medium, Sun‐Trine®) tested within 24 h using Allplex™ 2019‐nCoV assay (Seegene, South Korea) (Appendix S1: Online supplement 2). 24 An RT‐qPCR multiple variant assay was used for the detection of α and β/γ variants (ID™ SARS‐CoV‐2/UK/SA Variant Triplex (id Solutions) and for genome sequencing.
2.3.2. Secondary outcomes
COVID‐19 severity: Symptoms were those recorded in residents’ files by NH staff between 7 days before and 14 days after RT‐qPCR testing, according to previous studies 21 , 25 (Appendix S1: Online supplement 3). COVID‐19 was considered to be severe when residents had either (i) respiratory symptoms including shortness of breath, respiratory rate >24/min, oxygen saturation <90% or when oxygen therapy was used, or (ii) when they displayed other symptoms whose intensity was considered by the NH coordinating practitioner as sufficient to justify hospitalization or examination by the resident's General Practitioner. Other symptomatic residents had mild symptoms.
SARS‐CoV‐2 RBD and Nucleoprotein immunoglobulin levels: Blood samples were collected by venipuncture (serum tubes, Becton Dickinson, USA), stored at 4°C, and tested within 72 h. RBD‐IgG levels were measured using the SARS‐CoV‐2 IgG II Quant assay (Abbott Diagnostics). Results were expressed as arbitrary units per mL (AU/ml; positive threshold: 50 AU/ml; upper limit: 40,000 AU/ml). According to the manufacturer and to previous studies, a first threshold ≥1050 AU/ml was considered as a significant response, 26 and a second one ≥4160 AU/ml indicated a high neutralizing effect. 27 Nucleoprotein IgG (N‐IgG) levels were measured using the SARS‐CoV‐2 IgG assay (Abbott Diagnostics) (Appendix S1: Online supplement 4).
2.3.3. Nucleocapsid antigenemia
Blood N‐Ag was quantified in all RT‐qPCR‐positive residents seronegative for N‐IgG, using the COV‐QUANTO® kit (AAZ, France), a CE‐IVD marked immunoassay. 28
2.3.4. Exploratory outcomes
Neutralizing antibodies: Micro‐neutralization assays on blood were performed in 36 residents (Appendix S1: online supplement 5). 27
Inflammatory and kidney markers: Blood C‐reactive protein (hsCRP) and creatinine levels were measured (Appendix S1: online supplement 6).
2.4. Statistical analysis
In NH 4 (Table S1), blood sampling was not possible, and the residents’ data were used only to assess the link between vaccination status and SARS‐CoV‐2 infection and severe COVID‐19 incidence. Due to the very low number of missing data in the 6 NHs in which blood was collected, and to the fact that residents who did or did not contribute to blood collection did not differ in age, gender, and comorbidity status, no assumptions were made for missing data, and missing data were not replaced.
Categorical variables were described with frequency and proportions for each category. The description of continuous variables was performed using mean and standard deviation and/or median, with interquartiles, according to the distribution.
The statistical analysis plan was pre‐specified according to previous studies from our group, 6 , 7 with the following primary, secondary, and exploratory outcomes.
The primary outcome (incidence of positive RT‐qPCR) was estimated in the entire cohort with 95% confidence interval (CI), and compared according to the residents’ vaccination status (non‐vaccinated, one vaccine dose, or two vaccine doses) using the chi‐square test. Death was not considered as a competing risk as all deaths were related to COVID‐19 during the study period. COVID‐19–related deaths were considered as incident cases.
Secondary outcomes were compared in the whole cohort, according to the vaccination status, using the Fisher exact test for deaths and the Kruskal‐Wallis (KW) test for RBD‐IgG levels.
Other secondary outcomes according to the vaccination status were analyzed in RT‐qPCR‐positive residents: COVID‐19 symptom severity using the Fisher exact test, RBD‐IgG, and N‐Ag using the KW test. The relationship between levels of RBD‐IgG and risk of incident SARS‐CoV‐2 was analyzed using the Fisher exact test.
Exploratory analyses on the relationship between RBD IgG levels and serum neutralization in RT‐qPCR‐positive residents of one NH were performed using Fisher's exact test. Wilcoxon‐Mann‐Whitney 2‐sided tests were used to compare N‐Ag in asymptomatic, mild, and severe COVID‐19 residents.
Holm's correction was applied for the post hoc comparisons for each outcome. The statistical significance threshold was set at 5%.
We displayed the positive predicted values (PPVs) and negative predictive values (NPVs) of a positive RT‐qPCR testing for the accepted thresholds of IgG‐RBD values (positive threshold set at 50 AU/ml, significant threshold set at 1050 AU/ml, and 4160 AU/ml, indication of a high neutralizing effect). 26 , 27
Analyses were performed and illustrated using SAS Enterprise Guide, v7.3 (SAS Institute Inc) and GraphPad Prism 9.1.1 (GraphPad Software, Inc., San Diego, CA).
3. RESULTS
3.1. Characteristics of the residents
In the seven NHs, non‐vaccinated residents ranged from 3.9% to 32.6% and RT‐qPCR‐positive residents from 9.8% to 54.9%. Residents’ vaccination coverage was not significantly different across the NH sites (Table S1). A total of 396 residents (57 to 103 years, mean ± SD: 87.33 ± 9.17 years), with 312 females (78.8%), were included. The Charlson comorbidity index ranged from 2 to 15 (median, percentiles: 6.0, 5.0–7.0). Fifty‐one residents had prior COVID‐19, determined by a positive RT‐qPCR in 2020 or 2021, and only 2 residents without prior positive RT‐qPCR had detectable N‐protein‐IgG in the blood collected for this study. There were 48 (12.2%) non‐vaccinated residents, 31 (7.8%) with one vaccine dose, and 317 (80.0%) with two doses. Age, sex, Charlson comorbidity index, or previous RT‐qPCR results were comparable in the groups (Table 1)
TABLE 1.
Characteristics of the residents depending on their vaccination status
| Non‐vaccinated (a) (n = 48) | Vaccinated | p value | ||
|---|---|---|---|---|
| 1 vaccine dose (b) (n = 31) | 2 vaccine doses (c) (n = 317) | |||
| Age, mean (SD), year a | 87.2 (9.2) | 85.1 (9.2) | 87.6 (9.2) | .22 |
| Sex, n (%) b | ||||
| Female | 35 (72.9) | 25 (80.7) | 252 (79.5) | .56 |
| Male | 13 (27.1) | 6 (19.3) | 65 (20.5) | |
| Charlson index (median, IGR) [range] a |
6.0 (5.0–7.0) 2–15 |
6.0 (5.0–8.0) 3–15 |
6.0 (5.0–7.0) 2–15 |
.41 |
| Prior SARS‐CoV−2 positive RT‐qPCR, n (%) b | 5 (10.4) | 3 (9.7) | 43 (13.6) | .82 |
| Positive RT‐qPCR during the SARS‐CoV‐2 B.1.1.7 outbreak, n (%) b | 21 (43.7) | 10 (32.3) | 72 (22.7) |
.0059 .005 (a vs c) |
| N‐protein IgG >0.8 signal to cutoff ratio, n (%) c | 5 (20.0) | 5 (17.9) | 38 (14.0) | .57 |
| RBD‐IgG level, AU/ml, median (IQR) a |
n = 26 2 (1–415) |
n =28 534.00 (201.50; 2166.50) |
n =271 1,522.00 (444.00; 5389.00) |
.000001 .001 (a vs b) .01 (a vs c) .08: (b vs c) |
| COVID‐19–related deaths during the SARS‐CoV‐2 B.1.1.7 outbreak, n (%) c | 5 (10.4) | 2 (6.4) | 3 (0.9) |
.0007 .004 (a vs c) |
Analysis using Kruskal‐Wallis test.
Analysis using chi‐square test.
Analysis using Fisher's exact test.
Blood sampling could not be performed in residents from NH number 4 (Table 1, Table S1, and Figure 1). In the 6 NHs in which a blood sample was offered, residents who were (N = 325) or were not (N = 25) sampled were similar in age, gender, and Charlson index (data not shown).
FIGURE 1.
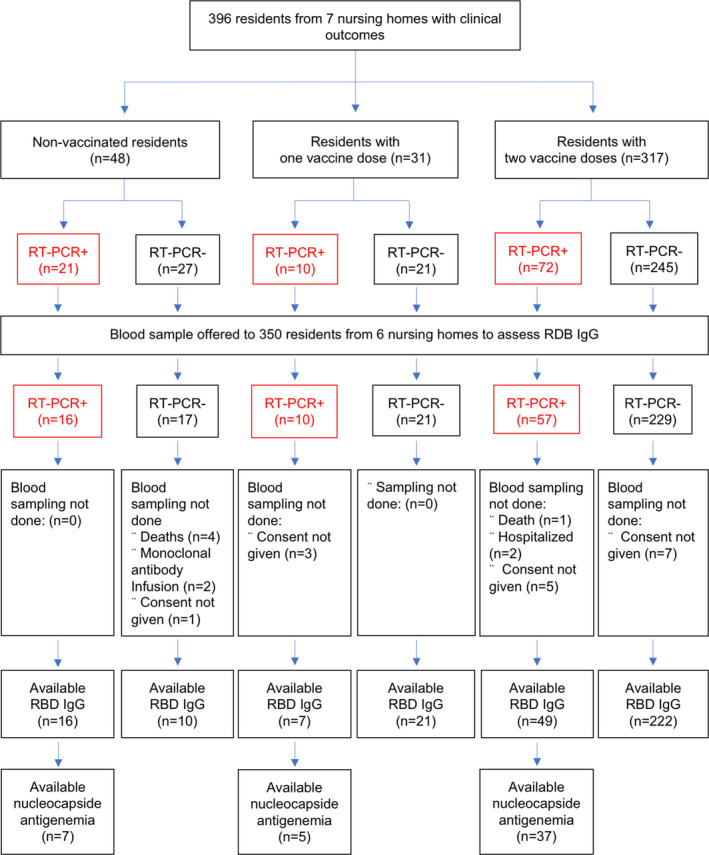
Flow Diagram of the sample of residents having faced a COVID‐19 outbreak at least 14 days after a BNT162b2 vaccination campaign
3.2. COVID‐19 incidence depending on vaccination status
RT‐qPCR was positive in 103 residents (with an incidence of 26% (95% CI: 21.7%‐30.3%). The RT‐qPCR cycle thresholds (Ct) were comparable in vaccinated and non‐vaccinated residents (Table 2). The VOC‐α variant was found in all NHs. Positive RT‐qPCR results were obtained in 22.7% of residents with two vaccine doses, 32.3% with one dose, and 43.7% in non‐vaccinated subjects.
TABLE 2.
Characteristics of the residents with RT‐qPCR positive results during the SARS‐CoV‐2 B.1.1.7 outbreak
| Non‐vaccinated (a) (n = 21) | Vaccinated | p value | ||
|---|---|---|---|---|
| 1 vaccine dose (b) (n = 10) | 2 vaccine doses (c) (n = 72) | |||
| Age, mean (SD), year b | 90.3 (7.4) | 83.8 (8.4) | 89.1 (7.8) | .06 |
| Sex, n (%) d | ||||
| Female | 15 (71.4) | 8 (80.0) | 56 (77.8) | .87 |
| Male | 6 (28.6%) | 2 (20.0) | 16 (22.2) | |
| Charlson index (median, IQR) [range] b |
6 (6.0–8.0) [4–15] |
7.0 (5.0–8.0) 5–9 |
7.0 (6.0–8.0) 3–15 |
.96 |
| Prior SARS‐CoV−2‐positive RT‐qPCR, n (%) c | 1 (4.8) | 0 (0) | 3 (4.2) | 1.00 |
| First positive RT‐qPCR cycle threshold, median (IQR) d | 26.0 (23.0–32.0) | 24.85 (20.4; 28.0) | 24.00 (19.2; 28.6) | .74 |
| Cycle threshold of the second RT‐qPCR a , median (IQR) d | 30.3 (27–35.2) | 30.7 (23.1; 34.5) | 27.00 (21.0; 31.7) | .21 |
| Symptoms, n (%) c | .002 | |||
| Asymptomatic | 2 (9.5) | 6 (60.0) | 31 (43.7) | .01 (a vs b), |
| Mild | 4 (19.1) | 2 (20.0) | 23 (31.0) | .002 (a vs c) |
| Severe | 10 (47.6) | 0 | 15 (21.1) | .09 (b vs c) |
| Deaths, n (%) c | 5 (23.8) | 2 (20.0) | 3 (4.2) | .002 (a vs c) |
| SARS‐CoV‐2 IgG levels | n =10 | n =7 | n =49 | .29 |
| N‐protein IgG >0.8 signal to cutoff ratio, n (%) b | 2 (20.0) | 1 (14.3) | 4 (8) | |
| SARS‐RBD‐IgG (AU/ml) e |
.01 .01 (a vs c) |
|||
| <50 | 6 (60.0) | 1 (14.3) | 5 (10.2) | |
| 51–1050 | 3 (30.0) | 5 (71.4) | 22 (44.9) | |
| 1051–4160 | 0 (0) | 1 (14.3) | 15 (30.6) | |
| >4160 | 1 (10) | 0 (0) | 7 (14.3) | |
| Nucleocapide antigenemia titer b , median (IQR)[range] f |
n = 7 43.0 (2.9–150.2) [0.7–555.2) |
n =5 1.8 (0.5–3.0) [0.2–12.5] |
n =37 1.5 (−0.4–16–6) [−1.5–328–4] |
.03 .004 (a vs c) |
Analysis using Kruskal‐Wallis test.
Analysis using ANOVA.
Analysis using Fisher's exact test.
The second RT‐qPCR was performed 7 days after the first positive RT‐qPCR result in 47 residents.
Analysis using Mann‐Whitney test.
level of detection of the assay is 2.95 ng/ml. Samples with Nucleocapsid Ag level over 180 pg/ml were diluted. Titer is the number of dilutions possible, still reaching the detection threshold. According to the manufacturer of the SARS‐CoV‐2 IgG II Quant assay (Abbott Diagnostics), the positive threshold is of 50 AU/ml RBD‐IgG levels. check Based on previous studies, an RBD‐IgG level ≥1050 AU/ml is considered as a significant antibody response to the vaccine, 26 and a level ≥4160 AU/ml indicates a high neutralizing effect against SARS‐CoV‐2. 27
3.3. COVID‐19–related deaths depending on vaccination status
Incident deaths during the outbreak, all due to COVID‐19, were higher in non‐vaccinated (10.4%) than in vaccinated residents (6.4% and 0.9% for those vaccinated with one or two doses, Table 1). In RT‐qPCR‐positive residents, the non‐vaccinated died more frequently (25% vs 4.3%; Table 2). Two non‐vaccinated residents received monoclonal antibodies and recovered completely.
3.4. COVID‐19–related symptoms depending on vaccination status
In RT‐qPCR‐positive residents during the SARS‐CoV‐2 B.1.1.7 outbreak, the non‐vaccinated developed significantly more severe symptoms than the vaccinated (47.6% vs 21.1%) (Table 2).
3.5. Clinical outcomes depending on RBD IgG levels
Serum samples were available for 325/350 residents from 6 NHs in which the blood collected could be organized (92.9%). Causes of missing data are displayed in Figure 1. RBD‐IgG levels of residents with prior COVID‐19 were not significantly different between NHs. RBD‐IgG levels of residents without prior COVID‐19 were also comparable (data not shown).
By comparison with residents who received two vaccine doses, the RBD‐IgG level was lower in non‐vaccinated residents and in those with one dose (Table 1).
Residents with higher levels of RBD‐IgG had a lower risk of developing SARS‐CoV‐2 during the outbreak (Table 3). The PPV and NPV of a positive RT‐qPCR by RBD‐IgG levels over 1050 AU/ml (significant response 26 ) were 0.86 and 0.24, respectively, with a sensitivity and specificity of 0.63 and 0.54 (Table S2).
TABLE 3.
Incidence rate of positive RT‐qPCR testing and serum neutralizing activity depending on SARS‐CoV‐2 RBD IgG levels
| SARS‐CoV‐2 RBD IgG levels, AU/ml a | p value | ||||
|---|---|---|---|---|---|
| <50 | 51–1050 | 1051–4160 | >4160 | ||
| RT‐qPCR testing result, n (%) | n = 12 | n = 97 | n = 82 | n = 80 | .01 |
| Positive | 5 (41.67) 7 (58.33) | 22 (22.68) | 15 (18.29) | 7 (8.75) | |
| Negative | 5 (41.67) 7 (58.33) | 75 (77.32) | 67 (81.71) | 73 (91.25) | |
| Serum micro‐neutralization titer against SARS‐CoV−2 wild type, median (IQR)[range] b |
n = 3 0 (0–0) [0–0] |
n = 10 0 (0–0) [0–0] |
n = 10 10.0 (0–10) [0–20] |
n = 5 160 (40–320) [20–640] |
.005 |
| Serum neutralization against SARS‐CoV−2 B.1.1.7 (VOC α), median (IQR)[range] b |
n = 3 0 (0–0) [0–0] |
n = 10 0 (0–0) [0–0] |
n = 10 10.0 (10–20) [0–40] |
n = 5 320 (80–640) [40–640] |
.005 |
Analysis using the Fisher exact test.
Analysis using the Kruskal‐Wallis test; micro‐neutralization titers are expressed as the serial dilution for which 50% neutralization is obtained.
Among the 49 RT‐qPCR‐positive vaccinated (two doses) residents with blood results, RBD‐IgG levels tended to be higher in asymptomatic residents than in those with mild or severe symptoms (median (IQR)[range] 1249 AU/ml (337; 3027) [11.00; 25,453.00] vs 517 AU/ml (150; 1289) [3; 14,631] vs 358 AU/mL (128; 1339) [93; 5824], respectively).
No in vitro serum neutralization activity was found for RBD‐IgG levels under 1050 AU/ml for both SARS‐CoV‐2 and VOC‐α. Above this threshold, the RBD‐IgG levels were associated with serum neutralization activity (Figure 2).
FIGURE 2.
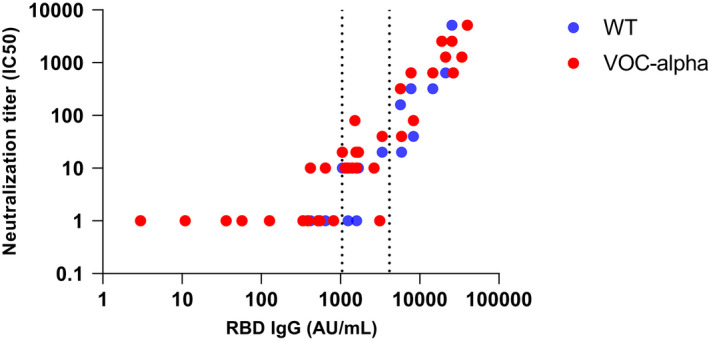
SARS‐CoV‐2‐specific neutralizing antibody levels against SARS‐CoV‐2 wild type (WT) and VOC‐alpha depending on RBD‐IgG levels in 36 residents. Micro‐neutralization titers are expressed as the serial dilution for which 50% neutralization is measured
Among the 49 RT‐qPCR‐positive residents with N‐Ag measurement, median N‐Ag levels were higher in non‐vaccinated residents than in those having received 2 vaccine doses (Table 2), and higher in residents with severe symptoms (Figure S1A). The RBD‐IgG level was predictive of N‐Ag in SARS‐CoV‐2‐infected residents (Figure S1B).
3.6. Inflammatory and kidney biomarkers
RT‐qPCR‐positive residents with elevated CRP (>10 mg/L, Figure S1C) or reduced glomerular filtration rates (GFR) (<60 ml/min/1.73 m2) had higher N‐Ag levels (Figure S1D).
4. DISCUSSION
Among 396 residents from 7 NHs facing a VOC‐α outbreak over 14 days after the end of a BNT162b2 vaccination campaign, 103 had a positive RT‐qPCR test. After two vaccine doses, the risk of incident VOC‐α infection was reduced by 48% when compared to non‐vaccinated residents, and by 26% in residents with one dose. In RT‐qPCR‐positive residents, two vaccine doses reduced the risk of severe symptoms by 56% (from 47.6% to 21.1%) and COVID‐19 death by 82.4% (from 23.8% to 4.2%). Post‐vaccine RBD‐IgG levels under 1050 AU/ml were associated with (i) an increased risk of incident VOC‐α infection, (ii) a low serum neutralizing effect against SARS‐CoV‐2 wild type and VOC‐α, and (iii) high levels of N‐Ag that were associated with a higher risk of severe COVID‐19 (severe symptoms, high C‐reactive protein, low glomerular filtration rate).
The 48% reduction of SARS‐CoV‐2 infection after two vaccine doses found in the present study is consistent with NH studies reporting a VOC‐α outbreak, even if the percentage of infected residents differed among the vaccinated and non‐vaccinated. 2 , 4 , 9 The reduction in COVID‐19 risk was estimated at 65% after a single BNT162b2 vaccine dose in a national data study in England. 29 The strong reduction of severe symptoms and death found in vaccinated residents was also reported. 4 , 9
In contrast to two previous studies, 2 , 30 we did not find any difference in viral load, estimated by the mean Ct. Given that Ct values, vaccine regimen, and COVID‐19 severity strongly differed between the three studies, further investigations are needed to determine the reduction of the viral load in infected residents after vaccination. This information is crucial to minimize the duration and consequences of isolation in vaccinated infected residents.
A novel result of the present study is that post‐vaccine RBD‐IgG levels can partly predict the efficacy of the vaccine to prevent incident SARS‐CoV‐2 and severe symptoms. This result is also consistent with the lack of neutralizing effect observed in the serum against SARS‐CoV‐2 in vitro under a threshold of 1050 AU/ml, and the association between RBD‐IgG levels and serum neutralization activity found above this threshold. In addition, lower RBD‐IgG levels were significantly associated with higher N‐Ag levels which were associated with a higher risk of COVID‐19 severity (more severe symptoms, higher C‐reactive protein levels and lower glomerular filtration rate—two biological markers of COVID‐19 severity) 18 , 19 which is in line with previous studies conducted in the general population. 31 Our results are consistent with studies in the general population showing a correlation between RBD‐IgG and serum neutralization. 32 , 33 These studies combine to suggest that (i) low RBD‐IgG levels following vaccination may not necessarily possess the key footprints required to block viral infection, and (ii) a minimal post‐vaccine RBD‐IgG level, possibly over 1050 AU/mL, may be required to block VOC‐α infection and/or prevent severe symptoms.
The strengths of this study include the sample size, and all studied NHs followed the same IPC guidance, making it possible to compare the results. 21 , 22
This study also has several limitations: (i) RBD‐IgG levels were measured using an immunoassay and results were expressed in arbitrary units. However, the assay used in our study can probably be considered as a quantitative assay (<3.5% imprecision). 34 (ii) RBD‐IgG levels were measured within five days after the diagnosis of the first RT‐qPCR‐positive resident of the NH. Even if this delay was as short as possible, RBD‐IgG levels may also reflect a possible rapid anamnestic response to infection. This bias tends, however, to reinforce our results, since we found a relationship between low levels of RBD‐IgG and a higher risk of SARS‐CoV‐2. (iii) Although the NH residents of the present study were comparable to the French NH population in terms of mean age, gender, and comorbidity status, our results obtained in residents exposed to a high risk of infection may not be extrapolated to all NH residents. (iv) Our results are only valuable for NHs facing a VOC‐α outbreak. Further studies are needed in NH residents facing other variants [particularly B.1.351(β), P1 (Γ), Delta (B.1.617.2), B.1.525 (Eta), Iota (B.1.526), B.1.617.1 (kappa), and C.37 (Lambda)] (https://www.who.int/en/activities/tracking‐SARS‐CoV‐2‐variants/). Indeed, RBD‐IgG produced by vaccinated residents seems less effective for binding and neutralizing in vitro variants 13 , 14 , 15 , 16 , 17 , 35 than the SARS‐CoV‐2 “wild type”. 14 , 15 , 16 , 17 , 35 , 36 (v) The results obtained are restricted to the BNT162b2 vaccination in NH residents and further studies with other vaccines are therefore necessary. In this study, 8.7% of residents with RBD‐IgG levels of over 4,160 AU/ml developed a positive RT‐qPCR. This suggests that vaccinated residents, even with high levels of S‐protein IgG after two vaccine doses, may participate in SARS‐CoV‐2 transmission while most often being asymptomatic or pauci‐symptomatic. These results suggest that vaccinated residents should be included in the wide‐facility testing strategy when a resident is infected.
5. CONCLUSIONS
These results confirm the effectiveness of two BNT162b2 doses to significantly reduce the incidence and severity of COVID‐19 in NH residents facing a VOC‐α outbreak. They tend to validate the quantification of the post‐vaccine antibody response as an estimate of BNT162b2 efficacy against SARS‐CoV‐2 VOC‐α.
CONFLICT OF INTEREST
The authors declare no conflicts of interest/competing interests.
Supporting information
Appendix S1
Fig S1
ACKNOWLEDGEMENTS
We thank (i) Drs. Vincent Foulongue and Monsef Benkirane (Montpellier) for providing the SARS‐CoV‐2/CHU Montpellier/France strain, and (ii) Drs. Olivier Terrier, Andres Pizzorno and Manuel Rosa‐Calatrava (CIRI, Centre International de Recherche en Infectiologie, Team VirPath, Inserm, U1111, Université Claude Bernard Lyon 1, CNRS, UMR5308, ENS de Lyon, Lyon) for providing the Gisaid UK (B.1.1.7): EPI_ISL_900512 strain, referred to as hCoV‐19/France/ARA‐SC2118/2020. The authors would also like to thank Anna Bedbrook, Fabienne Portejoie (MACVIA France), and Eva Pons (Master Métiers de l’Enseignement, de l'Education et de la Formation; Education Nationale française, Lyon) for their editorial assistance; Joy Martin, Isabelle Bussereau, and Marie‐Suzanne Léglise, Secours Infirmiers (Department of Geriatrics, Montpellier University Hospital), Véronique Vera and Florence Biblocque (admissions office, Montpellier University Hospital) for material support; Xavier Basagaña for his methodological support; and the residents and staff members of the nursing homes involved in the study. None of these contributors received any compensation for their help in carrying out the study.
Blain H, Tuaillon E, Gamon L, et al. Receptor binding domain‐IgG levels correlate with protection in residents facing SARS‐CoV‐2 B.1.1.7 outbreaks. Allergy. 2022;77:1885–1894. 10.1111/all.15142
Hubert Blain and Edouard Tuaillon equally contributed to the work.
Funding information
This work was not supported by a grant.
REFERENCES
- 1. McMichael TM, Currie DW, Clark S, et al. Epidemiology of COVID‐19 in a long‐term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005‐2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bailly B, Guilpain L, Bouiller K, et al. BNT162b2 mRNA vaccination did not prevent an outbreak of SARS COV‐2 variant 501Y.V2 in an elderly nursing home but reduced transmission and disease severity. Clin Infect Dis. 2021;16:ciab446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nanduri S, Pilishvili T, Derado G, et al. Effectiveness of Pfizer‐BioNTech and Moderna Vaccines in Preventing SARS‐CoV‐2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS‐CoV‐2 B.1.617.2 (Delta) Variant — National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb Mortal Weekly Report. 2021;70(34):1163–1166. doi: 10.15585/mmwr.mm7034e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA‐Based COVID‐19 vaccine candidates. N Engl J Med. 2020;383:2439‐2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blain H, Tuaillon E, Gamon L, et al. Spike antibody levels of nursing home residents with or without prior COVID‐19 3 weeks after a single BNT162b2 vaccine dose. JAMA. 2021;325(18):1898‐1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blain H, Tuaillon E, Gamon L, et al. Antibody response after one and two jabs of the BNT162b2 vaccine in nursing home residents: The CONsort‐19 study. Allergy. 2022;77(1):271‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA COVID‐19 vaccine in solid‐organ transplant recipients. N Engl J Med. 2021;385(7):661‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cavanaugh AM, Fortier S, Lewis P, et al. COVID‐19 Outbreak Associated with a SARS‐CoV‐2 R.1 Lineage Variant in a Skilled Nursing Facility After Vaccination Program — Kentucky, March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):639‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Canaday DH, Carias L, Oyebanji OA, et al. Reduced BNT162b2 mRNA vaccine response in SARS‐CoV‐2‐naive nursing home residents. Clin Infect Dis. 2021;16:ciab447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Teran RA, Walblay KA, Shane EL, et al. Postvaccination SARS‐CoV‐2 infections among skilled nursing facility residents and staff members ‐ Chicago, Illinois, December 2020‐March 2021. Am J Transplant. 2021;21:2290‐2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krause PR, Fleming TR, Longini IM, et al. SARS‐CoV‐2 Variants and Vaccines. N Engl J Med. 2021;385:179‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCallum M, Bassi J, De Marco A, et al. SARS‐CoV‐2 immune evasion by the B.1.427/B.1.429 variant of concern. Science. 2021;373(6555):648‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Planas D, Bruel T, Grzelak L, et al. Sensitivity of infectious SARS‐CoV‐2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med. 2021;27:917‐924. [DOI] [PubMed] [Google Scholar]
- 15. Becker M, Dulovic A, Junker D, et al. Immune response to SARS‐CoV‐2 variants of concern in vaccinated individuals. Nat Commun. 2021;12:3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harvey WT, Carabelli AM, Jackson B, et al. SARS‐CoV‐2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marot S, Malet I, Leducq V, et al. Neutralization heterogeneity of United Kingdom and South‐African SARS‐CoV‐2 variants in BNT162b2‐vaccinated or convalescent COVID‐19 healthcare workers. Clin Infect Dis. 2021;29:ciab492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elshazli RM, Toraih EA, Elgaml A, et al. Diagnostic and prognostic value of hematological and immunological markers in COVID‐19 infection: A meta‐analysis of 6320 patients. PLoS One. 2020;15:e0238160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uribarri A, Nunez‐Gil IJ, Aparisi A, et al. Impact of renal function on admission in COVID‐19 patients: an analysis of the international HOPE COVID‐19 (Health Outcome Predictive Evaluation for COVID 19) Registry. J Nephrol. 2020;33:737‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rolland Y, Benetos A, Villars H, Braun H, Blain H. Editorial: A COVID‐19 support platform for long term care facilities. J Nutr Health Aging. 2020;24:461‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blain H, Rolland Y, Tuaillon E, et al. Efficacy of a test‐retest strategy in residents and health care personnel of a nursing home facing a COVID‐19 outbreak. J Am Med Dir Assoc. 2020;21:933‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blain H, Rolland Y, Schols J, et al. August 2020 Interim EuGMS guidance to prepare European Long‐Term Care Facilities for COVID‐19. Eur Geriatr Med. 2020;11:899‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 24. Liotti FM, Menchinelli G, Marchetti S, et al. Evaluation of three commercial assays for SARS‐CoV‐2 molecular detection in upper respiratory tract samples. Eur J Clin Microbiol Infect Dis. 2021;40:269‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blain H, Rolland Y, Benetos A, et al. Atypical clinical presentation of COVID‐19 infection in residents of a long‐term care facility. Eur Geriatr Med. 2020;11:1085‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Praet JT, Vandecasteele S, De Roo A, De Vriese AS, Reynders M. Humoral and cellular immunogenicity of the BNT162b2 mRNA COVID‐19 Vaccine in nursing home residents. Clin Infect Dis. 2021;7;ciab300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27(7):1205‐1211. [DOI] [PubMed] [Google Scholar]
- 28. Hingrat QL, Visseaux B, Laouenan C, et al. Detection of SARS‐CoV‐2 N‐antigen in blood during acute COVID‐19 provides a sensitive new marker and new testing alternatives. Clin Microbiol Infect. 2020;27:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shrotri M, Krutikov M, Palmer T, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV‐19 and BNT162b2 against SARS‐CoV‐2 infection in residents of long‐term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021;S1473–3099(21):289‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McEllistrem MC, Clancy CJ, Buehrle DJ, Lucas A, Decker BK. Single dose of a mRNA SARS‐CoV‐2 vaccine is associated with lower nasopharyngeal viral load among nursing home residents with asymptomatic COVID‐19. Clin Infect Dis. 2021;26:ciab263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fajnzylber J, Regan J, Coxen K, et al. SARS‐CoV‐2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11:5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS‐CoV‐2 seroconversion in humans. Nat Med. 2020;26:1033‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bartsch YC, Fischinger S, Siddiqui SM, et al. Discrete SARS‐CoV‐2 antibody titers track with functional humoral stability. Nat Commun. 2021;12:1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. English E, Cook LE, Piec I, Dervisevic S, Fraser WD, John WG. Performance of the Abbott SARS‐CoV‐2 IgG II Quantitative Antibody Assay Including the New Variants of Concern, VOC 202012/V1 (United Kingdom) and VOC 202012/V2 (South Africa), and First Steps towards Global Harmonization of COVID‐19 Antibody Methods. J Clin Microbiol. 2021;59:e0028821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS‐CoV‐2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130‐135. [DOI] [PubMed] [Google Scholar]
- 36. Collier DA, De Marco A, Ferreira I, et al. Sensitivity of SARS‐CoV‐2 B.1.1.7 to mRNA vaccine‐elicited antibodies. Nature. 2021;593:136‐141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Fig S1


