To the Editor:
A number of studies have emphasized the weak humoral response to COVID-19 m-RNA vaccination in hemodialysis patients and kidney transplant recipients naïve to SARS-CoV-2.1 , 2 Firket et al.3 recently reported in this journal that kidney transplant recipients previously infected with SARS-CoV-2 develop similar levels of anti-S1/S2 IgG titers compared with healthy controls following of BNT162b2 mRNA vaccination, suggesting that natural infection may prime the immune system for optimal antibody response to COVID-19 mRNA vaccination.
We would like to report on a larger prospective observational study of the antibody response to the BNT162B2 mRNA vaccine (intramuscular injection of 30 µg mRNA on day 0 and day 21) in hemodialysis patients, kidney transplant recipients and healthy adults controls, naïve or previously infected with SARS-CoV-2, as documented by PCR or serology (IRB-approved studies, CTA 2021-000412-28 and CTA 2021-000461-33). Healthy adult controls were staff from nursing homes.4 SARS-CoV-2 receptor-binding domain (RBD)-specific IgG titers were measured by ELISA (Wantai Bio-Pharm) before and 21, 28, and 49 days after administration of the first vaccine dose.
The mean duration of dialysis and mean interval since transplantation were 47 and 122 months, respectively. In these patients, the mean time between COVID-19 and vaccination was 170 ± 16 days. Most COVID-19 were mild, except in 12 patients who required oxygenotherapy, 3 of them were critically ill. Patients received hydroxychloroquine (n = 3), dexamethasone (n = 3), and convalescent plasma therapy (n = 1, 224 days before vaccination). Transplant recipients received steroids (80%), antimetabolite immunosuppressants (78%), anticalcineurin (76%), and mTOR inhibitors (30%). There was no rejection episode during the year before vaccination.
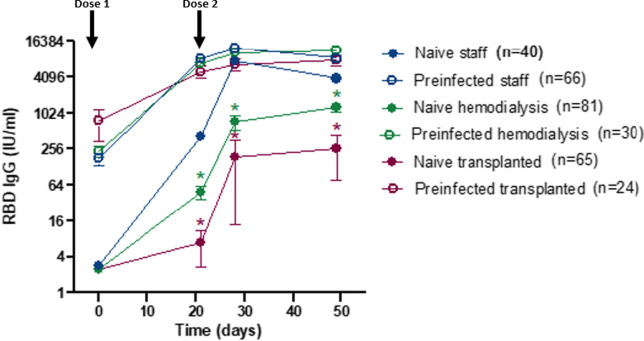
Lower levels of RBD IgG were detected in SARS-CoV-2 naive hemodialysis patients and kidney transplant recipients as compared to healthy adults ( Figure 1). In contrast, levels of RBD IgG levels were high and similar in hemodialysis patients, kidney transplant recipients, and adult controls previously infected with SARS-CoV-2, already after a single dose of mRNA vaccine. No serious adverse event was observed in any of the study groups.
FIGURE 1.
RBD IgG titers (mean and standard error of mean) to BNT162B2 vaccination in hemodialysis patients, kidney transplant recipients, and healthy adult controls naïve or previously infected with SARS-CoV-2. Arrows indicate time of administration of the two vaccine doses. *p < .001 as compared to naïve staff [Color figure can be viewed at wileyonlinelibrary.com]
Our results are in line with previously published studies describing weak immune responses to vaccine in COVID-19-naïve transplanted or hemodialysis patients.1, 2, 3 More importantly, they indicate that the immune suppression of hemodialysis patients and kidney transplant recipients does not reduce the antibody response to mRNA vaccination following priming by natural infection, confirming the observation of Firket et al. in a smaller cohort of kidney transplanted patients.3 Immunity to SARS-CoV-2 induced by the combination of natural infection and vaccination has been named hybrid immunity in healthy adults.5 Although the mechanism underlying hybrid immunity remains to be investigated, this process likely depends on the induction of memory B cells and CD4+ T cells by natural infection, leading to up to 100 times higher antibody titers following vaccination. One limitation of our study is that it does not include assessment of cell-mediated immunity. The observation that hybrid immunity can be induced in immunocompromised patients opens an important opportunity for the rational development of vaccines in this vulnerable population. It also provides reassurance that kidney transplant recipients and hemodialysis patients acquire robust immunity if they survive COVID-19.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Stumpf J, Siepmann T, Lindner T, et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine [published online ahead of print July 23, 2021]. Lancet Reg Health Eur. 2021. doi: 10.1016/j.lanepe.2021.100178 [DOI] [PMC free article] [PubMed]
- 2.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. https://www.nejm.org/doi/full/10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Firket L, Descy J, Seidel L, et al. Serological response to mRNA SARS- CoV- 2 BNT162b2 vaccine in kidney transplant recipients depends on prior exposure to SARS- CoV-2. Am J Transplant. 2021:1–2. doi: 10.1111/ajt.16726. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pannus P, Neven KY, Craeye SD, et al. Poor antibody response to BioNTech/Pfizer COVID-19 vaccination in SARS-CoV-2 naïve residents of nursing homes. medRxiv. 2021. doi: 10.1101/2021.06.08.21258366 [DOI]
- 5.Crotty S. COVID-19 vaccine responses provide insights into how the immune system perceives threats. Science. 2021;372(6549):1392–1393. [Google Scholar]