Abstract
Introduction
Studies directly comparing preterm birth rates in women with and without severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection are limited. Our objective was to determine whether preterm birth was affected by SARS‐CoV‐2 infection within a large integrated health system in New York with a universal testing protocol.
Material and methods
This retrospective cohort study evaluated data from seven hospitals in New York City and Long Island between March 2020 and June 2021, incorporating both the first and second waves of the coronavirus disease 2019 (COVID‐19) pandemic in the USA. All patients with live singleton gestations who had SARS‐CoV‐2 polymerase chain reaction (PCR) testing at delivery were included. Deliveries before 20 weeks of gestation were excluded. The rate of preterm birth (before 37 weeks) was compared between patients with positive and negative SARS‐CoV‐2 test results. This analysis was performed separately for resolved prenatal infections and infections at delivery, with the latter group subdivided by symptom status. Multiple logistic regression analysis was used to examine the association between SARS‐CoV‐2 infection and preterm birth, adjusting for maternal age, race‐ethnicity, parity, history of preterm birth, body mass index, marital status, insurance type, medical co‐morbidities, month of delivery, and wave of pandemic.
Results
A total of 31 550 patients were included and 2473 (7.8%) had laboratory‐confirmed infection. Patients with symptomatic COVID‐19 at delivery were more likely to deliver preterm (19.0%; adjusted odds ratio 2.76, 95% CI 1.92–3.88) compared with women with asymptomatic infection (8.8%) or without infection (7.1%). Among preterm births associated with symptomatic infection, 72.5% were medically indicated compared with 44.1% among women without infection (p < 0.001). Risk of preterm birth in patients with resolved prenatal infection was unchanged when compared with women without infection. Among women with infection at delivery, preterm birth occurred more frequently during the second wave compared with the first wave (13.6% vs. 8.7%, respectively; p < 0.006). However, this was not significant on multiple regression analysis after adjusting for other explanatory variables.
Conclusions
Pregnant women with symptomatic COVID‐19 are more than twice as likely to have a preterm delivery than patients without infection. Asymptomatic infection and resolved prenatal infection are not associated with increased risk.
Keywords: antibodies, coronavirus disease 2019, pregnancy, prematurity, preterm birth, race‐ethnicity, severe acute respiratory syndrome coronavirus 2
Abbreviations
- CDC
Centers for Disease Control and Prevention
- COVID‐19
coronavirus disease 2019
- PCR
polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
Key Message.
Pregnant patients with symptomatic COVID‐19 are more than twice as likely to have a preterm birth compared with patients without infection. Asymptomatic infection and resolved prenatal infection are not associated with an increased risk for preterm delivery.
1. INTRODUCTION
Several investigators have evaluated whether preterm birth rates have changed during the coronavirus disease 2019 (COVID‐19) pandemic compared with historical cohorts. 1 , 2 , 3 , 4 These studies have focused on the impact of mitigation measures such as lockdowns and disruptions in the provision of healthcare services. A recent meta‐analysis concluded that the pandemic was associated with a 9% decline in the preterm birth rate in high‐income countries, but no change was seen elsewhere. 5 Fewer studies have attempted to ascertain whether actual infection with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) affects the risk of preterm birth. Publications by the USA Centers for Disease Control and Prevention (CDC) have reported an increased frequency of preterm birth among pregnant women with SARS‐CoV‐2 infection compared with national averages. 6 , 7 A limitation of these and other large registry studies is that they do not compare pregnancy outcomes with those of patients without infection from the same populations and time period. 8 Thus, any increase in preterm birth attributed to viral infection is speculative. Furthermore, some of these studies are vulnerable to selection bias by over‐inclusion of symptomatic cases with more severe illness. Notably, the CDC publication by Woodworth et al confirmed that asymptomatic SARS‐CoV‐2 infection in pregnancy constituted less than 10% of the cases in their sample. 6 , 7 Using universal testing protocols, multiple studies have demonstrated that the majority of pregnant patients with SARS‐CoV‐2 infection are asymptomatic. 9 , 10
To date, studies directly evaluating the effect of SARS‐CoV‐2 infection on preterm birth are limited. 11 , 12 What is needed are large studies that compare women with and without infection from the same populations and time period, and in sufficient numbers to adjust for severity of infection and known associations with preterm birth. Recently, a prospective multinational cohort study attempted to do this. 13 The authors concluded that preterm birth was increased only in cases of symptomatic COVID‐19, but the study had several limitations that we subsequently review.
The objective of our study was to determine whether preterm birth, spontaneous preterm birth, or medically indicated preterm birth occur more frequently in pregnant women with SARS‐CoV‐2 infection compared with those without infection in a large integrated health system in New York with a universal testing protocol. We aim to clarify whether disease severity or resolved prenatal infection is associated with these outcomes, and whether results were different between the first and second waves of the pandemic.
2. MATERIAL AND METHODS
This retrospective cohort study evaluated data from seven hospitals in New York City and Long Island between March 15, 2020 and June 28, 2021, incorporating both the first and second waves of the pandemic in the USA. The first wave started in March 2020 and peaked in April 2020. The second wave started in November 2020 and peaked in January 2021. All patients with live singleton gestations who underwent SARS‐CoV‐2 polymerase chain reaction (PCR) testing during their delivery hospitalization were included. Patients who were evaluated at the hospital but sent home without admission were not included unless they subsequently delivered during the study period. Deliveries that occurred at less than 20 weeks of gestation were excluded. Universal PCR testing was implemented in April 2020. As a result of limited testing capabilities in March and early April, only symptomatic patients, and those with known or suspected exposure to the virus, received PCR testing. Serological testing for antibodies to the virus was also offered starting in June 2020. Antibody results after December 2020 were not evaluated in this study because of the widespread availability of SARS‐CoV‐2 vaccination, which induces a similar immune response.
Clinical data were obtained from the enterprise electronic health record system (Sunrise Clinical Manager; Allscripts). Data collected included patient demographic information, clinical characteristics, and pregnancy outcomes, including gestational age at delivery. Self‐reported race and ethnicity data were collected from prespecified categories. Participants’ records were manually reviewed to categorize preterm births as spontaneous or medically indicated. Spontaneous preterm birth was defined as resulting from preterm labor, preterm prelabor rupture of membranes, or cervical insufficiency. Cervical insufficiency was defined as painless cervical dilation, without contractions or labor, resulting in either second‐trimester delivery or cerclage placement. Medically indicated preterm birth was defined as being initiated by a clinician based on a maternal, fetal, or placental/uterine condition. 14 If there were characteristics of both categories, the primary factor driving the timing of delivery was used. Records were also manually reviewed to classify severity of illness for patients with positive SARS‐CoV‐2 PCR testing using the National Institutes of Health severity of illness categories. 15 Patients who reported no symptoms consistent with SARS‐CoV‐2 infection during their hospitalization were considered asymptomatic. Mild illness was defined as having various signs and symptoms of COVID‐19 (eg fever, cough, sore throat, malaise, headache, loss of taste and smell) but without shortness of breath, dyspnea, or abnormal chest imaging. Moderate illness consisted of lower respiratory disease but oxygen saturation that remained at 94% or more on room air. Severe illness had an oxygen saturation below 94% on room air, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (Pao 2/Fio 2) less than 300 mm Hg, respiratory frequency more than 30 breaths/minute, or lung infiltrates more than 50%. Critical illness was defined by respiratory failure, septic shock, and/or multiple organ dysfunction.
The rate of preterm birth (before 37 weeks of gestation) was determined for patients with positive and negative SARS‐CoV‐2 test results. Results from the first and second waves of the pandemic were compared. The analysis was performed separately for resolved prenatal infections and infections at delivery, with the latter group subdivided by symptom status. Infection at delivery is based on positive SARS‐CoV‐2 PCR testing during delivery hospitalization. Resolved prenatal infection was defined as negative SARS‐CoV‐2 PCR testing at delivery and either positive immunoglobulin G antibody testing or previous positive PCR testing. Negative for resolved prenatal infection is based on negative antibody testing at delivery and the absence of positive PCR testing. Symptom data were not collected for patients with resolved SARS‐CoV‐2 infection because it was not consistently documented, and was more prone to recall bias.
Descriptive statistics were used to characterize the data. Categorical variables were expressed as frequency and percentage. Each categorical outcome was examined using the chi‐squared test or Fisher's exact test, as appropriate. Comparisons for continuous variables were performed using either the Mann–Whitney U test or Student’s t test, as appropriate. Simple and multiple logistic regression analyses were used to examine the association between SARS‐CoV‐2 infection and preterm birth, adjusting for the following variables: maternal age, race‐ethnicity, parity, history of preterm birth, body mass index, marital status, insurance type, chronic hypertension, diabetes (gestational or pregestational), asthma or other chronic pulmonary disease, hypertensive disorders of pregnancy (including gestational hypertension, preeclampsia, HELLP syndrome, eclampsia), month of delivery, and wave of pandemic. We adjusted for month of delivery (March 2020 to June 2021) to account for whether clinical management changed from the early months of the pandemic to the later months, and because previous studies have observed seasonal patterns in preterm birth. 16 , 17 We adjusted for wave of pandemic because there may be factors associated with the second wave, which began in November 2020, that affect the outcome of preterm birth, including new virus strains, easing of lockdown and mitigation measures, altered patient behavior, and modified clinical practice. Unadjusted and adjusted odds ratios (OR) and 95% CI were calculated. Two‐sided p values less than 0.05 indicated statistical significance. Statistical analyses were performed with rstudio ® 1.1.463 built on R® 3.5.1 and/or SAS® Studio 3.8 Enterprise Edition build on SAS® 9.04 (SAS Inc., Cary, NC, USA).
2.1. Ethical approval
The Northwell Health Institutional Review Board approved this study as minimal‐risk research using data collected for routine clinical practice and waived the requirement for informed consent (IRB # 20‐0890; initial approval October 22, 2020). Some patients in this study were included in previous publications characterizing SARS‐CoV‐2 in pregnancy. 9 , 18 , 19 , 20
3. RESULTS
A total of 33 629 pregnant women were admitted for delivery during the study period, and 31 550 singleton live‐born deliveries with SARS‐CoV‐2 PCR testing were included for analysis (Figure 1). PCR results were not available for 1278 patients, of whom 97% (n = 1238) delivered in the first month of the study period when testing capabilities were limited to symptomatic patients or those with known or suspected exposure to the virus. Of the remaining patients without PCR results (n = 40), some may have had outpatient testing or testing may not have been performed. Patient demographics and clinical characteristics are presented in Table 1. A total of 2473 pregnant patients (7.8%) had laboratory‐confirmed infection: 1261 at delivery and 1212 resolved before delivery (Table 2). Patients with symptomatic COVID‐19 at delivery, which represented 10.8% of all infections, were more likely to deliver preterm (19%; adjusted OR 2.76, 95% CI 1.92–3.88; Table 3) compared with women without infection (7.1%). Among these preterm births associated with symptomatic infection, 72.5% (n = 37/51) were medically indicated compared with 44.1% (n = 944/2140) among women without infection (p < 0.001 on two‐tailed chi‐squared test). Patients with asymptomatic infection at delivery were not more likely to deliver preterm compared with women without infection (8.8% vs. 7.1%). Similarly, those with resolved prenatal infection were not at increased risk for preterm birth compared with those without resolved prenatal infection (6.0% vs. 6.1%).
FIGURE 1.
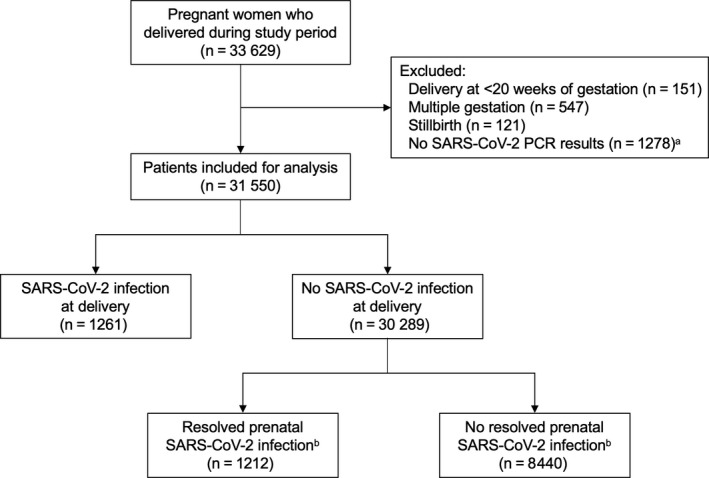
Flowchart of study patients. aMost patients missing SARS‐CoV‐2 PCR results delivered before mid‐April 2020 (n = 1238; 97%). bResolved prenatal infection was not evaluated after December 2020 because widespread availability of SARS‐CoV‐2 vaccination renders positive antibody results uninterpretable. PCR, polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
TABLE 1.
Patient demographics and clinical characteristics
| Characteristic | Term delivery (n = 29 272) | Preterm delivery (n = 2278) | p value |
|---|---|---|---|
| Maternal age, y | 31.6 ± 5.3 | 32.1 ± 5.7 | <0.001 |
| ≥35 | 8760 (29.9) | 815 (35.8) | <0.001 |
| Race and ethnicity | |||
| Non‐Hispanic White | 13 172 (45.0) | 757 (33.2) | <0.001 |
| Non‐Hispanic Black | 3385 (11.6) | 459 (20.1) | |
| Hispanic/Latino | 5403 (18.5) | 473 (20.8) | |
| Other | 7312 (25.0) | 589 (25.9) | |
| Parity | |||
| 0 | 12 939 (44.7) | 1055 (46.9) | <0.001 |
| 1 | 9482 (32.7) | 634 (28.2) | |
| 2 | 4127 (14.3) | 347 (15.4) | |
| ≥3 | 2413 (8.3) | 214 (9.5) | |
| History of preterm birth | 1197 (4.1) | 314 (14.0) | <0.001 |
| BMI, kg/m2 | 30.1 (26.9–34.0) | 30.7 (27.1–35.5) | <0.001 |
| ≥30 | 14 190 (50.9) | 1,222 (56.5) | <0.001 |
| Married | 20 891 (73.8) | 1357 (63.4) | <0.001 |
| Public health insurance | 10 653 (36.4) | 917 (40.3) | <0.001 |
| Medical co‐morbidities | |||
| Chronic hypertension | 898 (3.1) | 284 (12.532) | <0.001 |
| Diabetes, pregestational or gestational | 2960 (10.1) | 346 (15.2) | <0.001 |
| Asthma or other chronic pulmonary disease | 1825 (6.2) | 226 (9.9) | <0.001 |
| Hypertensive disorder of pregnancy | 3240 (11.1) | 770 (33.8) | <0.001 |
| SARS‐CoV‐2 infection a | 2262 (7.7) | 211 (9.3) | <0.009 |
Data are median (interquartile range), n (%), and mean ± SD.
Missing data for 4.7% of BMI, 3.5% of marital status, 1.1% of parity, and 1.1% of history of preterm birth.
Abbreviations: BMI, body mass index; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Either at delivery or resolved prenatal infection.
TABLE 2.
Preterm birth in pregnancies with and without SARS‐CoV‐2 infection, stratified by race and ethnicity—seven New York Hospitals, March 15 to June 28, 2021 a
| Outcome | SARS‐CoV‐2 infection status at delivery, N (%) b | p value e | Resolved prenatal SARS‐CoV‐2 infection status, N (%) c | p value e | |||
|---|---|---|---|---|---|---|---|
| Symptomatic Positive d (n = 268) | Asymptomatic Positive (n = 993) | Negative (n = 30 289) | Positive (n = 1212) | Negative (n = 8440) | |||
| Preterm birth | 51 (19.0) | 87 (8.8) | 2140 (7.1) | <0.001 | 73 (6.0) | 505 (6.0) | 0.96 |
| Non‐Hispanic White | 13/80 (16.3) | 24/367 (6.5) | 720/13 482 (5.3) | <0.001 | 14/383 (3.7) | 189/4102 (4.6) | 0.39 |
| Non‐Hispanic Black | 8/37 (21.6) | 14/119 (11.8) | 437/3688 (11.8) | 0.19 | 25/191 (13.1) | 85/872 (9.7) | 0.17 |
| Hispanic | 17/89 (19.1) | 23/298 (7.7) | 433/5489 (7.9) | <0.001 | 20/391 (5.1) | 92/1352 (6.8) | 0.23 |
| Other race/ethnicity | 13/62 (21.0) | 26/209 (12.4) | 550/7630 (7.2) | <0.001 | 14/247 (5.7) | 139/2114 (6.6) | 0.58 |
| Spontaneous preterm birth f | 14/231 (6.1) | 49/955 (5.1) | 1196/29 345 (4.1) | 0.09 | 36/1175 (3.1) | 299/8234 (3.6) | 0.33 |
| Medically indicated preterm birth g | 37/254 (14.6) | 38/944 (4.0) | 944/29 093 (3.2) | <0.001 | 37/1176 (3.1) | 206/8141 (2.5) | 0.22 |
Abbreviations: PCR, polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Self‐reported race and ethnicity data were collected from prespecified categories on hospitalization.
Infection at delivery is based on positive SARS‐CoV‐2 PCR testing during delivery hospitalization.
Resolved prenatal SARS‐CoV‐2 infection is based on negative PCR testing at delivery and either positive immunoglobulin G antibody testing (n = 1112) or previous positive PCR testing (n = 100). Negative for resolved prenatal infection is based on negative antibody testing at delivery (n = 8440).
Symptomatic patients met criteria for mild, moderate, severe or critical illness using NIH COVID‐19 Treatment Guidelines (https://www.covid19treatmentguidelines.nih.gov).
Calculated using two‐tailed chi‐squared test.
Denominator for spontaneous preterm birth rate excludes medically indicated preterm births.
Denominator for medically indicated preterm birth rate excludes spontaneous preterm births.
TABLE 3.
Logistic regression analysis to predict preterm birth in pregnancies with SARS‐CoV‐2 infection
| Characteristic | OR (95% CI) | p value | Adjusted OR (95% CI) a | Adjusted p value a |
|---|---|---|---|---|
| SARS‐CoV−2 infection status at delivery | ||||
| PCR negative at delivery (n = 30 289) | Reference | |||
| PCR positive at delivery (n = 1261) | 1.62 (1.34–1.93) | <0.001 | 1.53 (1.24–1.87) | <0.001 |
| Symptomatic (n = 268) | 3.09 (2.25–4.17) | <0.001 | 2.76 (1.92–3.88) | <0.001 |
| Asymptomatic (n = 993) | 1.26 (1.00–1.57) | 0.04 | 1.23 (0.96–1.56) | 0.10 |
| Resolved prenatal SARS‐CoV‐2 infection status | ||||
| PCR and antibody negative at delivery (n = 8440) | Reference | |||
| PCR negative at delivery, resolved prenatal infection (n = 1212) | 1.01 (0.78–1.29) | 0.96 | 0.94 (0.70–1.25) | 0.67 |
Abbreviations: OR, odds ratio; PCR, polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Adjusted for maternal age, race‐ethnicity, parity, history of preterm birth, body mass index (BMI), marital status, insurance type, chronic hypertension, diabetes, asthma or other chronic pulmonary disease, hypertensive disorders of pregnancy, month of delivery, and wave of pandemic. Missing data for 4.7% of BMI, 3.5% of marital status, 1.1% of parity, and 1.1% of history of preterm birth. Imputation was not performed. Complete data set was used for predictive modeling.
Maternal conditions were the most common medical indications for preterm delivery in patients with and without SARS‐CoV‐2 infection (64.0% vs. 62.0%, respectively; p = 0.73), and preeclampsia with severe features was the most common maternal condition in both groups (56.3% vs. 87.2%, respectively; p < 0.001). There was no difference in the overall frequency of preeclampsia between symptomatic and asymptomatic SARS‐CoV‐2‐positive patients (15.7% vs. 12.8%; p = 0.22). Among women with symptomatic COVID‐19, 5.6% (n = 15/268) were delivered in the preterm period for worsening respiratory function, which constituted 40.5% (n = 15/37) of this group's medically indicated preterm births.
Differences were observed between the first and second waves of the pandemic. Most notably, among women with SARS‐CoV‐2 infection at delivery, preterm birth occurred more frequently during the second wave compared with the first wave (13.6% vs. 8.7%, respectively; unadjusted OR 1.65, 95% CI 1.16–2.36, p < 0.006). However, on multiple logistic regression modeling, when other explanatory variables were considered, the second wave was no longer associated with increased risk (adjusted OR 1.48, 95% CI 0.22–10.15, p = 0.68). The rate of symptomatic cases decreased from 27% (n = 186/688) in the first wave to 14% (n = 82/573) in the second wave (p < 0.001). Among patients with asymptomatic infection, preterm birth occurred more frequently in the second wave than the first wave (12.0% vs. 5.6%, respectively; unadjusted OR 2.31, 95% CI 1.45–3.73, p < 0.001) but this finding was not significant after adjustment for other predictors of preterm birth (adjusted OR 3.24, 95% CI 0.34–73.10, p = 0.35). No significant sociodemographic differences were noted between waves.
4. DISCUSSION
In contrast to reports by the CDC, we observed that only a subset of patients with SARS‐CoV‐2 infection in pregnancy, those with symptomatic COVID‐19 at delivery, are at increased risk for preterm birth. After adjustment for confounding factors, we did not detect significant changes in preterm birth rates associated with resolved prenatal infection or asymptomatic SARS‐CoV‐2 infection at delivery. These groups did not differ from uninfected pregnant women. Although preterm birth occurred more frequently among women with SARS‐CoV‐2 infection in the second wave of the pandemic compared with the first, this difference was related to other underlying risk factors for preterm birth. Racial and ethnic minority groups have an increased baseline risk of preterm birth, 21 and have been disproportionately affected by the COVID‐19 pandemic. 22 Therefore, it is not surprising that preterm birth rates among women with SARS‐CoV‐2 infection will exceed the national average of 10% in publications reporting registry data, which are at substantial risk for selection bias. 23
Some large cohort studies have now demonstrated that pregnant patients with symptomatic COVID‐19 are more likely to deliver preterm than those with asymptomatic SARS‐CoV‐2 infection 8 , 24 but they do not make comparisons with uninfected women. Most studies directly comparing pregnant patients with and without infection evaluated small sample sizes, did not differentiate by severity of disease, or have relied on diagnosis codes to identify cases. 11 , 25 Our findings are consistent with the aforementioned INTERCOVID multinational cohort study. 13 That study, by Villar et al, was an ambitious collaborative effort to delineate the effect of SARS‐CoV‐2 infection on pregnancy outcomes. However, it had limitations as it included patients from more than 40 institutions, each with distinct clinical management protocols, across 18 countries with heterogeneous populations, socio‐economic conditions, political circumstances, and clinical practice patterns. Race and ethnicity data were not collected. In an effort to reduce bias, after each infected patient was identified, the investigators immediately and concomitantly enrolled two unmatched, consecutive, non‐infected women from the same hospital. However, it is uncertain how well this reference group represented the general non‐infected pregnant population, and the authors acknowledged that it would have been preferable to include all deliveries.
A major strength of our study is the inclusion of all patients who tested negative for the virus under a universal testing protocol. Furthermore, all included hospital sites have unified clinical management guidelines and use a single electronic health record system, which reduces inconsistencies in patient care decisions, clinical documentation, and data collection. In addition, this is one of the first large studies to compare preterm birth rates during different waves of the COVID‐19 pandemic, and we included more than three times the number of laboratory‐confirmed cases as the INTERCOVID study. Our study also has limitations, including its retrospective design and the uncertain timing and severity of infection among the group with resolved prenatal infection. We cannot exclude the possibility of antibody waning affecting our results. Nevertheless, inclusion of such women further demonstrates that most SARS‐CoV‐2 infections in pregnancy are not associated with preterm birth.
5. CONCLUSION
This study found that symptomatic COVID‐19 in pregnancy is associated with an increased risk for medically indicated but not spontaneous preterm birth. The risk of preterm birth was unchanged in patients with resolved prenatal infection and asymptomatic SARS‐CoV‐2 infection when compared with women without infection.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Concept and design: MB, NM, BR. Acquisition, analysis, or interpretation of data: MB, AR, LP, WS, RG, MG, NM, BR. Drafting of the manuscript: MB, WS, NM, MR. Statistical analysis: WS, MB. Supervision: MB, BR. Critical revision of manuscript: MB, WS, BR.
ACKNOWLEDGEMENTS
We thank Fernando Suarez and Shreya Sanghani for assistance with clinical data retrieval.
Blitz MJ, Gerber RP, Gulersen M, et al. Preterm birth among women with and without severe acute respiratory syndrome coronavirus 2 infection. Acta Obstet Gynecol Scand. 2021;100:2253–2259. 10.1111/aogs.14269
REFERENCES
- 1. Handley SC, Mullin AM, Elovitz MA, et al. Changes in preterm birth phenotypes and stillbirth at 2 Philadelphia hospitals during the SARS‐CoV‐2 pandemic, March‐June 2020. JAMA. 2021;325:87‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Janevic T, Glazer KB, Vieira L, et al. Racial/Ethnic disparities in very preterm birth and preterm birth before and during the COVID‐19 pandemic. JAMA Netw Open. 2021;4:e211816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O'Brien P, Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID‐19 pandemic. JAMA. 2020;324(7):705‐706. 10.1001/jama.2020.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Main EK, Chang SC, Carpenter AM, et al. Singleton preterm birth rates for racial and ethnic groups during the coronavirus disease 2019 pandemic in California. Am J Obstet Gynecol. 2021;224:239‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chmielewska B, Barratt I, Townsend R, et al. Effects of the COVID‐19 pandemic on maternal and perinatal outcomes: a systematic review and meta‐analysis. Lancet Glob Health. 2021;9:e759‐e772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woodworth KR, Olsen EO, Neelam V, et al. Birth and infant outcomes following laboratory‐confirmed SARS‐CoV‐2 infection in pregnancy—SET‐NET, 16 jurisdictions, March 29‐October 14, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1635‐1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Delahoy MJ, Whitaker M, O'Halloran A, et al. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory‐confirmed COVID‐19—COVID‐NET, 13 States, March 1‐August 22, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1347‐1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Metz TD, Clifton RG, Hughes BL, et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID‐19). Obstet Gynecol. 2021;137:571‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blitz MJ, Rochelson B, Rausch AC, et al. Universal testing for coronavirus disease 2019 in pregnant women admitted for delivery: prevalence of peripartum infection and rate of asymptomatic carriers at four New York hospitals within an integrated healthcare system. Am J Obstet Gynecol MFM. 2020;2:100169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vintzileos WS, Muscat J, Hoffmann E, et al. Screening all pregnant women admitted to labor and delivery for the virus responsible for coronavirus disease 2019. Am J Obstet Gynecol. 2020;223:284‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adhikari EH, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3:e2029256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martinez‐Perez O, Prats Rodriguez P, Muner Hernandez M, et al. The association between SARS‐CoV‐2 infection and preterm delivery: a prospective study with a multivariable analysis. BMC Pregnancy Childbirth. 2021;21:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID‐19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175:817‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Medically indicated late‐preterm and early‐term deliveries: ACOG committee opinion summary, number 818. Obstet Gynecol. 2021;137:388‐391. [DOI] [PubMed] [Google Scholar]
- 15. COVID‐19 Treatment Guidelines Panel . Coronavirus Diseases 2019 (COVID‐19) Treatment Guidelines. National Institutes of Health. Accessed May 16, 2020. https://www.covid19treatmentguidelines.nih.gov/overview/management‐of‐covid‐19/ [Google Scholar]
- 16. Lee SJ, Steer PJ, Filippi V. Seasonal patterns and preterm birth: a systematic review of the literature and an analysis in a London‐based cohort. BJOG. 2006;113(11):1280‐1288. [DOI] [PubMed] [Google Scholar]
- 17. Weinberg CR, Shi M, DeRoo LA, Basso O, Skjaerven R. Season and preterm birth in Norway: a cautionary tale. Int J Epidemiol. 2015;44:1068‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blitz MJ, Rochelson B, Minkoff H, et al. Maternal mortality among women with coronavirus disease 2019 admitted to the intensive care unit. Am J Obstet Gynecol. 2020;223(595–599):e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gulersen M, Blitz MJ, Rochelson B, Nimaroff M, Shan W, Bornstein E. Clinical implications of SARS‐CoV‐2 infection in the viable preterm period. Am J Perinatol. 2020;37:1077‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prasannan L, Rochelson B, Shan W, et al. Social determinants of health and coronavirus disease 2019 in pregnancy. Am J Obstet Gynecol MFM. 2021;3:100349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Emeruwa UN, Spiegelman J, Ona S, et al. Influence of race and ethnicity on severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection rates and clinical outcomes in pregnancy. Obstet Gynecol. 2020;136:1040‐1043. [DOI] [PubMed] [Google Scholar]
- 23. Krumholz HM. Registries and selection bias: the need for accountability. Circ Cardiovasc Qual Outcomes. 2009;2:517‐518. [DOI] [PubMed] [Google Scholar]
- 24. Vousden N, Bunch K, Morris E, et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS‐CoV‐2 infection in the UK from March to September 2020: a national cohort study using the UK Obstetric surveillance system (UKOSS). PLoS One. 2021;16:e0251123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chinn J, Sedighim S, Kirby KA, et al. Characteristics and outcomes of women with COVID‐19 giving birth at US academic centers during the COVID‐19 pandemic. JAMA Netw Open. 2021;4:e2120456. [DOI] [PMC free article] [PubMed] [Google Scholar]


