Summary
The immunogenicity and safety of Pfizer‐BioNTech BNT162b2 mRNA vaccine in allogeneic haematopoietic stem cell transplantation (HSCT) recipients are unknown. We prospectively followed 152 HSCT recipients who were at least six months following transplantation and with no active acute graft‐versus‐host disease (GVHD). Blood samples were taken 2–4 weeks after the second vaccination and analyzed for receptor‐binding domain (RBD) antibodies and neutralizing antibodies (NA). 272 immunocompetent healthcare workers served as controls. At a median of 28 days after the second vaccination, 118 patients (77·6%) developed RBD immunoglobulin G (IgG) with a geometric mean titre (GMT) of 2·61 [95% CI (confidence interval), 2·16–3·16]. In the control group 269/272 (98·9%) developed RBD IgG, with a GMT of 5·98 (95% CI 5·70–6·28), P < 0·0001. The GMT of NA in HSCT recipients and controls was 116·0 (95% CI 76·5–175·9), and 427·9 (95% CI 354·3–516·7) respectively (P < 0001). Multivariate logistic regression analysis revealed that HSCT recipients with no chronic GVHD and no immunosuppressive therapy at the time of vaccination had significantly higher levels of NA following the second vaccination. Adverse events were minimal and were less common than in healthy controls. In conclusion; the BNT162b2 mRNA vaccination is safe and effective in HSCT recipients, especially those who are immunosuppression‐free. A significant fraction developed protecting NA.
Keywords: COVID‐19, vaccination, haematopoietic stem cell transplantation, neutralizing antibodies
Introduction
In December 2019, the first cases of coronavirus disease (COVID‐19) emerged in Wuhan, China, and within a few weeks a pandemic spread all over the world, affecting already over 232 million people by September 2021 and responsible for over 4·7 million deaths. 1 Results from the Pfizer‐BioNTech and Moderna vaccine clinical trials have shown 95% efficacy in preventing symptomatic laboratory‐confirmed COVID‐19 2 , 3 and they were approved by the FDA for emergency use in December 2020. Soon after the approval a vaccination programme was initiated in Israel with the BNT‐162b2 mRNA vaccine of Pfizer‐BioNTech as the only administered vaccine.
Recipients of autologous and allogeneic haematopoietic stem cell transplantation (HSCT) who develop COVID‐19 have poor overall outcomes. The Center for International Blood and Marrow Transplant Research (CIBMTR) found that severe disease requiring mechanical ventilation occurred in 45/318 (14%) HSCT recipients; 28/184 (15%) following allogeneic HSCT and 17/134 (13%) following autologous HSCT; thirty‐day survival was 68% and 67% respectively. 4 Another study found that mortality of HSCT recipients is lower in patients whose primary disease is in remission (4·2%) compared to those who are not in remission (22·2%). 5
The Pfizer and Moderna clinical trials excluded immunocompromised patients. 2 , 3 The Israeli Ministry of Health approved the vaccine for immunocompromised patients including patients following stem cell transplantation. 6 We report here the first study testing safety and neutralizing antibody (NA) production among 152 patients who underwent allogeneic stem cell transplantation following vaccination with the Pfizer‐BioNTech mRNA vaccine.
Methods
Study design and participants
In the first three months following authorization of BNT162b2 mRNA vaccine in Israel, we offered our allogeneic HSCT recipients who were scheduled for routine clinic visits the opportunity to participate in our study. All adult patients (>18 years) at least six months after transplantation who gave consent to participate in the study were included. Patients receiving anti‐CD20 monoclonal during six months prior to vaccination and patients with active acute graft‐versus‐host disease (GVHD) were excluded. Patients who had recovered from COVID‐19 or had active COVID‐19 at the time of the vaccination or up to seven days after the second vaccine were also excluded. Finally, patients were included if they had serology test results 2–4 weeks after the second dose of the vaccine. Ciclosporin was the only immunosuppressive therapy given for preventing GVHD at the time of vaccination. Ciclosporin, prednisone, mycophenolate and/or extracorporeal photopheresis (ECP) were used for treatment of chronic GVHD (CGVHD). The local vaccination guidelines in our HSCT recipients are according to standard guidelines, starting six months post transplantation, except for influenza vaccination, which is given in the fall season at least three months post transplantation. 7
Controls were 272 immunocompetent healthcare workers (HCW) tested for antibody response 2–4 weeks following the second vaccine.
Written informed consent was obtained from all participants. The protocol and informed consent were approved by the Institutional review board.
Data extraction
Relevant clinical data were retrieved from electronic medical records and included age, gender, underlying haematological diseases, transplant date, donor type, disease status [complete remission (CR/no CR)]. CGVHD grade was defined according to the NIH criteria. 8 Comorbidities were recorded including hypertension, ischaemic heart disease, diabetes mellitus, chronic obstructive pulmonary disease and other malignancies. Data on immunosuppressive therapy during the study were collected. Laboratory data included total white blood cell (WBC) count, absolute lymphocyte count, blood chemistry and ciclosporin blood levels. Patients were categorized according to ciclosporin blood levels [above or below therapeutic levels (150–350 µg/l)], and prednisone daily dosage (above or below 20 mg).
Safety
Adverse events (AE) were obtained using specific questionnaires. These events included local reactions (pain at injection site, erythaema, swelling) and systemic reactions (fever, fatigue, headache, myalgia, chills, nausea/vomiting, paraesthesia) within 30 days after vaccination. Patients were instructed to report any suspected AE and were actively screened for any other systemic and local complaints.
Serology assays
Samples from participants were tested with an enzyme‐linked immunosorbent assay (ELISA) that detects immunoglobulin G (IgG) antibodies against the receptor‐binding domain (RBD) of SARS‐CoV‐2. 9 , 10 Titres >1·1 were defined as positive.
A SARS‐CoV‐2 pseudovirus neutralization assay was performed using a propagation‐competent vesicular stomatitis virus (VSV)‐spike similar to that previously published, 11 which was kindly provided by Gert Zimmer, University of Bern, Switzerland. Sera not capable of reducing viral replication by 50% at a 1:8 dilution or below were considered non‐neutralizing. All samples that were positive for RBD IgG were tested for NA. Negative RBD IgG tests were not tested, since these have previously shown to yield negative NA tests.
Statistical methods
Continuous variables were assessed for normality by the Kolmogorov–Smirnov test and are presented as means ± standard deviation (SD) or medians with interquartile range (IQR), where appropriate. Titres are presented as geometric mean (GMT) and 95% CIs. Categorical variables are presented as frequencies and percentages. For group comparisons, non‐parametric statistical tests (Kruskall–Wallis test) were used for continuous variables and the chi‐square test for categorical variables, with adjustment for multiple comparisons according to Tukey. Multivariable logistic analysis was used to identify factors associated with vaccine‐induced antibody response among the entire cohort (HSCT recipients and immunocompetent controls), and among the HSCT recipients' cohort.
The whole group was adjusted in the statistical models for timing of serology since the second vaccine dose, age, gender, time from transplant to vaccination and underlying comorbidities. Results are presented as odds ratio (OR), 95% CIs, and P values. All P values reflect the results of two‐sided tests. All data analyses were performed with SAS 9.4 software (Cary, NC, USA).
A scatter plot of log‐transformed IgG and NA was obtained using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA). The correlation between IgG and log‐transformed NA was analyzed using Spearman's correlation by two‐tailed parametric t‐test means with 95% CIs.
Results
Baseline characteristics
Our cohort included 176 patients. 24 were excluded, due to lack of available serology testing at the appropriate timing (9), acute GVHD (6), declined vaccination (4), treatment with anti‐CD20 during the last six months (2) and past COVID‐19 infection (3). The final study population consisted of 152 allogeneic HSCT recipients. Demographic, clinical and laboratory data are included in Table I.
Table I.
Baseline characteristics of HSCT recipients: demographic, clinical and laboratory characteristics stratified by antibody response.
| Variable | Total cohort (n = 152) | Non‐responsive serology (n = 34) | Responsive serology (n = 118) | P value | |
|---|---|---|---|---|---|
| HSCT recipients’ characteristics | |||||
| Age, years (mean ± SD) | 58·4 ± 14·0 | 58·6 ± 14·4 | 58·4 ± 14·0 | 0·94 | |
| Male, n (%) | 96 (63·2) | 22 (64·7) | 74 (62·7) | 0·83 | |
| Any comorbidity, n (%) | 40 (26·3) | 8 (23·5) | 32 (27) | 0·67 | |
| Underlying haematological disease | |||||
| AML, n (%) | 68 (44·7) | 13 (38·2) | 55 (46·6) | 0·66 | |
| MDS, n (%) | 23 (15·1) | 5 (14·7) | 18 (15·3) | ||
| MPD, n (%) | 16 (10·5) | 5 (14·7) | 11 (9·3) | ||
| ALL, n (%) | 12 (7·9) | 2 (5·9) | 10 (8·5) | ||
| NHL, n (%) | 24 (15·8) | 6 (17·6) | 18 (15·3) | ||
| HL, n (%) | 6 (3·9) | 3 (8·8) | 3 (2·5) | ||
| CLL, n (%) | 2 (1·3) | 0 (0) | 2 (1·7) | ||
| AA, n (%) | 1 (0·7) | 0 (0) | 1 (0·8) | ||
| Status of disease | |||||
| Complete remission, n (%) | 145 (95·5) | 32 (94·1) | 113 (95·8) | 0·69 | |
| Transplantation | |||||
| Years since transplantation (median ± IQR) | 3·4 (2–6·3) | 3·1 (1·9–5·5) | 3·6 (2·1–6·5) | 0·41 | |
| 6–12 months post‐transplant, n (%) | 5 (3·3) | 4 (12·1) | 1 (0·8) | 0·0145 | |
| 12–24 months post‐transplant, n (%) | 34 (22·5) | 6 (18·2) | 28 (23·7) | ||
| >24 months post‐transplant, n (%) | 113 (74·3) | 24 (70·6) | 89 (75·4) | ||
| Donor type, n (%) | |||||
| Matched unrelated donor | 84 (55·3) | 16 (47·1) | 68 (57·6) | 0·02 | |
| Sibling | 62 (40·8) | 14 (41·1) | 48 (40·7) | ||
| Haploidentical | 6 (3·9) | 4 (11·8) | 2 (1·7) | ||
| Conditioning, n (%) | |||||
| RTC | 81 (53·2) | 14 (41·2) | 67 (56·8) | 0·0526 | |
| RIC | 50 (32·9) | 17 (50·0) | 33 (28·0) | ||
| MAC | 21 (13·9) | 3 (8·8) | 18 (15·3) | ||
| ATG, n (%) | |||||
| Yes | 97 (63·8) | 22 (64·7) | 75 (63·3) | 0·902 | |
| No | 55 (36·2) | 12 (35·3) | 43 (36·4) | ||
| Variable | Total cohort (n = 152) | No‐responsive serology (n = 34) | Responsive serology (n = 118) | P value |
|---|---|---|---|---|
| CGVHD n (%) | ||||
| None | 85 (55·9) | 13 (38·2) | 72 (61·0) | 0·002 |
| Mild | 39 (25·7) | 8 (23·6) | 31 (26·3) | |
| Moderate–severe | 28 (18·4) | 13 (38·2) | 15 (12·7) | |
| Prior AGVHD, n (%) | ||||
| Yes | 25 (16·4) | 6 (17·6) | 19 (16·1) | 0·834 |
| No | 127 (83·6) | 28 (82·4) | 99 (83·9) | |
| Lymphocyte absolute (K/μl) | 2·46 ± 1·44 | 2·25 ± 1·67 | 2·52 ± 1·37 | 0·33 |
| Immunosuppressive therapy, n (%) | ||||
| No therapy | 63 (41·4) | 4 (11·8) | 59 (50·0) | <0·0001 |
| Ciclosporin | 32 (21·1) | 16 (47·1) | 16 (13·6) | <0·0001 |
| Prednisone | 64 (42·1) | 21 (61·8) | 43 (36·4) | 0·084 |
| Mycophenolate | 8 (5·3) | 7 (20·6) | 1 (0·8) | <0·0001 |
AA, aplastic anaemia; AGVHD, acute graft‐versus‐host disease; ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; ATG, anti‐thymocyte globulin; CGVHD, chronic graft‐versus‐host disease; CLL, chronic lymphocytic leukaemia; HL, Hodgkin lymphoma; HSCT, haematopoietic stem cell transplantation; IQR, interquartile range; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MPD, myeloproliferative disease; NHL, non‐Hodgkin lymphoma; RIC, reduced intensity conditioning; RTC, reduced toxicity conditioning; SD, standard deviation.
The mean age was 58·4 ± 14·0 (range 22–82) years, 63% were males, 26% had comorbidities [hypertension 10 (6·6%), ischaemic heart disease nine (5·9%), diabetes mellitus eight (5·3%), other malignancies six (4%), COPD four (2·6%)].
Acute myelogenous leukaemia (AML) and myelodysplastic syndrome (MDS) were the leading diagnoses in 68 (44·7%) and 23 (15·1%) patients respectively. 32 (21·7%) patients had lymphoproliferative disease [non‐Hodgkin lymphoma (24), Hodgkin lymphoma (6), chronic lymphocytic lymphoma (CLL) (2)], 16 had myeloproliferative disorders [13 (8·6%) had myelofibrosis and three (2%) patients had chronic myelogenous leukaemia (CML)]. 12 patients (8%) had acute lymphoblastic lymphoma (ALL) and one had aplastic anaemia (0·7%). Stem cell source was a matched unrelated donor (MUD) in 55·3%, a sibling donor in 40·8%, and a haploidentical donor in 3·9% of patients. Conditioning was reduced toxicity in 81 (53·3%), reduced intensity in 50 (32·9%), and myeloablative in 21 patients (13·8%). Anti‐thymocyte globulin was given in 97 patients (63·8%). Median time from transplantation was 3·4 (IQR 2–6.3) years, 74·3% were more than 24 months after transplantation. At the time of vaccination 85 (55·9%) patients did not have any GVHD, 39 (25·7%) had mild CGVHD, 23 (15·1%) had moderate CGVHD and 5 (3·3%) had severe CGVHD. Twenty‐five patients (16·4%) had prior acute GVHD (AGVHD). Mean lymphocyts count was 2·46 ± 1·44 (K/μl).
Sixty‐three (41·4%) patients were not treated by any immunosuppressive treatment at the time of vaccination; 32 (21·1%) were on ciclosporin, 29 on low dose and three on therapeutic doses; 64 (42·1%) HSCT recipients were treated with prednisone, 92·2% (59/64) on less than 20 mg a day. Only eight patients received mycophenolate and four were on ECP treatment.
Twenty‐six patients (17·1%) following HSCT were treated for their underlying disease to prevent relapse; 16 were given azacitidine for MDS/AML, two tyrosine kinase inhibitor (TKI) for Philadelphia positive (Ph+) leukaemia, three FMS‐like tyrosine kinase 3 (FLT3) inhibitors for FLT3 positive AML. Only seven patients (4·5%) had active haematologic disease at the time of vaccination.
The control group included 272 immunocompetent healthcare workers; their demographic characteristics are shown in Table II.
Table II.
Comparison between HSCT recipients and immunocompetent controls.
| HSCT recipients (n = 152) |
Control (n = 272) |
P value | |
|---|---|---|---|
| Gender, female, n (%) | 56 (36·8) | 206 (75·7) | <0·0001 |
| Age, years (mean ± SD) | 58·4 ± 14·1 | 55·6 ± 14·2 | 0·05 |
| Days from second vaccine to serology (median) | 28 (20‐45) | 26 (24–27) | 0·0009 |
| Positive IgG RBD, n (%) | 118 (77·6) | 269 (98·9) | <0·0001 |
| IgG RBD GMT (95% CI) | 2·61 (2·16, 3·16) | 5·98 (5·70, 6·28) | <0·0001 |
| Neutralizing antibodies GMT (95% CI) | 116·0 (76·5, 175·9) | 427·9 (354·3, 516·7) | <0·0001 |
CI, confidence interval; GMT, geometric mean titre; HSCT, haematopoietic stem cell transplantation; IgG, immunoglobulin G; RBD, receptor‐binding domain; SD, standard deviation.
Safety
Vaccine‐related serious AEs were not observed in the study. We have not seen any exacerbation of GVHD at a mean follow‐up of 30 days following the second dose. Allergic responses were not observed. The frequencies of local AEs (AE) following the first and second vaccines were 9·9% and 11·8% respectively (Table III). The most common local reaction was pain at the injection site, which was mild in most cases and subsided within 24 h. Systemic AEs were more common following the second vaccine (5·3% vs 13·2%) and included mostly fatigue and headache. Immunocompetent HCW experienced significantly more local and systemic AEs than HSCT recipients (P < 0·0001). The response rates to the questionnaires regarding AEs in the HSCT recipients and control group were 82% and 75%,respectively.
Table III.
Adverse events following the first dose and the second dose of BNT162b2 mRNA vaccine in HSCT recipients and in immunocompetent healthcare worker controls.
| Adverse events |
HSCT n = 152 |
Controls n = 272 |
P value |
|---|---|---|---|
| Local AE, n (%) | |||
| Any local AE after first vaccine | 15 (9·9) | 199 (73) | P < 0·0001 |
| Any local AE after second vaccine | 18 (11·8) | 223 (82) | P < 0·0001 |
| Systemic AE, n (%) | |||
| Any systemic AE after first vaccine | 8 (5·3) | 57 (21) | P = 0·0006 |
| Any systemic AE after second vaccine | 20 (13·2) | 150 (55) | P < 0·0001 |
AE, adverse event; HSCT, haematopoietic stem cell transplantation.
Immunogenicity following BNT162b2 vaccination
Antibody responses after the second vaccine dose are summarized in Table IV and Fig 1. At a median time of 28 days (IQR 8–69) after the second vaccination, 118 patients (77·6%) developed RBD IgG with a GMT of 2·61 (95% CI, 2·16, 3·16). In the control group 269/272 (98·9%) developed RBD IgG antibodies, with a GMT of 5·98 (95% CI 5·70, 6·28) at a median of 26 days (IQR 24–27) after the second vaccination. The GMT of NA in HSCT recipients and controls was 116·0 (95% CI, 76·5, 175·9), and 427·9 (95% CI 354·3, 516·7) respectively (P < 0001). Multivariate logistic regression analysis was used to determine the influence of age, gender and underlying immunosuppressive disease, on the magnitude of response to the second dose of the vaccine among the entire cohort (Table IV). Underlying immunosuppression was significantly associated with a non‐reactive response of IgG antibodies [OR 0·04 (95% CI 0·01, 0·13, P < 0·0001); C‐statistics 0·84].
Table IV.
Multivariate logistic regression model for positive RBD IgG among HSCT recipients versus immunocompetent controls (n = 424).
| Effect | Odds ratio | Lower 95% CI | Upper 95% CI | P value |
|---|---|---|---|---|
| Gender female vs male | 1·36 | 0·63 | 2·94 | 0·441 |
| Age <65 years vs >65 years | 1·61 | 0·77 | 3·36 | 0·203 |
| Days after second vaccine | 1·02 | 1·00 | 1·05 | 0·113 |
| HSCT recipients vs control group | 0·04 | 0·01 | 0·13 | <0·0001 |
CI, confidence interval; HSCT, haematopoietic stem cell transplantation; IgG, immunoglobulin G; RBD, receptor‐binding domain.
Fig 1.
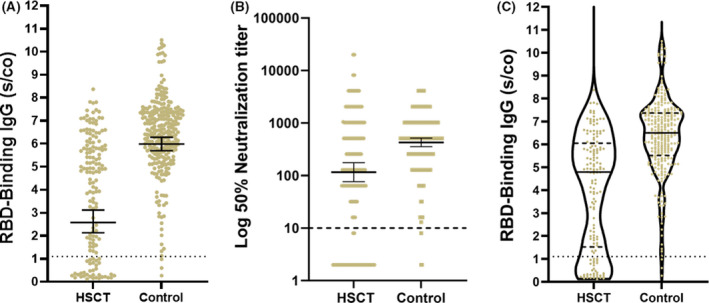
Quantitation of IgG following the second dose of the BNT162b2 vaccine in HSCT recipients and immunocompetent healthcare worker controls. (A) RBD IgG Levels, GMT B) Neutralizing antibodies above the cut‐off. C) Violin plot demonstrating the median RBD IgG titres among HSCT recipients and controls. The dotted black line indicates the limit level of positive antibodies. The short black line indicates GMT and 95% CI. GMT, geometric mean titres; HSCT, haematopoietic stem cell transplantation; RBD, receptor binding domain; S/CO, sample/cut‐off ratio. [Colour figure can be viewed at wileyonlinelibrary.com]
The following variables were associated with decreased antibody response (Table 1): immunosuppressive therapy [45/69 (65·2%) vs 73/83 (88%); P = 0·0008]; presence of moderate–severe CGVHD [15/28 (53·6%) vs 31/39 (79·5%) of those with mild CGVHD, and 72/85 (84·7%) of those without CGVHD; P = 0·0027]; timing of vaccination following transplantation [1/5 (20%), 28/34 (82%) and 89/113 (78·8%) among those vaccinated 6‐12 months, 12–24 months and >24 months after transplantation respectively; P = 0·0145]; haploidentical transplant; conditioning protocol [33/50 (66·0%) of patients who received reduced intensity conditioning (RIC) developed antibodies vs 67/81 (82·7%) and 18/21 (85·7%) of patients who received reduced toxicity conditioning (RTC) or myeloablative conditioning (MAC) respectively]. Preventive treatment did not have an influence on response to vaccine; however, the group was too small (26 patients). There was also no difference between underlying disease and patients with or without co‐morbidities (Table 1).
Multivariate logistic regression analysis found that among HSCT recipients, RIC and immunosuppressive treatment were the only statistically significant variables that were associated with poor antibody response, C‐statistics = 0·79 (Table V).
Table V.
Multivariate logistic regression analysis for negative RBD IgG antibody response following second vaccination among HSCT recipients.
| Effect | Odds ratio | Lower 95% CI | Upper 95% CI | P value |
|---|---|---|---|---|
| Gender female vs male | 0·88 | 0·34 | 2·26 | 0·7845 |
| Age >65 years vs <65 years | 1·34 | 0·47 | 3·80 | 0·5689 |
| Any comorbidity | 0·94 | 0·30 | 2·96 | 0·9171 |
| Years after transplantation | 1·02 | 0·91 | 1·15 | 0·6905 |
| Days after 2nd vaccination | 0·98 | 0·95 | 1·01 | 0·1117 |
| CGVHD moderate to severe vs no to mild | 2·36 | 0·81 | 6·87 | 0·1142 |
| Donor haploidentical vs other | 1·09 | 0·68 | 1·75 | 0·7275 |
| Immunosuppressive treatment | 6·42 | 1·88 | 21·98 | 0·0030 |
| Conditioning RIC vs RTC and MAC | 3·52 | 1·26 | 9·83 | 0·0164 |
CGVHD, chronic graft‐versus‐host disease; CI, confidence interval; HSCT, haematopoietic stem cell transplantation; IgG, immunoglobulin G; MAC, myeloablative conditioning; RBD, receptor‐binding domain; RIC, reduced intensity conditioning; RTC, reduced toxicity conditioning.
Interestingly, a bimodal distribution of the antibody response can be observed (Fig 1C); some patients have minimal response, mainly those on immunosuppressive treatment [RBD IgG GMT (1·83, 95% CI 1·39, 2·42); NA GMT (55·8, 31·5–98·8)], while those who did not require any immunosuppressive treatment develop higher antibody levels [RBD IgG GMT (4·32, 95% CI 3·60, 5·18); NA GMT (332·1, 200·9–549·2), P < 0·0001]. The difference in GMT between HSCT recipients without therapy and the control group was still statistically significant (P < 0·001).
We found high correlation, r = 0·70 (95% CI 0·59, 0·78; P < 0·0001) between RBD‐binding IgG and NA (Fig 2).
Fig 2.
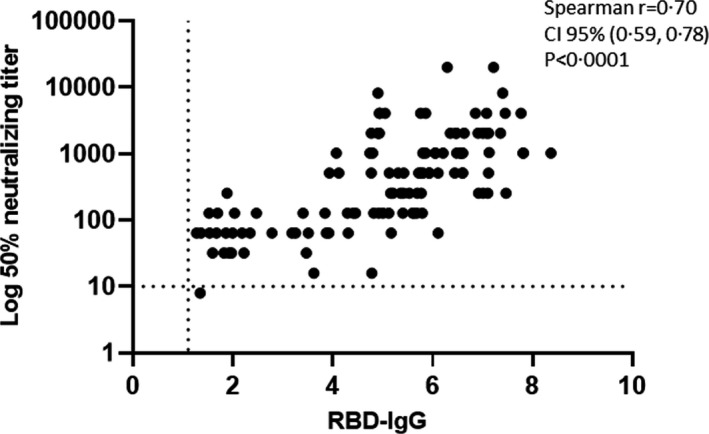
Correlation between RBD IgG and log‐transformed neutralizing antibodies. The correlation was analyzed two‐four weeks following the second vaccination, using Spearman's correlation by two‐tailed parametric t‐test means with a 95% CI. CI, confidence interval; IgG, immunoglobulin; RBD, receptor‐binding domain.
No patient was infected in our cohort with SARS‐CoV‐2 after the second dose of the vaccine with a median follow‐up of 108 (104–114) days.
Discussion
We found that the rate of the humoral response to the BNT162b2 vaccine among HSCT recipients was lower (77·6% vs 98·9%) compared to the response in immunocompetent control subjects. However, compared to other immunosuppressed populations, the efficacy of the vaccine in HSCT recipients was better. Only 18% of heart transplant recipients developed antibodies following the vaccine, 12 and 37% of kidney transplant recipients. 13 , 14 The response rate in patients with CLL was 55% among treatment‐naïve patients and 16% in patients under treatment at the time of vaccination. 15 The GMT of RBD IgG was also lower in our patients compared to the controls (2·61 vs 5·98), as well as of NA (116·0 vs 427·9). Among HSCT recipients a bimodal distribution of antibody response was noted: those with high titre include recipients who do not need immunosuppression, while those with low titre include patients who need immunosuppression, mainly prednisone and ciclosporin, including those with CGVHD. In our cohort 41·4% of recipients were not treated with immunosuppression anymore and thus had better humoral response. The relatively high response rate in our cohort may be related to the inclusion of patients at least six months following transplant and excluding patients with active AGVHD or those who were treated with anti‐CD20 six months prior to vaccination. Patients with AGVHD require high‐dose immune suppression, affecting both B and T cells, and therefore are not expected to be able to mount antibody response.
The efficacy of BNT162b2 vaccine in a mixed population of 63 patients after HSCT and CD19‐based chimaeric antigen receptor T‐cell (CART) therapy was found as 47/57 (75%) for RBD IgG and 7/37 (19%) for T‐cell response. 16
Another recent study reported the efficacy and safety of a first injection of BNT162b2 in 112 allo‐HSCT patients and showed that a first dose of the vaccine is safe and provides a 55% rate of seroconversion in allotransplanted patients compared to 100% for healthy controls (P < 0·001). 17
We report the rate and predictors of the humoral response to the BNT162b2 vaccine in HSCT recipients using not only RBD IgG but also NA. Despite the high correlation detected between RBD IgG and NA, NA is the best correlate of protection. 18 , 19 NA shows antibody functionality, not only binding but neutralization and protection. Bergwerk et al. showed that low NA titres were strongly correlated with a higher risk of infection after vaccination. 20 They documented 39 SARS‐CoV‐2 breakthrough infections among 1,497 fully vaccinated HCW. NA titres in case patients were lower in the peri‐infection period as well as in peak levels 2–3 weeks after vaccination, consistent with the hypothesis that the NA is a correlate of protection. The correlation was stronger with NA than with RBD IgG. 20 Thus, the use of NA is of paramount importance to increase the accuracy of humoral response assessment.
The ideal post‐transplant timing for COVID19 vaccination has not been determined. The response rate to vaccines during the first months or years after HSCT is usually lower than that in healthy individuals of the same age, but it improves over time to become close to normal 2–3 years after transplantation. 21 Influenza, conjugated pneumococcal and Haemophilus influenzae type b vaccines have been found to induce a humoral response as early as three months after transplantation, leading to recent guidelines which recommend starting these crucial vaccinations as early as three months after transplantation, irrespective of whether the patient has developed GVHD or received immunosuppressive therapy. 7 , 21 The response to three doses of pneumococcal conjugate vaccine (PCV) was 64–98% and comparable between patients who were vaccinated from three months and those who were vaccinated from nine months after transplant. 22 A fourth dose of PCV13 administered at 9–12 months after the procedure still increased the geometric mean concentrations. Patients vaccinated three months after the procedure might have lower antibody titres at 24 months than those vaccinated after nine months. 22
The response rates to inactivated influenza vaccine were 10–40% within six months of transplantation and improved to 10–72% after six months following transplantation with the seasonal flu and 37–84% with pandemic adjuvanted or non‐adjuvanted H1N1 vaccines. Two years following transplantation the response rates become close to the response rates of healthy individuals. The response was negatively affected by lymphopenia, hypogammaglobulineemia, GVHD, immunosuppressants and rituximab. 23 , 24 All other inactivated vaccines are recommended 6–12 months following transplantation. Live attenuated vaccines are recommended from 24 months after transplantation, only in seronegative patients with no GVHD, no immuno‐suppressants, no relapse, and no recent administration of immunoglobulins. 7 , 21
Recently, the European Society for Blood and Marrow Transplantation and the American Society of Hematology/American Society for Transplantation and Cellular Therapy recommended waiting until six months after transplantation to initiate COVID‐19 vaccination if transmission in the surrounding society is well controlled. They also recommended vaccinating patients with moderate–severe GVHD. Reasonable criteria to postpone COVID‐19 vaccination are severe, uncontrolled AGVHD grades III–IV, recipients who have received anti‐CD20 antibodies such as rituximab or other B‐cell‐depleting therapy during the past six months, CAR T‐cell patients with B‐cell aplasia earlier than six months after treatment, recent therapy with anti‐thymocyte globulin or alemtuzumab. 25 , 26
During a follow‐up period of 108 days no vaccinated patient was infected with SARS‐CoV‐2 but owing to a rapid vaccination programme in Israel the epidemic was fading at the same period and the chances for infection decreased. Patients that did not mount antibodies to the vaccination were instructed to strictly follow social distancing guidance.
We found that the BNT162b2 mRNA vaccine was safe without any episode of GVHD or allergy. Immunocompetent HCW experienced significantly more AEs than HSCT recipients.
The current study has several limitations. First, we used humoral response as a surrogate for vaccine efficacy but did not check T‐cell activity against the virus. Cell‐mediated immunity is a critical determinant of protection. Indeed, loss of antibodies does not necessarily imply loss of clinical protection and immune memory can persist, even in individuals with low antibody concentrations. 27 Due to the small population of immunosuppressed patients within a growing proportion of herd immunity we could not show clinical efficacy in preventing disease. Second, our study population was defined in advance to include patients with good potential to respond to vaccination since they were at least six months post transplant and with no AGVHD. Moreover, the majority of the patients were more than a year post transplant. Additional research is needed on patients who receive the vaccine earlier (three or six months to 12 months after transplant). Third, we have no data on immune reconstitution status and response to other vaccination in these patients.
In this study we have demonstrated that this potent vaccine is highly immunogenic in HSCT recipients, mainly those who did not require any immunosuppressive therapy. The high efficacy of the BNT162b2 mRNA Covid‐19 vaccine, together with its minimal toxicity in HSCT recipients, may convince hesitant patients to receive vaccination.
Recent data from Israel show that immunity declines with time even in the healthy population 28 and were the basis of the decision by the Israeli Ministry of Health to give a third dose of COVID‐19 vaccine to people aged 60 or over who were vaccinated for at least five months starting 30 July 2021. On 12 August the FDA authorized additional vaccine doses for certain immunocompromised individuals.
References
- 1. COVID‐19 Map [Internet, https://coronavirus.jhu.edu/]. Johns Hopkins Coronavirus Resource Center. [cited 2021 June 9].
- 2. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. NEJM. 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. NEJM. 2021;384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharma A, Bhatt NS, St Martin A, Abid MB, Bloomquist J, Chemaly RF, et al. Clinical characteristics and outcomes of COVID‐19 in haematopoietic stem‐cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8:e185–e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coll E, Fernández‐Ruiz M, Sánchez‐Álvarez JE, Martínez‐Fernández JR, Crespo M, Gayoso J, et al. COVID‐19 in transplant recipients: the Spanish experience. Am J Transplant. 2021;21:1825–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corona dashboard [Internet, https://datadashboard.health.gov.il/COVID‐19/general]. [cited 2021 Apr 9].
- 7. Carpenter PA, Englund JA. How I vaccinate blood and marrow transplant recipients. Blood. 2016;127:2824–32. [DOI] [PubMed] [Google Scholar]
- 8. Lee SJ. Classification systems for chronic graft‐versus‐host disease. Blood. 2017;129:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oved K, Olmer L, Shemer‐Avni Y, Wolf T, Supino‐Rosin L, Prajgrod G, et al. Multi‐center nationwide comparison of seven serology assays reveals a SARS‐CoV‐2 non‐responding seronegative subpopulation. EClinicalMedicine. 2020;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zettl F, Meister TL, Vollmer T, Fischer B, Steinmann J, Krawczyk A, et al. Rapid QUANTIFICATION of SARS‐CoV‐2‐neutralizing antibodies using propagation‐defective vesicular stomatitis virus pseudotypes. Vaccines (Basel). 2020;8:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dieterle ME, Haslwanter D, Bortz RH, Wirchnianski AS, Lasso G, Vergnolle O, et al. A replication‐competent vesicular stomatitis virus for studies of SARS‐CoV‐2 spike‐mediated cell entry and its inhibition. Cell Host Microbe. 2020;28:486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peled Y, Ram E, Lavee J, Sternik L, Segev A, Wieder‐Finesod A, et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40:759–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grupper A, Rabinowich L, Schwartz D, Schwartz IF, Ben‐Yehoyada M, Shashar M, et al. Reduced humoral response to mRNA SARS‐CoV‐2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21:2719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rozen‐Zvi B, Yahav D, Agur T, Zingerman B, Ben‐Zvi H, Atamna A, et al. Antibody response to SARS‐CoV‐2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect. 2021;27:1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ram R, Hagin D, Kikozashvilli N, Freund T, Amit O, Bar‐On Y, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID‐19 vaccine in patients after allogeneic HCT or CD19‐based CART therapy—A Single‐Center Prospective Cohort Study. Transplant Cell Ther. 2021;27:788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chevallier P, Coste‐Burel M, Le Bourgeois A, Peterlin P, Garnier A, Béné MC, et al. Safety and immunogenicity of a first dose of SARS‐CoV‐2 mRNA vaccine in allogeneic hematopoietic stem‐cells recipients. eJHaem. 2021;2:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dispinseri S, Secchi M, Pirillo MF, Tolazzi M, Borghi M, Brigatti C, et al. Neutralizing antibody responses to SARS‐CoV‐2 in symptomatic COVID‐19 is persistent and critical for survival. Nat Commun. 2021;12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krammer F. Correlates of protection from SARS‐CoV‐2 infection. Lancet. 2021;397:1421–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid‐19 breakthrough infections in vaccinated health care workers. New Engl J Med. 2021;385:1474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cordonnier C, Einarsdottir S, Cesaro S, Di Blasi R, Mikulska M, Rieger C, et al. Vaccination of haemopoietic stem cell transplant recipients: guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19:e200–e212. [DOI] [PubMed] [Google Scholar]
- 22. Cordonnier C, Labopin M, Chesnel V, Ribaud P, De La Camara R, Martino R, et al. Randomized study of early versus late immunization with pneumococcal conjugate vaccine after allogeneic stem cell transplantation. Clin Infect Dis. 2009;48:1392–401. [DOI] [PubMed] [Google Scholar]
- 23. Natori Y, Humar A, Lipton J, Kim DD, Ashton P, Hoschler K, et al. A pilot randomized trial of adjuvanted influenza vaccine in adult allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2017;52:1016–21. [DOI] [PubMed] [Google Scholar]
- 24. Ambati A, Einarsdottir S, Magalhaes I, Poiret T, Bodenstein R, LeBlanc K, et al. Immunogenicity of virosomal adjuvanted trivalent influenza vaccination in allogeneic stem cell transplant recipients. Transpl Infect Dis. 2015;17:371–9. [DOI] [PubMed] [Google Scholar]
- 25. COVID vaccines version 5.02 ‐ 2021‐02‐21.pdf . Available from: https://www.ebmt.org/sites/default/files/2021‐02/COVID%20vaccines%20version%205.02%20‐%202021‐02‐21.pdf
- 26. ASH‐ASTCT COVID‐19 Vaccination for HCT and CAR T cell recipients ‐ Hematology.org . Available from: https://www.hematology.org:443/covid‐19/ash‐astct‐covid‐19‐vaccination‐for‐hct‐and‐car‐t‐cell‐recipients
- 27. Dan JM, Mateus J, Kato YU, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS‐CoV‐2 assessed for up to 8 months after infection. Science. 2021;371:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to BNT162b2 Covid‐19 vaccine over 6 months. New Engl J Med. 2021; 10.1056/NEJMoa2114583. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


