Dear Editor,
The development and manufacturing of effective vaccines against COVID‐19 has been an epic achievement in record time, and we believe that the vaccine will help stop the pandemic. As with other medical interventions, vaccines may carry a small risk of adverse reactions (ARs), especially when used on large populations outside the highly controlled setting of Phase 3 clinical trials. We report five cases of different rare and severe cutaneous conditions arising in close connection with COVID‐19 vaccination (Table 1).
Table 1.
Brief summary of the demographic and clinical features of the patients.
| Patient | Diagnosis | Sex | Age, years | Type of COVID‐19 vaccine | Time lag, days | Comorbidities | Clinical course |
|---|---|---|---|---|---|---|---|
| 1 | PRP | F | 62 |
Moderna, first dose (second dose not administered) |
5 | Metabolic syndrome, T2DM, hypertensive heart disease, hypothyroidism, CKD | Progressive remission with systemic prednisone (1 mg/kg/day for 2 weeks, then tapered) and topical steroids at 1‐month follow‐up. Hospitalization for COVID‐19 infection 4 months after PRP onset |
| 2 | PRP | F | 82 |
Pfizer–BioNTech, first dose (second dose not administered) |
7 | Plaque and nail psoriasis, CLL, T2DM, hypertension, COPD | Clinical improvement achieved with subcutaneous MTX 15 mg/weekly. Residual PP hyperkeratosis and scaly plaques on head and neck at the 4‐month follow‐up |
| 3 | SS | F | 69 |
Oxford–AstraZeneca, first dose (second dose not administered) |
12 | Overweight, hypertension, dyslipidaemia, iron‐deficiency anaemia | Treated with steroid administration (prednisone 1 mg/kg/day for 4 weeks, then slow tapering). At 3‐month follow‐up, complete healing of the ulcerated plaques with residual hyperpigmentation |
| 4 | PLEVA | M | 70 |
Pfizer–BioNTech, second dose |
5 | Acute lymphocytic leukaemia in complete remission | Treated with topical combination of fusidic acid 2% plus betamethasone cream 0.1%. Complete remission within 10 weeks |
| 5 | EM | F | 76 |
Pfizer–BioNTech, first dose (second dose administered) |
4 |
Lung adenocarcinoma (Stage IV), arterial hypertension, T2DM, COPD |
Topical prescription of methylprednisolone 0.1% cream twice daily for 10 days. Complete clearance achieved in 10 days. No recurrence with the second vaccine dose |
CKD, chronic kidney disease; CLL, chronic lymphocytic leukaemia; COPD, chronic obstructive pulmonary disease; EM, erythema multiforme; PLEVA, pityriasis lichenoides et varioliformis acuta; PP, palmoplantar; PRP, pityriasis rubra pilaris; T2DM, Type 2 diabetes mellitus; MTX, methotrexate; SS; Sweet syndrome.
By April 2021, about 15% of the 849 000 inhabitants of the province of Vicenza (in the Veneto region of northeast Italy) had been vaccinated, with a male : female ratio of 3 : 2. The priority was accorded to older people (> 80 years), healthcare workers and school staff. Overall, 187 adverse events were recorded. Besides the more common reactions reflecting aspecific activation of the immune system such as urticaria and cutaneous rash, we observed four rare acute conditions in five recently vaccinated patients [pityriasis rubra pilaris (PRP) in two patients, and Sweet syndrome (SS), pityriasis lichenoides et varioliformis acuta (PLEVA) and erythema multiforme (EM) in one patient each] (Fig. 1). All these conditions were confirmed histologically (Fig. 2) and appeared within the first 2 weeks following the first dose of the COVID‐19 vaccine.
Figure 1.
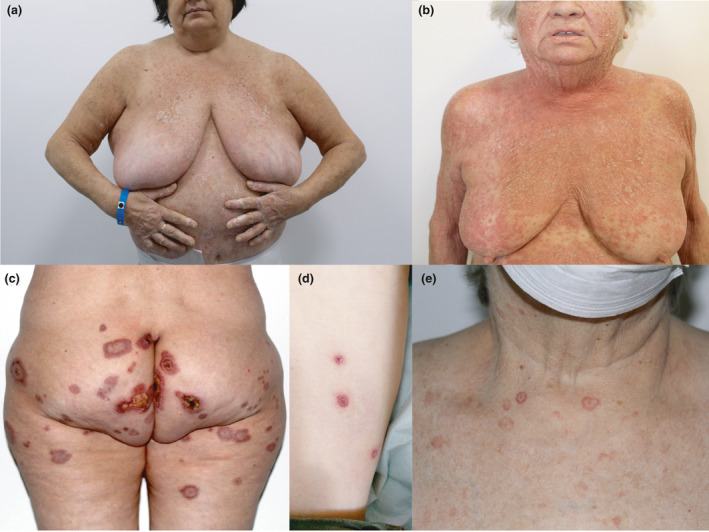
(a–e) Clinical features of the five cases with possible COVID‐19 vaccine‐related adverse reactions: (a,b) pityriasis rubra pilaris (two patients, both confirmed histologically) manifesting as widespread distributed erythematous patches and concomitant unaffected ‘islands’, resolving with lamellar desquamation; (c) Sweet syndrome, showing evolutive polymorphism of early annular lesions evolving into large, thickened ulcerated plaques; (d) pityriasis lichenoides et varioliformis acuta, manifesting as scattered, nonfolliculocentric, rapidly evolving papules showing erythematous, raised borders and an eroded centre, covered by a haemorrhagic crust; and (e) erythema multiforme, showing noncoalescing target‐like lesions mainly distributed on the limbs.
Figure 2.
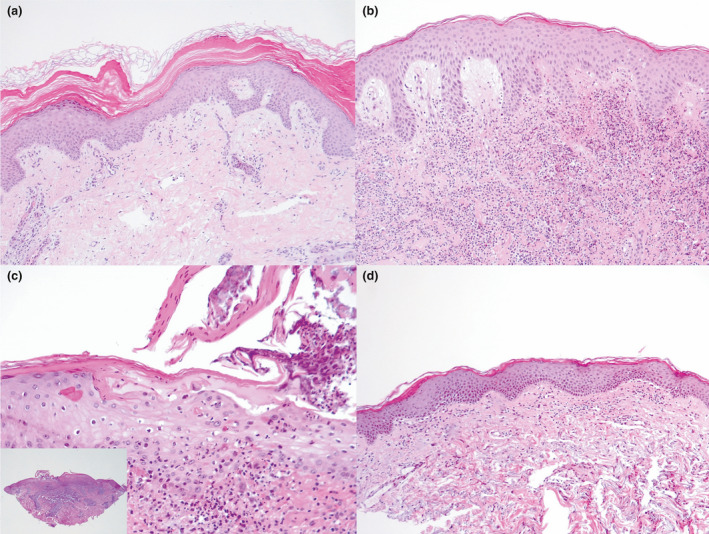
(a–e) Histological analysis of incisional cutaneous biopsy taken from the patients: (a) pityriasis rubra pilaris, showing acanthotic epidermis with fusion and broadening of rete ridges and alternating orthokeratosis and parakeratosis; (b) mildly acanthotic epidermis with subepidermal oedema and intense interstitial neutrophilic infiltrate in the dermal layer in a patient with Sweet syndrome; (c) pityriasis lichenoides et varioliformis acuta, showing focal epidermal ulceration, spongiosis, parakeratosis, and interface inflammation within a wedge‐shaped dermal inflammatory cell infiltrate; and (d) vacuolar interface dermatitis with a dermal inflammatory lymphohistiocytic infiltrate and a mild epidermal spongiosis in a patient with erythema multiforme. Haematoxylin and eosin, original magnification (a) × 200; (b,c) × 50; (c) × 400, inset × 25.
To better characterize the relationship between these possible ARs and vaccine administration, we used the Naranjo Adverse Drug Reaction Probability Scale, 1 obtaining a score for each patient indicating a ‘probable’ causality link (Table 2). All patients had undergone a COVID‐19 throat swab test 2 weeks prior to the vaccine, which was negative, and none of them had been infected since the pandemic started. None of the patients had any medical history of previous ARs to drugs or vaccines, or of any dermatological disorders.
Table 2.
Naranjo Adverse Drug Reaction Probability Scale 1 : the reaction is considered definite if the score is ≥ 9, probable if 5 to 8, possible if 1 to 4, and doubtful if 0 or less.
| Question: | Patient | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 1 |
Are there previous conclusive reports on this reaction? Yes: +1; No: 0; Don’t know: 0 |
0 | 0 | +1 | 0 | +1 |
| 2 |
Did the adverse event appear after the suspected drug was administered? Yes: +2; No: −1; Don’t know: 0 |
+2 | +2 | +2 | +2 | +2 |
| 3 |
Did the adverse reaction improve when the drug was discontinued, or a specific antagonist was administered? Yes: +1; No: 0; Don’t know: 0 |
+1 | +2 | +1 | +1 | +1 |
| 4 |
Did the adverse event reappear when the drug was re‐administered? Yes: +2; No: −1; Don’t know: 0 |
0 | 0 | 0 | 0 | −1 |
| 5 |
Are there alternative causes that could on their own have caused the reaction? Yes: −1; No: +2; Don’t know: 0 |
+2 | +2 | +2 | +2 | +2 |
| 6 |
Did the reaction reappear when a placebo was given? Yes: −1; No: +1; Don’t know: 0 |
0 | 0 | 0 | 0 | 0 |
| 7 |
Was the drug detected in blood (or other fluids) in concentrations known to be toxic? Yes: +1; No: 0; Don’t know: 0 |
0 | 0 | 0 | 0 | 0 |
| 8 |
Was the reaction more severe when the dose was increased or less severe when the dose was decreased? Yes: +1; No: 0; Don’t know: 0 |
0 | 0 | 0 | 0 | 0 |
| 9 |
Did the patient have a similar reaction to the same or similar drugs in any previous exposure? Yes: +1; No: 0; Don’t know: 0 |
0 | 0 | 0 | 0 | 0 |
| 10 |
Was the adverse event confirmed by any objective evidence? Yes: +1; No: 0; Don’t know: 0 |
+1 | +1 | +1 | +1 | +1 |
| Total score | 6 | 7 | 7 | 6 | 6 | |
A number of cutaneous conditions following COVID‐19 immunization have been reported, most of which were mild and self‐limiting such as local injection‐site reactions, and urticarial and morbilliform eruptions. In addition, some rare reactions have been observed including chilblains, cosmetic filler reactions, flares of herpes zoster or simplex, pityriasis rosea‐like reactions, EM and SS. 2 , 3 Our observations confirm the possible association of COVID‐19 vaccination with both EM and SS. In addition, this case series expands on the spectrum of possible vaccine ARs to include PRP and PLEVA.
There is a debate about the capacity of COVID‐19 vaccines to trigger immune‐mediated conditions, either with exacerbation of pre‐existing or new onset of immune‐mediated disorders. These events may result from upregulated inflammatory immunological pathways or crossreactivity between viral or adjuvant molecules and self‐antigens. Other vaccines have been associated with immune‐mediated cutaneous ARs, including EM, cutaneous lupus, Gianotti–Crosti syndrome, lichenoid eruption and granuloma annulare, 4 and also a few cases of SS, PRP and PLEVA. 5
The causal relationship between mRNA vaccines and cutaneous immunological reactions is still under debate, and we cannot exclude that the events we have reported were purely coincidental. Systemic surveillance and accurate reporting are essential to estimate associations and better qualify the potentially at‐risk population, defining effective management strategies.
Conflict of interest: the authors declare that they have no conflicts of interest.
AS and EP contributed equally to this work and should be considered joint first authors.
References
- 1. Naranjo CA, Busto U, Sellers EM et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239–45. [DOI] [PubMed] [Google Scholar]
- 2. McMahon DE, Amerson E, Rosenbach M et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol 2021; 85: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Darrigade AS, Théophile H, Sanchez‐Pena P et al. Sweet syndrome induced by SARS‐CoV2 Pfizer‐BioNTech mRNA vaccine. Allergy 2021; 76: 3194–6. 10.1111/all.14981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stone CA Jr, Rukasin CRF, Beachkofsky TM, Phillips EJ. Immune‐mediated adverse reactions to vaccines. Br J Clin Pharmacol 2019; 85: 2694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gunatheesan S, Ferguson J, Moosa Y. Pityriasis lichenoides et varioliformis acuta: a rare association with the measles, mumps and rubella vaccine. Australas J Dermatol 2012; 53: 76–8. [DOI] [PubMed] [Google Scholar]


