Abstract
Many safe and effective severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccinations dramatically reduce risks of coronavirus disease 2019 (COVID‐19) complications and deaths. We aimed to describe cases of SARS‐CoV‐2 infection among patients with chronic liver disease (CLD) and liver transplant (LT) recipients with at least one prior COVID‐19 vaccine dose. The SECURE‐Liver and COVID‐Hep international reporting registries were used to identify laboratory‐confirmed COVID‐19 in CLD and LT patients who received a COVID‐19 vaccination. Of the 342 cases of lab‐confirmed SARS‐CoV‐2 infections in the era after vaccine licensing, 40 patients (21 with CLD and 19 with LT) had at least one prior COVID‐19 vaccination, including 12 who were fully vaccinated (≥2 weeks after second dose). Of the 21 patients with CLD (90% with cirrhosis), 7 (33%) were hospitalized, 1 (5%) was admitted to the intensive care unit (ICU), and 0 died. In the LT cohort (n = 19), there were 6 hospitalizations (32%), including 3 (16%) resulting in mechanical ventilation and 2 (11%) resulting in death. All three cases of severe COVID‐19 occurred in patients who had a single vaccine dose within the last 1‐2 weeks. In contemporary patients with CLD, rates of symptomatic infection, hospitalization, ICU admission, invasive ventilation, and death were numerically higher in unvaccinated individuals. Conclusion: This case series demonstrates the potential for COVID‐19 infections among patients with CLD and LT recipients who had received the COVID‐19 vaccination. Vaccination against SARS‐CoV‐2 appears to result in favorable outcomes as attested by the absence of mechanical ventilation, ICU, or death among fully vaccinated patients.

Abbreviations
- CLD
chronic liver disease
- COVID‐19
coronavirus disease 2019
- GI
gastrointestinal
- ICU
intensive care unit
- LT
liver transplant
- MMF
mycophenolate mofetil
- mRNA
messenger RNA
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
Since the onset of the coronavirus disease 2019 (COVID‐19) pandemic, we have learned much about the sequelae of COVID‐19 in patients with liver disease.( 1 ) Patients with decompensated cirrhosis are at increased risk of hospitalization, intensive care unit (ICU) admission, and death from COVID‐19.( 2 , 3 , 4 ) Although liver transplant (LT) recipients may not be at higher risk of COVID‐19 mortality,( 5 , 6 ) some data suggest that these patients might have an increased risk of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection.( 7 ) Meanwhile, vaccine development has progressed at a rapid rate, and vaccine rollouts have tended to prioritize vulnerable patients, including immunocompromised patients.( 8 ) While the endorsement and potential value of SARS‐CoV‐2 vaccination is well‐established in patients with liver disease,( 9 , 10 , 11 ) more data are needed on vaccine response and sequelae of postvaccine infections in patients with chronic liver disease (CLD) and LT recipients.( 12 )
There is an excellent immune response to the SARS‐CoV‐2 vaccination in the general population; in vaccinated individuals, complications of COVID‐19, including ICU admission and death, are incredibly rare.( 13 , 14 , 15 , 16 ) However, vaccine immunogenicity may not be as robust for immunocompromised or immunosuppressed patients. Patients with advanced liver disease have deficiencies in innate and humoral immunity, referred to as cirrhosis‐associated immune dysfunction.( 17 , 18 ) Similarly, LT recipients require immunosuppressant medications and have blunted antibody responses following influenza, hepatitis A, hepatitis B, and pneumococcal vaccinations.( 19 , 20 , 21 , 22 ) Early data suggest that the antibody response to the SARS‐CoV‐2 vaccination is attenuated in CLD( 23 ) and solid organ recipients.( 24 , 25 ) However, real‐world data are still lacking on the patterns and outcomes of postvaccination infections in patients with CLD and LT recipients.
We used data from two large international registries to describe cases of laboratory‐confirmed COVID‐19 among patients with CLD and LT recipients who had received vaccination against SARS‐CoV‐2.
Patients and Methods
We established an online reporting system for cases of laboratory‐confirmed SARS‐CoV‐2 in patients with CLD or prior LT. For this study, data were collected between March 5, 2021, and August 2, 2021 (era after vaccine licensing), through two collaborating registries (SECURE‐Liver supported by the American Association for the Study of Liver Diseases and COVID‐Hep.net supported by the European Association for the Study of the Liver). These registries were coordinated from the University of North Carolina (United States) and the University of Oxford (United Kingdom). Given that all data submitted to these registries were de‐identified, both the University of Oxford Clinical Trials and Research Governance and the University of North Carolina Office of Human Research Ethics deemed that this project was not human research and did not require formal institutional review board approval.
As previously described,( 2 , 5 ) clinicians were invited to submit online case report forms providing de‐identified clinical information on cases of laboratory‐confirmed SARS‐Cov‐2 in patients with prior LT or CLD. Case report forms included demographics, liver disease etiology and severity, SARS‐Cov‐2 vaccination details, and COVID‐19 outcomes, including hospitalization, ICU stay, and death. Clinicians were instructed to submit forms after patients had COVID‐19 for a long enough duration to experience recovery from COVID‐19 or death. Here we describe the cases of SARS‐CoV‐2 infection among patients with at least one prior COVID‐19 vaccine dose compared with contemporary (i.e., submitted March 5, 2021, to August 2, 2021) unvaccinated patients with CLD and prior LT.
Results
A total of 342 cases of lab‐confirmed SARS‐CoV‐2 infection among patients with CLD and prior LT were submitted to SECURE‐Liver and COVID‐Hep 2.0 from March 5, 2021, and August 2, 2021. Among these, 40 (12%) patients (n = 21 with CLD, n = 19 with LT) had at least one prior SARS‐CoV‐2 vaccination, 14 had received both doses (all received the same type of vaccine for both doses), and 12 occurred ≥2 weeks after the second vaccine dose (Fig. 1). Characteristics of CLD and LT cases are found in Tables 1 and 2, respectively, and the description of vaccinations received are shown in Fig. 1.
FIG. 1.
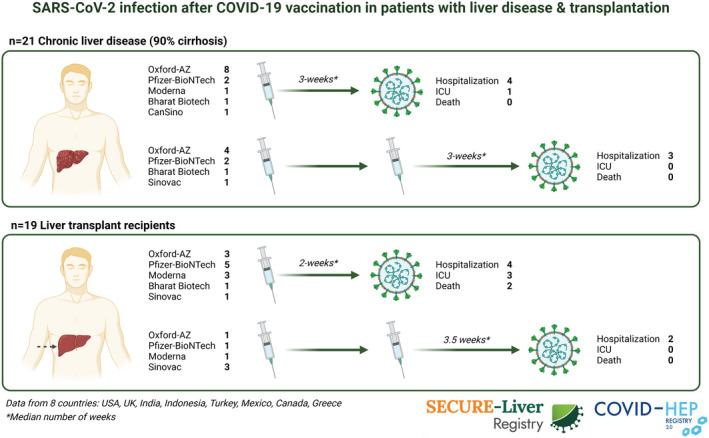
SARS‐CoV‐2 infection after COVID‐19 vaccination in patients with liver disease and transplantation.
TABLE 1.
Patient Characteristics and Information on Vaccination and COVID‐19 Outcomes Among Patients With CLD
| Age (years) | Sex | Etiology | CLD Severity | Vaccine Type | Vaccine Doses | Weeks Between v1 & SARS‐CoV‐2 | Weeks Between v2 & SARS‐CoV‐2 | Hospitalized | Invasive Ventilation | ICU | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 60‐69 | M | HCV | CP‐A | Oxford‐AstraZeneca | 1 | <1 | – | No | – | – | No |
| 50‐59 | M | NAFLD | CP‐B | Oxford‐AstraZeneca | 1 | 1 | – | No | – | – | No |
| 40‐49 | M | NAFLD | CP‐A | Oxford‐AstraZeneca | 1 | 1 | – | Yes | No | No | No |
| 40‐49 | M | Other | Noncirrhotic CLD | Pfizer‐BioNTech | 1 | 2 | – | No | – | – | No |
| 60‐69 | M | NAFLD | CP‐A | Oxford‐AstraZeneca | 1 | 2 | – | Yes | No | Yes | No |
| 50‐59 | F | NAFLD | CP‐A | Oxford‐AstraZeneca | 1 | 3 | – | Yes | No | No | No |
| 60‐69 | M | HBV | CP‐A | Oxford‐AstraZeneca | 1 | 3 | – | No | – | – | No |
| 70‐79 | M | Other | Noncirrhotic CLD | Pfizer‐BioNTech | 1 | 3 | – | No | – | – | No |
| 60‐69 | M | ALD | CP‐A | Moderna | 1 | 4 | – | No | – | – | No |
| 50‐59 | M | HCV | CP‐A | Bharat Biotech | 1 | 4 | – | No | – | – | No |
| 50‐59 | F | Other | CP‐A | CanSino | 1 | 4 | – | No | – | – | No |
| 40‐49 | F | Other | CP‐B | Oxford‐AstraZeneca | 1 | 6 | – | No | – | – | No |
| 40‐49 | M | ALD | CP‐B | Oxford‐AstraZeneca | 1 | 8 | – | Yes | No | No | No |
| 70‐79 | F | Other | CP‐B | Sinovac | 2 | 5 | 1 | No | – | – | No |
| 50‐59 | F | Other | CP‐C | Bharat Biotech | 2 | 9 | 1 | No | – | – | No |
| 60‐69 | M | HCV | CP‐A | Oxford‐AstraZeneca | 2 | 9 | 2 | Yes | No | No | No |
| 60‐69 | F | Other | CP‐B | Oxford‐AstraZeneca | 2 | 6 | 3 | No | – | – | No |
| 50‐59 | M | NAFLD | CP‐A | Pfizer‐BioNTech | 2 | 9 | 3 | No | – | – | No |
| 70‐79 | F | NAFLD | CP‐A | Pfizer‐BioNTech | 2 | 9 | 6 | Yes | No | No | No |
| 60‐69 | M | NAFLD | CP‐B | Oxford‐AstraZeneca | 2 | 12 | 8 | Yes | No | No | No |
| <40 | M | Other | CP‐A | Oxford‐AstraZeneca | 2 | 18 | 6 | No | – | – | No |
Full vaccinated patients in gray.
Abbreviations: ALD, alcohol‐associated liver disease; CP‐A, Child‐Pugh A; CP‐B, Child‐Pugh B; CP‐C, Child‐Pugh C; F, female; HBV, hepatitis B virus; HCV, hepatitis C virus; M, male; NAFLD, nonalcoholic fatty liver disease; v1, first vaccine dose; v2, second vaccine dose.
TABLE 2.
Patient Characteristics Information on Vaccination and COVID‐19 Outcomes Among LT Recipients
| Age (years) | Sex | Time Since Transplant (years) | Immunosuppression | Vaccine Type | Vaccine Doses | Weeks Between Last Dose & SARS‐CoV‐2 | Weeks Between v2 & SARS‐CoV‐2 | Hospitalized | Invasive Ventilation | ICU | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50‐59 | M | >10 | Tacrolimus, MMF | Moderna | 1 | 1 | – | Yes | Yes | Yes | Yes |
| <40 | M | 5‐10 | Tacrolimus | Oxford‐AstraZeneca | 1 | 1 | – | No | – | – | No |
| 40‐49 | M | 1‐5 | Tacrolimus | Sinovac | 1 | 1 | – | No | – | – | No |
| 40‐59 | F | 1‐5 | Tacrolimus, MMF | Oxford‐AstraZeneca | 1 | 1 | – | No | – | – | No |
| <40 | M | 5‐10 | Tacrolimus | Pfizer‐BioNTech | 1 | 2 | – | No | – | – | No |
| 60‐69 | M | <1 | Sirolimus, MMF | Moderna | 1 | 2 | – | Yes | Yes | Yes | Yes |
| 70‐79 | F | >10 | Sirolimus | Pfizer‐BioNTech | 1 | 2 | – | Yes | Yes | Yes | No |
| 60‐69 | F | >10 | Tacrolimus | Pfizer‐BioNTech | 1 | 2 | – | No | – | – | No |
| 60‐69 | F | 1‐5 | Tacrolimus, Everolimus, MMF | Bharat Biotech | 1 | 2 | – | No | – | – | No |
| 60‐69 | M | 1‐5 | Tacrolimus | Pfizer‐BioNTech | 1 | 2 | – | No | – | – | No |
| 40‐49 | F | >10 | Prednisone, tacrolimus, MMF | Pfizer‐BioNTech | 1 | 3 | – | No | – | – | No |
| 60‐69 | M | 1‐5 | Tacrolimus | Moderna | 1 | 3 | – | No | – | – | No |
| 70‐79 | M | 6‐10 | Tacrolimus, azathioprine | Oxford‐AstraZeneca | 1 | 5 | – | Yes | No | No | No |
| <40 | M | 1‐5 | Prednisone, tacrolimus | Moderna | 2 | 6 | 2 | No | – | – | No |
| 60‐69 | M | >10 | Tacrolimus, MMF | Pfizer‐BioNTech | 2 | 12 | 2 | Yes | No | No | No |
| 60‐69 | F | 1‐5 | Not known | Sinovac | 2 | 7 | 3 | No | – | – | No |
| 50‐59 | F | 5‐10 | Tacrolimus | Sinovac | 2 | 8 | 4 | Yes | No | No | No |
| 60‐69 | F | 1‐5 | Tacrolimus | Sinovac | 2 | 8 | 4 | No | – | – | No |
| 40‐49 | M | 1‐5 | Tacrolimus, MMF | Oxford‐AstraZeneca | 2 | 20 | 12 | No | – | – | No |
Full vaccinated patients in gray.
Abbreviations: F, female; M, male; v1, first vaccine dose; v2, second vaccine dose.
COVID‐19 in Patients with CLD With ≥1 Vaccine Dose Versus Unvaccinated
In patients with CLD who received at least one vaccination (n = 21), the median age was 59 years (range 28‐72), 67% were male, and cases were from North America (n = 10, 45%), India (n = 6, 27%), the United Kingdom (n = 2, 9%), Turkey (n = 1, 5%), Indonesia (n = 1, 5%), and Greece (n = 1, 5%) (Table 1). The most common CLD etiology reported was nonalcoholic fatty liver disease (n = 7, 33%), and 90% of patients had cirrhosis (Child Pugh A/B/C, 63%/32%/5%). SARS‐CoV‐2 infection was diagnosed after one vaccine dose in 13 (62%) and after two doses in 8 (38%). Viral vector vaccines (Oxford‐AstraZeneca, CanSino) were received by 13 (62%), messenger RNA (mRNA) vaccines (Pfizer‐BioNTech, Moderna) by 5 (24%), and inactivated vaccines (Bharat Biotech, Sinovac) by 3 (14%). Among those who received a single vaccine dose, the median time from vaccination to infection was 3 weeks (range 0‐8), and for those who received both doses, the median time from the second dose to infection was 3 weeks (range 1‐8). Regarding symptoms at presentation, 11 (57%) had respiratory symptoms, 1 (5%) had gastrointestinal (GI) symptoms, 1 (5%) had GI and respiratory symptoms, and 8 (38%) reported no GI or respiratory symptoms (Table 3). Of these 21 patients who received prior vaccination, 7 (33%) were hospitalized, 1 (5%) was admitted to the ICU, 0 required invasive ventilation, and 0 died. Among patients with cirrhosis (n = 19), 9 (47%) developed a new decompensating event (8 new/worsening ascites, 7 hepatic encephalopathy, 4 spontaneous bacterial peritonitis), including 5 patients who were fully vaccinated (≥2 weeks after second dose).
TABLE 3.
Symptoms, Hospitalization, ICU Admission, Invasive Ventilation, and Death by Liver Disease and Vaccination Status
| All CLDs, ≥1 Vaccine Dose (n = 21) | All CLDs, Unvaccinated (n = 225) | Cirrhosis, ≥1 Vaccine Dose (n = 19) | Cirrhosis, Unvaccinated (n = 159) | Prior LT, ≥1 Vaccine Dose (n = 19) | Prior LT, Unvaccinated (n = 77) | |
|---|---|---|---|---|---|---|
| GI and/or respiratory symptoms (n, %) | 13 (62%) | 172 (76%) | 12 (63%) | 118 (74%) | 17 (89%) | 68 (88%) |
| Hospitalization (n, %) | 7 (33%) | 162 (72%) | 7 (37%) | 118 (74%) | 6 (32%) | 33 (43%) |
| ICU admission (n, %) | 1 (5%) | 20 (9%) | 1 (5%) | 15 (9%) | 3 (16%)* | 7 (9%) |
| Invasive ventilation (n, %) | 0 (0%) | 12 (5%) | 0 (0%) | 9 (6%) | 3 (16%)* | 9 (12%) |
| Death (n, %) | 0 (0%) | 18 (8%) | 0 (0%) | 15 (9%) | 2 (11%)* | 6 (8%) |
All among patients with a single dose within 1‐2 weeks of SARS‐CoV‐2 diagnosis.
In comparison, among the 225 unvaccinated patients with CLD with COVID‐19 (median age 59 years, 62% male, 72% cirrhosis, Child‐Pugh A/B/C 47%/31%/22%), 185 (76%) had respiratory and/or GI symptoms from COVID‐19 compared with 57 (24%) who did not endorse GI or respiratory symptoms. Of these unvaccinated CLD cases, 162 (72%) were hospitalized, 20 (9%) were admitted to the ICU, 12 (5%) required invasive ventilation, and 18 (8%) died, primarily from COVID‐19 lung disease (n = 11 of 18, 61%) (Table 3). Similarly, rates of symptomatic infection, hospitalization, ICU admission, invasive ventilation, and death were numerically higher in unvaccinated compared to vaccinated patients with cirrhosis (Table 3).
COVID‐19 in LT Recipients With ≥1 Vaccine Dose Versus Unvaccinated
Among LT recipients who received at least one vaccination (n = 19), the median age was 60 years (range 32‐79), 58% were male, and most were from North America (n = 14, 74%) followed by Turkey (n = 2, 11%), India (n = 2, 11%), and the United Kingdom (n = 1, 5%) (Table 2). The median time from LT to infection was 4 years (range 1‐24), and the most common immunosuppression regimen prior to diagnosis was tacrolimus monotherapy (n = 8, 42%). A total of 7 (37%) were on mycophenolate mofetil (MMF). The most frequent vaccine type was mRNA (n = 10, 53%), and the remaining patients received inactivated (n = 5, 26%) and viral vector vaccines (n = 4, 21%). Among those who received a single vaccine dose, the median time from the vaccination to infection was 2 weeks (range 1‐5), and for those who received two doses, the median time from the second vaccine dose to infection was 3.5 weeks (range 2‐12). Most (n = 13, 68%) patients had respiratory symptoms at diagnosis, and 1 (5%) had no respiratory or GI symptoms (Table 3). There were 6 hospitalizations (32%), including 3 (16%) resulting in mechanical ventilation and ICU admission and 2 (11%) resulting in death. Deaths were from COVID‐19 lung disease in 1 patient and cardiogenic shock in 1 patient. Of note, all three cases of severe COVID‐19 occurred in patients who had a single vaccine dose within the last 1‐2 weeks (i.e., not fully vaccinated). Among fully vaccinated LT recipients (n = 5), there were 2 hospitalizations and 0 ICU admissions or deaths.
There were 77 unvaccinated LT recipients with lab‐confirmed COVID‐19 (median age = 53 years, 55% male, median time from transplant = 6 years [range <1‐31 years], 48% on tacrolimus monotherapy/29% on prednisone/22% on MMF). Respiratory symptoms were reported in 29 (38%), GI symptoms in 3 (4%), both GI/respiratory symptoms in 36 (47%), and neither respiratory nor GI symptoms in 8 (10%). Of these unvaccinated LT recipients, 33 (43%) were hospitalized, 7 (9%) were admitted to the ICU, 9 (12%) required mechanical ventilation, and 6 (8%) died, all from COVID‐19 lung disease (Table 3).
Discussion
In the general population, SARS‐CoV‐2 vaccination has demonstrated excellent safety and efficacy, resulting in a robust immune response and significantly reduced risks of COVID‐19 infection and complications.( 16 ) As we have previously shown, patients with decompensated cirrhosis have increased risks of COVID‐19 complications resulting in ICU admission and death.( 2 ) Given the potential for higher risks of COVID‐19 in CLD and LT recipients, guidelines strongly recommend vaccination in patients with liver diseases.( 9 , 10 , 11 ) In patients with CLD and prior LT, there is a lingering concern of low vaccine immunogenicity, given underlying immune dysfunction.( 12 ) This case series adds to recently published literature on breakthrough infections in transplant recipients,( 26 ) demonstrating the potential for breakthrough SARS‐CoV‐2 infections in patients with CLD and LT recipients. Yet, reassuringly, among patients who were fully vaccinated, there were no cases resulting in ICU admission, invasive ventilation, or death. Furthermore, among patients with CLD and cirrhosis, rates of hospitalization, ICU admission, invasive ventilation, and death were numerically lower among patients who had received at least one vaccine dose compared with those who were unvaccinated. These findings are consistent with recently published papers reporting that SARS‐CoV‐2 vaccination reduces severe disease in patients with cirrhosis( 27 ) and solid organ transplant recipients.( 28 )
Data on COVID‐19 vaccine response primary consist of reports of postvaccination antibody titers in LT recipients.( 24 ) Antibody titers are not the only correlate of protection against SARS‐CoV‐2 infection and may not be a sufficient measure of protection against COVID‐19 in patients with CLD and LT recipients. It may be that antibodies help prevent infections, which could explain breakthrough infections in our series. However, T‐cell responses, which may be preserved and sufficient in patients with CLD and LT recipients, might help attenuate the COVID‐19 disease course. This could underlie the low rates of ICU admission, mechanical ventilation, and death in patients with CLD who received the full COVID‐19 vaccine series in this data set. Supporting these observations, a recent report suggests that heart and liver transplant recipients develop adequate humoral or cellular responses to an mRNA vaccine.( 29 ) Postvaccination T‐cell responses in patients with CLD have yet to be fully characterized.
The few cases of severe COVID‐19 resulting in ICU admission and death reported in this series occurred after a single dose of SARS‐CoV‐2 vaccination and were diagnosed within 1‐2 weeks after the vaccine dose. SARS‐CoV‐2 immunity continues to improve beyond 2 weeks of the first mRNA vaccine dose and increases further after a second dose.( 30 ) It is also possible that some of these patients had SARS‐CoV‐2 infection at the time of vaccination. These data are important to inform patients on the need to complete the SARS‐CoV‐2 vaccination series and use other recommended strategies to prevent SARS‐CoV‐2 acquisition.
Among fully vaccinated patients, there were no cases of COVID‐19 resulting in ICU admission, mechanical ventilation or death, which should provide reassurance to patients and providers. However, there were some cases in our series of liver‐related decompensation events in fully vaccinated individuals, and the potential for breakthrough cases among an immunosuppressed population raises questions about improved strategies of protection against COVID‐19 in vulnerable populations. There has been interest in providing a booster dose to immunosuppressed patients, who may not mount a robust immune response. In a recent case series of vaccinated transplant recipients, a third of patients with negative antibody titers after two doses had increased titers after a third SARS‐CoV‐2 vaccination dose.( 31 ) However, a large proportion of patients had persistently low or negative anti‐spike antibody titers after a third booster dose. Until more data are available on the benefits of this strategy, this suggests that precautions including social distancing and masking should remain in place for patients at the highest risk of severe COVID‐19, including those with decompensated cirrhosis.( 2 )
This study is strengthened by its large, international, diverse patient data set. Additionally, the use of clinician reporting minimizes the risk of misclassification of liver disease, vaccination details, and COVID‐19 outcomes. However, the findings of this study have to be interpreted in the context of potential limitations. First, this cohort may be subject to selection bias inherent in registry studies, potentially resulting in an overrepresentation of patients with severe COVID‐19. Second, this study design does not allow for the identification of patients with liver disease who were vaccinated but did not test positive for SARS‐CoV‐2. Therefore, while these data demonstrate the potential for breakthrough infections, we are unable to determine the rates of COVID‐19 infections among vaccinated patients with liver disease. Third, as a comparison population, we included cases of SARS‐CoV‐2 among unvaccinated patients with CLD and liver transplant recipients. While these cases were submitted to registries during an identical time period to vaccinated cases (March 5, 2021, to July 22, 2021), it remains possible that some of the infections among unvaccinated individuals occurred before this time (such as before vaccines were available) and were logged after some delay. Finally, we were not able to adjust for potential center‐specific or region‐specific differences in the management of COVID‐19, which could influence hospitalization rates, ICU escalation decisions, and outcomes.
In summary, this case series demonstrates the potential for breakthrough COVID‐19 infections among patients with CLD and LT recipients. Although some fully vaccinated patients were hospitalized, reassuringly, COVID‐19 resulting in ICU admission or death was limited to patients who had received a recent, single SARS‐CoV‐2 dose. These data reinforce the existing guidelines recommending COVID‐19 vaccination for patients with CLD and LT recipients. However, well‐established strategies for COVID‐19 prevention, including physical distancing and masking, should be considered even for vaccinated patients with CLD and LT recipients. Given the remaining unknowns, ongoing research in these populations on postvaccine immune response and strategies such as booster doses will be important.
Supporting information
Table S1
Acknowledgment
The authors thank all of those who submitted cases to the registry (Supporting Table S1). They acknowledge the following endorsing societies: American Association for the Study of Liver Diseases, European Association for Study of the Liver, United European Gastroenterology, British Association for Study of the Liver, International Liver Cancer Association, British Society of Gastroenterology, Gastroenterological Society of Australia, British Liver Trust, European Liver Patients’ Association, Hellenic Association of the Study of the Liver, Hepatology Society of the Philippines, Chinese Portal Hypertension Diagnosis and Monitoring Study Group, and ERN RARE LIVER.
Supported by the Wellcome Trust (Clinical Research Training Fellowship), American Association for the Study of Liver Diseases (Advanced/Transplant Hepatology Award), and National Institutes of Health Clinical Center (T32 DK007634).
Potential conflict of interest: Dr. Dalekos advises and received grants from Pfizer, Genkyotex, Novartis, and Sobi. He received grants from AbbVie and Gilead. Dr. Barritt consults for Target RWE.
References
Author names in bold designate shared co‐first authorship.
- 1. Marjot T, Webb GJ, Barritt AS, Moon AM, Stamataki Z, Wong VW, et al. COVID‐19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol 2021;18:348‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marjot T, Moon AM, Cook JA, Abd‐Elsalam S, Aloman C, Armstrong MJ, et al. Outcomes following SARS‐CoV‐2 infection in patients with chronic liver disease: an international registry study. J Hepatol 2021;74:567‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, et al. High rates of 30‐day mortality in patients with cirrhosis and COVID‐19. J Hepatol 2020;73:1063‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ioannou GN, Liang PS, Locke E, Green P, Berry K, O’Hare AM, et al. Cirrhosis and severe acute respiratory syndrome coronavirus 2 infection in US veterans: risk of infection, hospitalization, ventilation, and mortality. Hepatology 2021;74:322‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, et al. Outcomes following SARS‐CoV‐2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol 2020;5:1008‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kulkarni AV, Tevethia HV, Premkumar M, Arab JP, Candia R, Kumar K, et al. Impact of COVID‐19 on liver transplant recipients—a systematic review and meta‐analysis. EClinicalMedicine 2021;38:101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colmenero J, Rodríguez‐Perálvarez M, Salcedo M, Arias‐Milla A, Muñoz‐Serrano A, Graus J, et al. Epidemiological pattern, incidence, and outcomes of COVID‐19 in liver transplant patients. J Hepatol 2021;74:148‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dooling K, Marin M, Wallace M, McClung N, Chamberland M, Lee GM, et al. The Advisory Committee on Immunization Practices’ Updated Interim Recommendation for Allocation of COVID‐19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep 2021;69:1657‐1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fix OK, Blumberg EA, Chang KM, Chu J, Chung RT, Goacher EK, et al. AASLD Expert Panel Consensus Statement: Vaccines to prevent COVID‐19 infection in patients with liver disease. Hepatology 2021;74:1049‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID‐19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol 2021;74:944‐951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Society of Transplantation . Updated joint AST/ASTS/ISHLT statement about vaccine efficacy in organ transplant recipients. https://www.myast.org/updated‐joint‐astastsishlt‐statement‐about‐vaccine‐efficacy‐organ‐transplant‐recipients. Accessed July 21, 2021.
- 12. Marjot T, Webb GJ, Barritt AS, Ginès P, Lohse AW, Moon AM, et al. SARS‐CoV‐2 vaccination in patients with liver disease: responding to the next big question. Lancet Gastroenterol Hepatol 2021;6:156‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med 2020;383:2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med 2021;384:403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Team CC‐VBCI . COVID‐19 vaccine breakthrough infections reported to CDC—United States, January 1‐April 30, 2021. MMWR Morb Mortal Wkly Rep 2021;70:792‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lebossé F, Gudd C, Tunc E, Singanayagam A, Nathwani R, Triantafyllou E, et al. CD8(+)T cells from patients with cirrhosis display a phenotype that may contribute to cirrhosis‐associated immune dysfunction. EBioMedicine 2019;49:258‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liaskou E, Hirschfield GM. Cirrhosis‐associated immune dysfunction: novel insights in impaired adaptive immunity. EBioMedicine 2019;50:3‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chong PP, Avery RK. A comprehensive review of immunization practices in solid organ transplant and hematopoietic stem cell transplant recipients. Clin Ther 2017;39:1581‐1598. [DOI] [PubMed] [Google Scholar]
- 20. Aggeletopoulou I, Davoulou P, Konstantakis C, Thomopoulos K, Triantos C. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev Med Virol 2017;27:e1942. [DOI] [PubMed] [Google Scholar]
- 21. McCashland TM, Preheim LC, Gentry MJ. Pneumococcal vaccine response in cirrhosis and liver transplantation. J Infect Dis 2000;181:757‐760. [DOI] [PubMed] [Google Scholar]
- 22. Harmala S, Parisinos CA, Shallcross L, O'Brien A, Hayward A. Effectiveness of influenza vaccines in adults with chronic liver disease: a systematic review and meta‐analysis. BMJ Open 2019;9:e031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hakimian D, Shafrir A, Milgrom Y, Masarwa M, Hazou W, Amer J, et al. Elderly with advanced liver fibrosis had lower response to Pfizer’s SARS‐CoV‐2 vaccine response. In: Proceedings of the European Association for the Study of the Liver International Liver Conference, 2021. [Google Scholar]
- 24. Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody response to 2‐dose SARS‐CoV‐2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021;325:2204‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rabinowich L, Grupper A, Baruch R, Ben‐Yehoyada M, Halperin T, Turner D, et al. Low immunogenicity to SARS‐CoV‐2 vaccination among liver transplant recipients. J Hepatol 2021;75:435‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qin CX, Moore LW, Anjan S, Rahamimov R, Sifri CD, Ali NM, et al. Risk of breakthrough SARS‐CoV‐2 infections in adult transplant recipients. Transplantation 2021;105:e265‐e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. John BV, Deng Y, Scheinberg A, Mahmud N, Taddei TH, Kaplan D, et al. Association of BNT162b2 mRNA and mRNA‐1273 vaccines with COVID‐19 infection and hospitalization among patients with cirrhosis. JAMA Intern Med 2021;181:1306‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ravanan R, Mumford L, Ushiro‐Lumb I, Callaghan C, Pettigrew G, Thorburn D, et al. Two doses of SARS‐CoV‐2 vaccines reduce risk of death due to COVID‐19 in solid organ transplant recipients: preliminary outcomes from a UK registry linkage analysis. Transplantation 2021;105:e263‐e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herrera S, Colmenero J, Pascal M, Escobedo M, Castel MA, Sole‐González E, et al. Cellular and humoral immune response after mRNA‐1273 SARS‐CoV‐2 vaccine in liver and heart transplant recipients. Am J Transplant 2021. Jul 22. 10.1111/ajt.16768. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med 2020;383:2439‐2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Werbel WA, Boyarsky BJ, Ou MT, Massie AB, Tobian AAR, Garonzik‐Wang JM, et al. Safety and immunogenicity of a third dose of SARS‐CoV‐2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med 2021;174:1330‐1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


