Dear Editor,
A 74‐year‐old Thai man presented with a rash that had appeared 25 h after he had received his first dose of the adenoviral‐vectored COVID‐19 vaccine, ChAdOx1 nCoV‐19 (Oxford–AstraZeneca). The lesions had appeared abruptly without any accompanying symptoms. The patient's medical history included end‐stage renal disease, atrial fibrillation and ischaemic stroke. The patient denied taking any new drugs, supplements or foods prior to this cutaneous eruption.
Physical examination revealed multiple, well‐defined, round to oval, erythematous to violaceous plaques with central dusky appearance and bullous formation on the trunk and both extremities (Fig. 1). There was no mucosal involvement.
Figure 1.
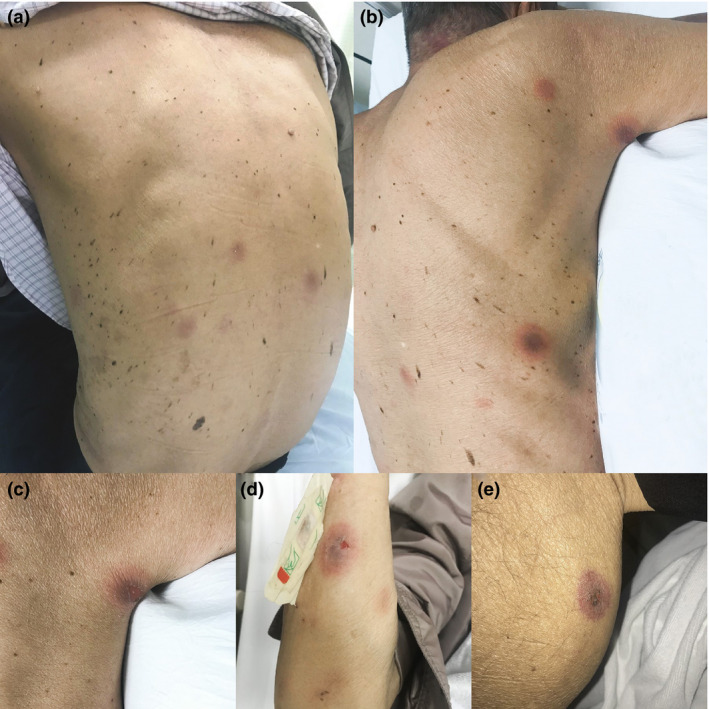
(a,b,d) Round to oval, erythematous to violaceous patches with central dusky appearance on the trunk and limbs; (c,d,e) large and well‐demarcated central erosions were also noted on (c) the axilla and trunk; (d) right forearm and (e) right leg. No mucosal lesions were observed and the lesions were found in > 2 different sites of the body.
A punch biopsy was taken, and histopathology findings were consistent with bullous fixed drug eruption (BFDE) (Fig. 2).
Figure 2.
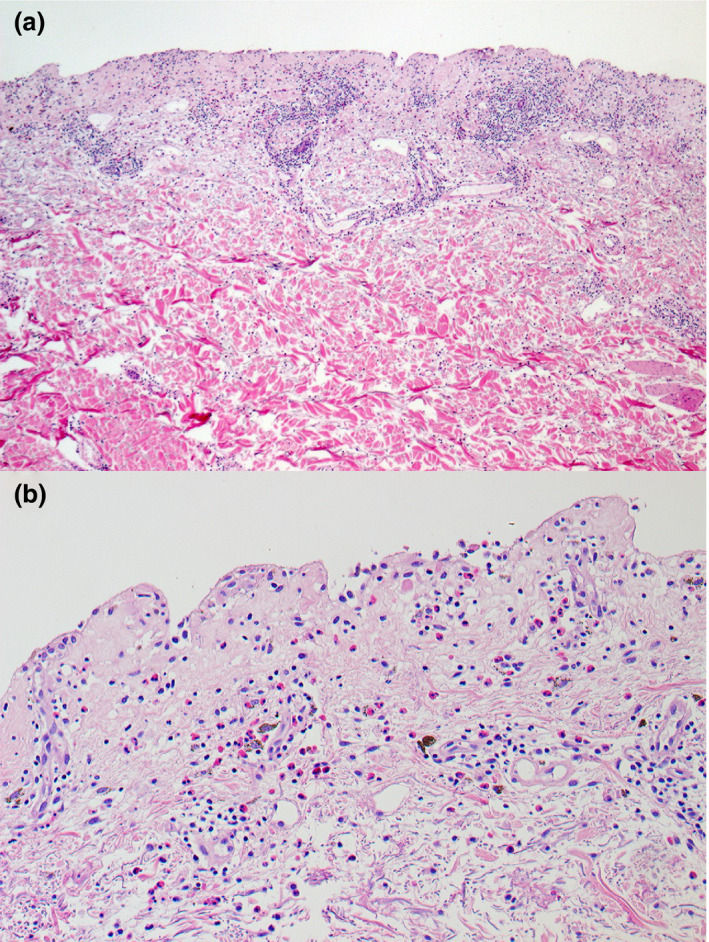
(a,b) Histological examination of a punch biopsy was performed from the lesion on the patient's back showed (a) subepidermal separation with superficial and deep perivascular inflammatory cell infiltration and (b) mixed inflammatory cells infiltrate, composing of lymphohistiocytes and numerous eosinophils. Melanophages were seen in the upper dermis. Haematoxylin and eosin, original magnification (a) × 50; (b) × 200.
Laboratory investigations did not show any definite internal organ involvement.
Given the clinical and histological features, a diagnosis of generalized BFDE (GBFDE) was made. Fixed drug eruption (FDE) (not bullous or generalized) typically presents within 1–2 weeks after the initial exposure, and in < 2 days for subsequent episodes, whereas GBFDE occurs with more sudden onset and typically within 24 h. 1 Based on the temporal relationship, the ChAdOx1 nCoV‐19 vaccine was considered as the eruption trigger, with a score of 5 (probable) on the Naranjo Adverse Drug Reaction Probability Scale.
Several vaccines have been implicated in triggering FDE, including the combined pentavalent DTaP‐IPV‐Hib (6‐in‐1) vaccine, yellow fever, influenza, human papillomavirus, recombinant adjuvant varicella zoster vaccine, and COVID‐19 vaccines. 2 , 3 , 4 , 5 Whereas FDE is usually self‐limiting and has a favourable prognosis, GBFDE is considered a severe cutaneous adverse reaction (SCAR) with a high mortality rate among elderly patients. 1 Despite the wide use of the COVID‐19 vaccinations, only eight cases of SCAR associated with these vaccines have been documented (Table 1).
Table 1.
Reported cases of severe cutaneous adverse reactions due to COVID‐19 vaccine administration.
| Patient | Sex | Age, years | Allergy | Vaccine platform | Dose | Timing of onset | Lag period after vaccination, days, days | Clinical phenotype | Supporting investigations | Outcome | Second dose administration |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 74 | Sulfa drugs, amoxicillin–clavulanic acid |
Viral vector vaccine (Janssen, Ad26.COV2.S) |
First | 3 days | 10 | AGEP |
Blood test: leucocytosis with neutrophilia and eosinophilia, normal creatinine level and liver enzymes Histology: epidermal spongiosis with subcorneal neutrophilic pustules and dermal neutrophilic inflammation with eosinophils. DIF: negative |
Improved with oral prednisolone 20 mg/day and topical steroid | NA |
| 2 | F | 43 | NA |
Viral vector vaccine (Oxford‐AstraZeneca, ChAdOx1) |
First | 3 days | NA | AGEP | Blood test: leucocytosis with eosinophilia. Histology: lichenoid interface dermatitis, intracorneal pustules, lymphocytic infiltrate with numerous eosinophils | Resolution with topical corticosteroid within 30 days | Platform changed to mRNA vaccine (Pfizer/BioNTech, BNT162b2); no recurrence of reaction |
| 3 | F | 32 | No |
Viral vector vaccine (Oxford‐AstraZeneca, ChAdOx1) |
First | 3 weeks | NA | AGEP | Blood test: leucocytosis with neutrophilia | Resolution with short course systemic corticosteroid within 2 weeks | Not mentioned |
| 4 | M | 38 | NA |
mRNA vaccine (Pfizer/BioNTech, BNT162b2) |
Second | 5 days | NA | AGEP |
Blood test: marked neutrophilia Histology: supportive of the diagnosis of AGEP |
Resolution with topical mometasone cream | NA |
| 5 | M | 60 | NA |
Viral vector vaccine (Oxford‐AstraZeneca, ChAdOx1) |
First | 3 days | 7 | SJS | Histology: moderate intraepidermal infiltration of lymphocytes and neutrophils with moderate spongiosis, scattered degenerated apoptotic keratinocytes, patchy areas of basal cell degeneration and interface dermatitis, perivascular and periadnexal inflammatory cell infiltrate along with extravasation of erythrocytes in dermis | Complete resolution with oral ciclosporin 300 mg/day after 7 days | Platform changed; no data on outcome |
| 6 | F | Middle‐aged | No |
mRNA vaccine (Pfizer/BioNTech, BNT162b2) |
Second | 5 days | NA | SJS | NA | Treated with oral prednisolone 30 mg/day; outcome unknown | NA |
| 7 | F | 49 | NA |
mRNA vaccine (Pfizer/BioNTech, BNT162b2) |
First | 7 days | NA | TEN | Histology: full‐thickness epidermal necrosis along with dermal–epidermal separation and necrotic keratinocytes | Treatment with 2 doses of etanercept 50 mg/mL (on Days 1 and 3); complete resolution in 22 days | Not mentioned |
| 8 | M | 66 | No |
mRNA vaccine (Moderna, mRNA‐1273) |
Second | 24 h | 5 | GBFDE |
Blood test: anti‐BP180 negative (8), anti‐BP230 negative (< 2) Histology: full‐thickness epidermal necrosis and a very sparse lymphocytic inflammatory infiltrate |
Improved with high‐dose oral prednisone | NA |
|
9 (our case) |
M | 74 | Penicillin (swollen lips) |
Viral vector vaccine (Oxford‐AstraZeneca, ChAdOx1) |
First | 25 h | 2 | GBFDE |
Histology: subepidermal separation with superficial and deep perivascular mixed inflammatory cells infiltration composing lymphohistiocytes and numerous eosinophils, melanophages were seen in the upper dermis IFN‐γ ELISpot assay: negative for polysorbate 80 |
Resolution with residual hyperpigmentation with topical desoximetasone within 2 weeks | Platform changed; no data on outcome |
AGEP, acute generalized exanthematous pustulosis; BP, bullous pemphigoid; DIF, direct immunofluorescence; GBFDE, generalized bullous fixed drug eruption; IFN, interferon; NA, not applicable; SJS, Stevens–Johnson syndrome; TEN, toxic epidermal necrolysis.
The treatment for GBFDE treatment is cessation of the causative agents and supportive care. 1 We treated our patient with topical 0.25% desoximetasone cream. The lesions gradually resolved within 2 weeks, leaving postinflammatory hyperpigmentation.
Use of patch testing on an area of residual hyperpigmentation after FDE resolution was considered as a method to confirm the culprit drug; unfortunately, testing could not be performed due to limited access to the vaccine and hospital areas during the COVID‐19 pandemic. As an alternative, an interferon (IFN)‐γ ELISpot assay was undertaken. This technique assesses the amount of IFN‐γ production from peripheral blood mononuclear lymphocytes after stimulation with the suspect agents. In this case, the vaccine excipient, polysorbate80 (dilutions of 1 : 2000 and 1 : 10 000), was tested and yielded negative results. Our patient also reported receiving an annual influenza vaccination, which contains a similar excipient (polysorbate), without any adverse reactions. This indicated that the GBFDE was a result of a hypersensitivity reaction to the ChAdOx1 nCoV‐19 vaccine rather than the excipient.
To our knowledge, this is the first report of ChAdOx1 nCoV‐induced GBFDE. Because of the potential recurrence of SCAR, the patient was advised to switch to a different COVID‐19 vaccine platform.
Acknowledgement
We thank the patient for providing informed consent for publication of their case details and images. We also thank the Skin and Allergy Research Unit for their support.
Conflict of interest: the authors declare that they have no conflicts of interest.
References
- 1. Patel S, John AM, Handler MZ et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol 2020; 21: 393–9. [DOI] [PubMed] [Google Scholar]
- 2. Savas Erdogan S, Cebeci KF. Fixed drug eruption after pentavalent diphtheria, tetanus, acellular pertussis, inactivated poliovirus and Haemophilus influenzae type b combined vaccine in an infant. Eur J Hosp Pharm 2021. Online ahead of print. 10.1136/ejhpharm-2021-002875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mintoff D, Pisani D, Betts A et al. SARS‐CoV‐2 mRNA vaccine‐associated fixed drug eruption. J Eur Acad Dermatol Venereol 2021; 35: e560–3. [DOI] [PubMed] [Google Scholar]
- 4. Thompson H, Nichols L, Gonzalez ST. Bullous fixed drug eruption following administration of the recombinant adjuvant Shingrix vaccine. BMJ Case Rep 2021; 14: e241293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farinazzo E, Ponis G, Zelin E et al. Cutaneous adverse reactions after m‐RNA COVID‐19 vaccine: early reports from Northeast Italy. J Eur Acad Dermatol Venereol 2021; 35: e548–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


