Abstract
A case of linear IgA bullous dermatosis developing 3 days after the second dose of Oxford AstraZeneca COVID‐19 vaccine in an adult patient, suggesting a possible causal association. It is worth keeping in mind that COVID‐19 vaccination could induce immune‐mediated bullous disease in susceptible people.
Dear Editor,
Linear IgA bullous dermatosis (LABD) is a subepithelial vesiculobullous disease, characterized by linear deposition of IgA along the basement membrane zone of the skin and/or mucosae. 1 Most cases of LABD are idiopathic, 1 but drugs, infections and malignancies have all been implicated as possible inductors. 2 To date, only two cases of LABD following vaccination have been reported, one after influenza 3 and one after human papillomavirus 3 vaccine administration. We describe the first case of LABD developing 3 days after the second dose of Oxford AstraZeneca COVID‐19 vaccine in an adult patient, suggesting a possible causal association.
A 61‐year‐old man presented with a 22‐day history of a bullous eruption, which had first appeared 3 days after his second dose of Oxford AstraZeneca COVID‐19 vaccine. He reported no symptoms of infection, medication intake or sources of stress in the previous weeks.
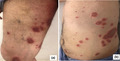
Physical examination revealed tense blisters with serous content on a background of erythematous, urticarial and purpuric skin on his legs, and target‐like lesions on his abdomen, trunk and thighs (Fig. 1). The oral and genital mucosae were also involved.
Figure 1.
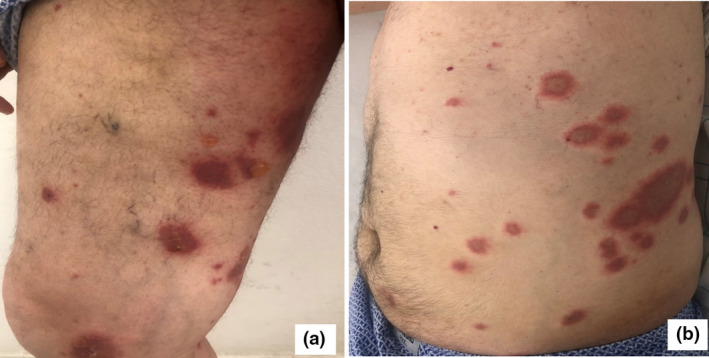
(a) Tense blisters on a background of urticarial skin; (b) multiple target‐like lesions on the abdomen and trunk.
Histopathological examination revealed a subepidermal split with an inflammatory infiltrate composed of lymphocytes, histiocytes and some eosinophilic polynuclear lymphocytes. Direct immunofluorescence demonstrated predominant linear IgA deposition at the dermoepidermal junction (Fig. 2), while indirect immunofluorescence revealed antibasement membrane zone IgA antibodies binding to the roof of salt‐split skin.
Figure 2.
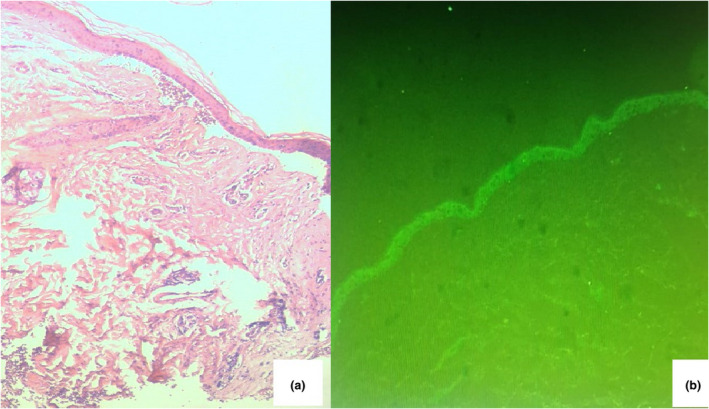
(a) Deep junctional detachment with bulla (haematoxylin and eosin, original magnification × 40); (b) direct immunofluorescence showed linear IgA deposits along the basement membrane zone (original magnification × 20).
Laboratory examination revealed eosinophilia (1450 cells/µL; normal < 500/µL). ELISA results for anti‐desmoglein (Dsg)1 and Dsg3, and for bullous pemphigoid (BP)180 antibodies were negative.
A diagnosis of LABD was made. The patient was treated with oral prednisolone, with marked clinical improvement and normalization of polynuclear eosinophil count.
There are multiple reports in the literature on drugs that cause LABD, particularly vancomycin. 2 Drug‐induced LABD is defined by its polymorphic clinical features, which are more severe than in spontaneous forms.
A large range of cutaneous adverse events after COVID‐19 vaccine is being reported as the vaccination programmes widen. Regarding bullous dermatoses, Coto‐Segura et al. reported three cases of BP and one case of LABD induced by the BioNTech/Pfizer mRNA COVID‐19 vaccine. 4 Tomayko et al. reported 12 cases of subepidermal blistering eruptions, including BP, following COVID‐19 vaccination. 5
Among the possible explanations for LABD developing after vaccination is molecular mimicry, where a viral antigen shares sequence or structural similarity with a host antigen. Another is direct or indirect activation of the host's immune system by viral antigens or cytokines, such as interleukins and transforming growth factor‐β, which increase IgA synthesis. 3
To our knowledge, this is the first case of LABD after Oxford AstraZeneca COVID‐19 vaccine (adenoviral vector vaccine). It is possible, given the absence of triggers, that the condition was spontaneous but considering the temporal association between COVID‐19 vaccination and the development of the eruption, we suggest that immunization was the most probable trigger. Vaccination is a rare trigger for this condition. While this case may be a simple coincidence, it is worth keeping in mind that COVID‐19 vaccination could induce immune‐mediated bullous disease in susceptible people.
Acknowledgement
We thank the patient for written informed consent to publication of their case details and images.
Conflict of interest: the authors declare that they have no conflicts of interest.
References
- 1. Chanal J, Ingen‐Housz‐Oro S, Ortonne N et al. Linear IgA bullous dermatosis: comparison between the drug‐induced and spontaneous forms. Br J Dermatol 2013; 169: 1041–8. [DOI] [PubMed] [Google Scholar]
- 2. Lammer J, Hein R, Roenneberg S et al. Drug‐induced linear IgA bullous dermatosis: a case report and review of the literature. Acta Derm Venereol 2019; 99: 508–15. [DOI] [PubMed] [Google Scholar]
- 3. Alberta‐Wszolek L, Mousette AM, Mahalingam M, Levin NA. Linear IgA bullous dermatosis following influenza vaccination. Dermatol Online J 2009; 15: 3. [PubMed] [Google Scholar]
- 4. Coto‐Segura P, Fernández‐Prada M, Mir‐Bonafé M et al. Vesiculobullous skin reactions induced by COVID‐19 mRNA vaccine: report of four cases and review of the literature. Clin Exp Dermatol 2021. 10.1111/ced.14835. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tomayko MM, Damsky W, Fathy R et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID‐19 vaccination. J Allergy Clin Immunol 2021; 148: 750–1. [DOI] [PMC free article] [PubMed] [Google Scholar]


