Abstract
Background
Emerged mutations can be attributed to increased transmissibility of the B.1.617 and B.1.36 Indian delta variants of SARS‐CoV‐2, most notably substitutions L452R/E484Q and N440K, respectively, which occur in the receptor‐binding domain (RBD) of the Spike (S) fusion glycoprotein.
Objective
We aimed to assess the effects of mutations L452R/E484Q and N440K (as well as the previously studied mutation E484K present in variants B.1.351 and P.1) on the affinity of RBD for ACE2, SARS‐CoV‐2 main receptor. We also aimed to assess the ability of antibodies induced by natural infection or by immunization with BNT162b2 mRNA vaccine to recognize the mutated versions of the RBD, as well as blocking the interaction RBD‐ACE2, an important surrogate readout for virus neutralization.
Methods
To this end, we produced recombinant wild‐type RBD, as well as RBD containing each of the mutations L452R/E484Q, N440K, or E484K (the latest present in variants of concern B.1.351 and P.1), as well as the ectodomain of ACE2. Using Biolayer Interferometry (BLI), we measured the binding affinity of RBD for ACE2 and the ability of sera from COVID‐19 convalescent donors or subjects immunized with BNT162b2 mRNA vaccine to block this interaction. Finally, we correlated these results with total anti‐RBD IgG titers measured from the same sera by direct ELISA.
Results
The binding assays showed L452R/E484Q double‐mutant RBD to interact with ACE2 with higher affinity (KD = 4.6 nM) than wild‐type (KD = 21.3 nM) or single mutants N440K (KD = 9.9 nM) and E484K (KD = 19.7 nM) RBDs. Meanwhile, the anti‐RBD IgG titration resulted in lower recognition of mutants E484K and L452R/E484Q by infection‐induced antibodies, whereas only mutant E484K was recognized less by antibodies induced by vaccination. More interestingly, sera from convalescent as well as immunized subjects showed reduced ability to block the interaction between ACE2 and RBD mutants E484K and L452R/E484Q, as shown by the inhibition assays.
Conclusion
Our data suggest that the newly emerged SARS‐CoV‐2 variant B.1.617, as well as the better‐studied variants B.1.351 and P.1 (all containing a mutation at position E484) display increased transmissibility both due to their higher affinity for the cell receptor ACE2 and their ability to partially bypass immunity generated against the wild‐type virus. For variant B.1.36 (with a point mutation at position N440), only increased affinity seems to play a role.
Keywords: affinity, neutralization, RBD, SARS‐CoV‐2, vaccine
Binding assays using Biolayer Interferometry showed that the RBD containing L452R/E484Q present in the new SARS‐CoV‐2 variant B.1.617 interacts with ACE2 with higher affinity than wild‐type or single mutants N440K and E484K. Sera from convalescent and BTN162b2 vaccinated individual showed reduced ability to block the interaction between ACE2 and RBD mutants E484K and L452R/E484Q suggesting that the new SARS‐CoV‐2 variant B.1.617 bypass immunity generated against the wild‐type virus. Abbreviations: ACE2, angiotensin‐converting enzyme 2; COVID‐19, coronavirus disease 2019; RBD, receptor binding domain; RBDE484K, RBD containing mutation E484K on S; RBDL452R/E484Q, RBD containing both mutations L452K and E484Q on S; RBDN440K, RBD containing mutation N440K on S; RBDWT, wild‐type RBD; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus type 2

Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- BLI
biolayer interferometry
- COVID‐19
coronavirus disease 2019
- RBD
receptor‐binding domain
- RBDE484K
RBD containing mutation E484K on S
- RBDL452R/E484Q
RBD containing both mutations L452K and E484Q on S
- RBDN440K
RBD containing mutation N440K on
- RBDWT
wild‐type RBD
- RBM
receptor‐binding motif
- S
SARS‐CoV‐2 spike glycoprotein
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus type 2
1. INTRODUCTION
Antibodies, in particular neutralizing antibodies, are the primary effector mechanism induced by anti‐viral vaccines. Hence, for vaccines against COVID‐19, neutralizing antibodies are expected to be the correlate of protection. 1 , 2 It is known that for SARS‐CoV‐2, the receptor‐binding domain (RBD) of the Spike fusion glycoprotein is the primary target for neutralizing antibodies, and that ELISA antibody titers against the RBD tightly correlate with virus neutralization. 3
The receptor‐binding motif (RBM) is the segment of the RBD in closest contact with the host receptor angiotensin‐converting enzyme 2 (ACE2, Figure 1A/B), and, because of its relevance for the virus attachment, it is the most important binding site for neutralizing antibodies. 4 The importance of RBD/RBM is underlined by the observation that prominent variants of concern (e.g., South Africa B.1.351 and Brazil P.1) show strongly reduced susceptibility to neutralizing antibodies due to equally reduced recognition by serum antibodies caused by E484K mutation located in the RBM. 5 , 6 In contrast, variants lacking the E484K mutation (e.g., UK B.1.1.7) are well recognized by convalescent and immunized sera, with the RBD‐ACE2 interaction barely affected by these antibodies. 7 It seems though that a slight increase in the affinity of the RBD for the receptor (1.6‐fold) can lead to increased viral infectivity.
FIGURE 1.
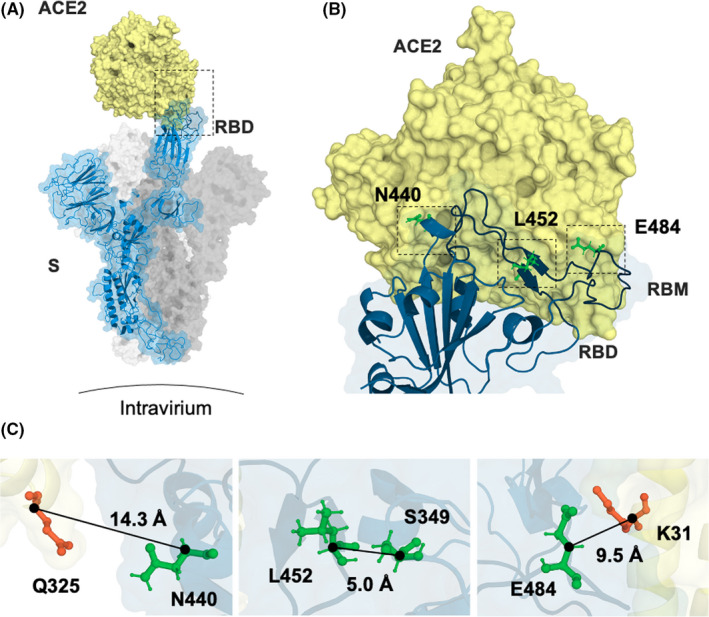
Positions of mutations N440K, L452R, and E484Q. (A) S monomer (blue ribbon and surface) bound to ACE2 ectodomain (yellow surface). (B) This interaction is shown in detail, highlighting residues N440, L452, and E484 (green sticks). Interestingly, both residues L452 and E484 are found in close contact with ACE (ie, at the RBM), whereas residue N440 is relatively distant from the interaction surface (ie, out of the RBM). (C) Distance from the nearest residue for N440, L452, and E484 (residues from the RBD shown in green and from ACE2 in orange). From PDB files 6ACG and 2AJF
Here, we show that the Indian delta variant B.1.617 of SARS‐CoV‐2 has acquired higher infectivity and transmissibility (therefore becoming the dominant strain in many countries) by both enhanced affinity for ACE2 and neutralizing immune evasion. In variant B.1.36, on the other hand, only increased affinity for ACE2 plays a role, making this variant more similar to the UK B.1.1.7.
2. MATERIAL AND METHODS
2.1. Gene cloning
The constructs for RBDWT and mutants E484K, L452R/E484Q, and N440K were all designed as single genes containing each protein of interest fused to an N‐terminal µ‐phosphatase signal peptide and a C‐terminal hexahistidine tag. An Avitag™ BAP sequence followed by an EPEA C‐tag replaced the 6xHis tag in the ACE2‐expressing construct. Gene synthesis was performed by Twist Biosciences, which, using the NotI/XbaI restriction sites, also inserted the sequences into its standard vector pTwist‐CMV‐BetaGlobin‐WPRE‐Neo.
2.2. Protein expression and purification
Using Expifectamine® (Thermo Fisher), all constructs were transfected into HEK293F cells. For in vivo biotinylation of the Avitag™ BAP, though, a second plasmid coding for BirA was co‐transfected with the ACE2 vector. Upon five days of incubation in suspension, the RBD constructs were purified by IMAC using a TALON Crude column, while ACE2 was purified by immunoaffinity using a CaptureSelect XP C‐tag column (Thermo Fisher). All proteins were further purified by SEC using a Superdex75 (RBD) and a Superdex200 (ACE2) column (Cytiva).
2.3. Human sera
Human sera were obtained from 5 COVID‐19 convalescent patients who were recruited at the University Hospital of Bern, Bern, Switzerland as described. 3 Participants were recruited via three different routes: (a) inpatients with a SARS‐CoV‐2 test result (real‐time PCR; RT‐PCR), (b) medical personnel of the Inselspital, and (c) residual material from patients stored at the Liquid Biobank Bern (www.biobankbern.ch). Inclusion criteria of inpatients are (a) hospitalization in Inselspital, (b) tested positive for SARS‐CoV‐2 using RT‐PCR (nasopharyngeal swab), (c) aged 18 or older, and (d) signed general consent. Human sera from 8 subjects immunized with BNT162b2 (Biontech/Pfizer) vaccine were recruited at the University Hospital of Zürich, Zürich, Switzerland.
2.4. RBD‐ACE2 binding kinetics
The binding kinetics assays were performed using and Octet RED96E (Sartorius). Briefly, SAX sensors were loaded to saturation with biotinylated ACE2 at 25 μg⋅ml−1 and subsequently quenched with biocytin. Association was carried out in 300 s, with the RBD serially diluted from 100 to 3.12 nM in 1:2 steps. Finally, dissociation was also performed in 300 s. All proteins were diluted in kinetics buffer (KB). A loaded sensor run in KB only served as drift control. The resulting curves were aligned to beginning of association, and a 1:1 model was used for global fitting.
2.5. RBD‐ACE2 binding inhibition
Inhibition of RBD‐ACE2 binding by either convalescent or immunized sera was also analyzed by Biolayer Interferometry using Octet. In this case, however, HIS1K sensors were loaded to saturation with 25 μg⋅ml−1 of each RBD tested, and two association steps were performed: a short one (120 s) with the sera samples diluted at 1:20 in KB; and a longer one (300 s) with ACE2 diluted to 100 nM also in KB. Drift control was performed with a loaded sensor run in KB only. Naïve serum was used as positive control, and inhibition indexes were calculated as the proportional reduction in the area under the curve of ACE2 association.
2.6. Anti‐RBD IgG titration
For the ELISA assays, high binding 96‐well plates were coated overnight with 1 μg⋅ml−1 of each of the RBDs tested; blocked for 2 h with 0.15% casein in PBS; and bound for 1 h with 1:20 convalescent or immunized sera serially diluted in 1:3 steps. Bound IgG antibodies were detected with goat anti‐human IgG‐POX antibody (Nordic MUbio). Development was performed with tetramethylbenzidine (TMB) and stopped with 1 M H2SO4. Absorbance was read 450 nm, and the resulting curves were fitted into a 4‐parameter non‐linear model. OD50 values were then calculated as the width at half height of each fitted curve.
2.7. Statistical analysis
Statistical tests were performed using GraphPad PRISM 9.0 (GraphPad Software, Inc.). Differences in ELISA titers and inhibition indexes were tested by non‐parametric one‐way ANOVA with no comparison correction (Fisher's LSD test). Statistical significance is displayed as p ≤ .05 (*), p ≤ .01 (**), p ≤ .001 (***).
3. RESULTS
3.1. Binding kinetics of RBD mutants to ACE2 show increased affinity due L452R/E484Q and E484K mutations
To address the questions of antibody binding strength and competition mechanistically, we have expressed recombinant versions of the RBD containing mutations E484K (shared between the Brazilian isolate P.1 8 and the South African isolate B.1.351 9 ), L452R/E484Q (Indian variant B.1.617), and N440K (variant B.1.36, also emerged in India). 10 The positions of these mutations relative to wild‐type RBD are shown in Figure 1.
All RBDs were purified to homogeneity, and the affinity to recombinant ACE2 ectodomain was determined by BLI (Figure 2 and Table 1). 11 The results show that the affinity of ACE2 for RBDL452R/E484Q (KD = 4.6 nM, Figure 2C) is ≈ fivefold higher than that measured for RBDWT (KD = 21.3 nM, Figure 2A). In between are the values for RBDE484K (KD = 19.7 nM, Figure 2B) 12 and RBDN440K (KD = 9.9 nM, Figure 2D). Interestingly, in all cases, the increase in affinity is due to a strongly enhanced association rate (kon, 3–5 times higher for the mutants than for RBDWT), rather than any significant changes in the dissociation constant (koff, Table 1).
FIGURE 2.
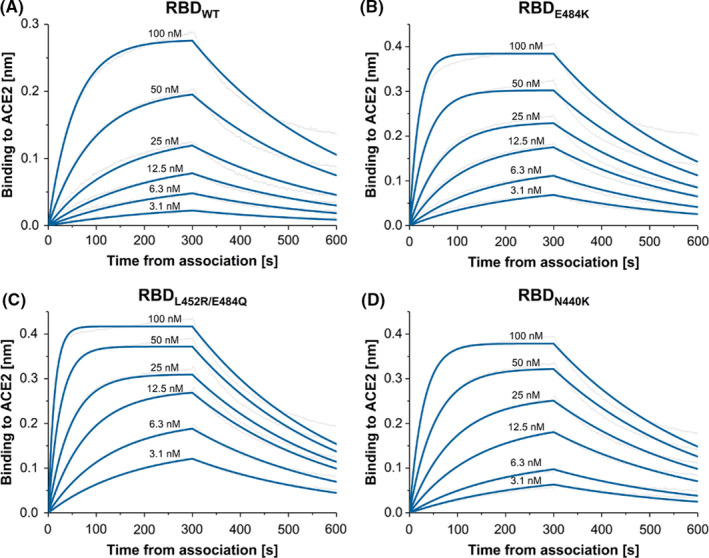
BLI sensorgrams illustrating the interaction between ACE2 and (A) wild‐type RBD (RBDWT); (B) E484K single‐mutant RBD (RBDE484K); (C) L452R/E484Q double‐mutant RBD (RBDL452R/E484Q; and (D)) N440 single‐mutant RBD (RBDN440K)
TABLE 1.
Kinetic parameters for the RBD‐ACE2 interaction calculated by BLI
| Analyte* | KD [M] | kon [M−1s−1] | koff [s−1] | R 2 |
|---|---|---|---|---|
| RBDWT | 21.3 × 10−9 | 1.5 × 105 | 3.2 × 10−3 | 0.9926 |
| RBDE484K | 19.7 × 10−9 | 1.6 × 105 | 3.1 × 10−3 | 0.9957 |
| RBDL452R/E484Q | 4.6 × 10−9 | 7.2 × 105 | 3.3 × 10−3 | 0.9934 |
| RBDN440K | 9.9 × 10−9 | 3.1 × 105 | 3.1 × 10−3 | 0.9954 |
*RBDWT=Receptor‐Binding Domain wild type, *RBDL452R/E484Q= Receptor‐Binding Domain L452R, E484Q mutations, *RBDE484K= Receptor‐Binding Domain E484K mutation, *RBDN440K= Receptor‐Binding Domain N440Kmutation.
3.2. RBD mutants L452R/E484Q and E484K evade recognition and neutralization by convalescent sera while neutralization by BNT162b2 immune sera is impaired only
Anti‐RBD IgG ELISA shows that antibodies induced by infection with wild‐type Wuhan SARS‐CoV‐2 fail to recognize the RBD mutants L452R/E484Q (variant B.1.617) and E484K (variants P.1 and B.1.351), while recognition of RBDN440K is unaltered. This observation is reproduced in the RBD‐ACE2 binding inhibition assays (Figure 3B), where the neutralizing activity of the convalescent sera is significantly affected by mutations E484K and L452R/E484Q and not by N440K.
FIGURE 3.
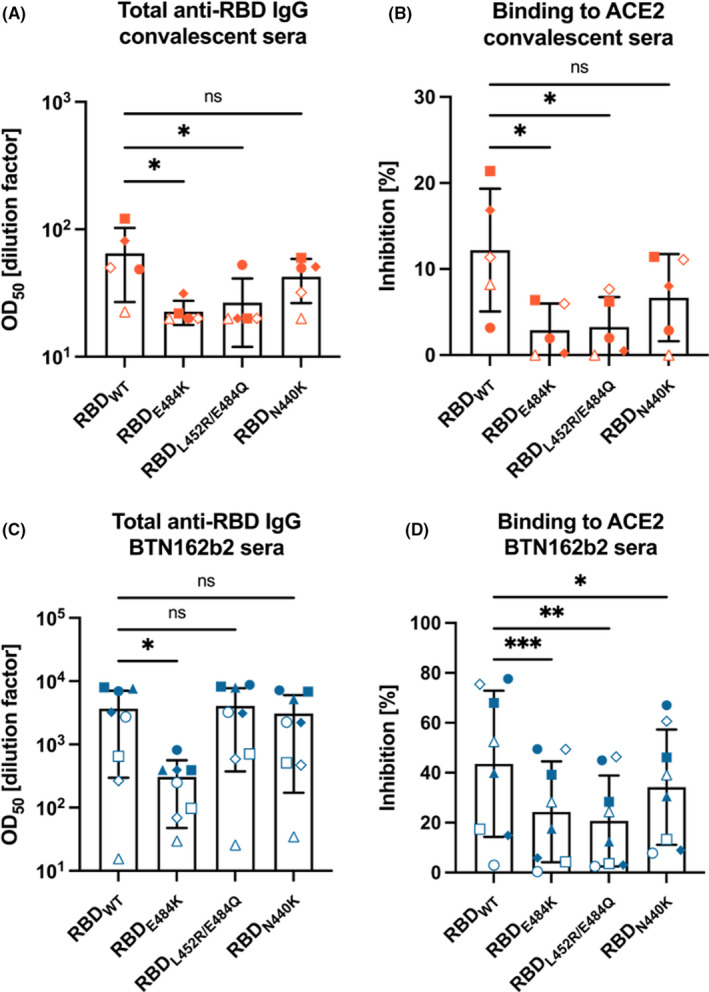
Comparison of total anti‐RBD IgG and RBD‐ACE2‐blocking antibodies between wild‐type RBD and mutants E484K, L452R/E484Q, and N440K. (A) Total anti‐RBD IgG titers of sera obtained from COVID‐19‐convalescent donors. (B) Inhibition of RBD‐ACE2 binding by 1:20 dilution of the same samples; (C) Total anti‐RBD IgG titers of for sera obtained from donors immunized with two doses of BNT162b2 mRNA COVID‐19 vaccine (Pfizer); (D) Inhibition of RBD‐ACE2 binding by 1:20 dilution of the same samples
As shown previously, 13 vaccination induces antibodies with broader specificity than viral infection, resulting in antibodies that showed reduced binding to RBD carrying mutation E484K while all other RBDs, including the Indian variants of concern were recognized well by the vaccine‐induced immune sera (Figure 3C).
In contrast to direct binding of immune sera to RBD, inhibition of the RBD‐ACE2 interaction was more severely affected and reduced for all mutations, most notably E484K and L452R/E484Q (Figure 3D). This most likely reflects the increased receptor affinity of the mutant RBDs for ACE2, rendering it more difficult to inhibit binding of RBD to ACE2.
4. DISCUSSION
Recently emerged in India, the variants B.1.617 and B.1.36 of SARS‐CoV‐2 have rapidly spread across Southeast Asia and Northeast Africa, quickly becoming the predominant strain also in North America and Europe. 14 In the UK, for example, the detection frequency of lineage B.1.617.2 has raised from ≈ 20% in early May to ≈ 100% in late June, which strongly indicates enhanced viral infectivity/transmissibility.
Several properties contribute to the prevalence of a particular viral strain, including its fitness and ability to escape existing immunity. Fitness is mostly influenced by viral transmissibility and replication competence, which may be affected by many factors, including viral receptor affinity. In contrast, immune evasion is normally simply driven by reduced recognition by neutralizing antibodies, with serotype formation being the most extreme form of this mechanism (whereby antibodies induced against serotype 1 of SARS‐CoV completely fail to neutralize the serotype 2 of the same virus). Overall, mutations on viral surface proteins involved in cell attachment and/or fusion have been postulated to be the main driver of enhanced transmissibility and/or virulence. 15
Here, we assessed whether the increasing prevalence of the Indian variants of concern B.1.617 and B.1.36 could be explained by any of these 2 properties. Our data show that the affinity of RBD for ACE2 is fivefold increased by the point mutations L452R and E484Q (Figure 2C and Table 1), whereas mutation N440K has the effect of doubling this value, when compared to RBDWT. In both cases though, a K‐Q H‐bond is likely formed, as RBD residues E484 and N440 distance by only ≈10 and ≈ 14 Å Cα‐Cα from residues K31 and Q325 of ACE2, respectively (Figure 1). We are also tempted to speculate that mutation L452R contributes to the increased affinity observed for the double‐mutant RBD mostly by stabilizing the RBM rather than by forming new bonds with the receptor. This is because L452 sits in the middle of β‐sheet β5/6, the only structured region of the RBM. Its side chain also faces the internal S349, and it seems unlikely that a drastic change in φ angle could occur at this position, as Y451 is deeply embedded in the internal structure of the RBD.
While these biophysical differences are truly impressive, it remains to be seen whether this increased affinity fully translates to increased viral infectivity. This is because, in oppose to what happens in vitro using soluble proteins, interaction between RBD and ACE2 in vivo is largely influenced by the environment surrounding the S glycoprotein on the virion. Nevertheless, it is reasonable to assume that significant changes in this parameter would inevitably be reflected in the dynamics between virions and host cells. This principle illustrated by the fact that the RBD of the more transmissible variant B.1.1.7 binds ACE2 with affinity twice higher than RBDWT, which, in turn, interacts with the receptor four times more strongly than the RBD from the quickly controlled SARS‐CoV‐1.
Regarding the recognition of RBD by convalescent sera, a sharp decay is observed for mutants E484K and L452R/E484Q (Figure 3A). This result is not surprising, as mutation E484K has already been shown to evade immunity in the context of variants B.1.351 and P.1. 12 , 13 , 16 Likewise point mutations conferred by L452R or E484Q alone have been shown to reduce sensitivity to antibodies elicited by BNT162b2 vaccine. 17 Mutation N440K, on the other hand, has caused no significant effect on recognition of RBD by antibodies induced by infection (Figure 3A). With the data in hands, we can only conjecture that this mutation does not alter the surface properties of the RBM sufficiently to impact its immune recognition. A similar trend appears in the results from the RBD‐ACE2 binding inhibition assays (Figure 3B).
Intriguingly, the same assays performed with sera from BNT162b2‐immunized donors tell a slightly different story. In this case, only mutant E484K evades recognition by anti‐RBD antibodies, while all others exhibit IgG titers equivalent to those obtained for RBDWT (Figure 3C). This result may be due to the change in charges (from negative to positive) that occur for E484K only, as the glutamine in E484Q is neutral at physiological pH. This might lead to a lower binding recognition of antibodies to the E484K as it has been recently postulated by free energy calculation performed by Wu et al. 18 Moreover, the overall difference in recognition patterns observed for convalescent and vaccinated sera, however, can be simply attributed to the much lower titers measured from the first group of samples, which would make slight differences more noticeable.
More interestingly though, inhibition of RBD‐ACE2 interaction by vaccine‐induced antibodies significantly falls for all mutants, most markedly for E484K and L452R/E484Q (Figure 3D), indicating that the increased receptor affinity renders neutralization more difficult.
Based on these results, we suggest that the RBDs of the Indian variants of concern B.1.617 and B.1.36 may have acquired enhanced infectivity/transmissibility through two distinct mechanisms: B.1.617 by increased affinity for ACE2 and immune evasion; and B.1.36 by increased affinity for ACE2 only.
CONFLICT OF INTERESTS
M. F. Bachmann is a board member of Saiba AG, involved in the development of RBD‐CuMV, a vaccine against COVID‐19. All other authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
G.A., L.X, and S.K. performed and interpreted experiments. M.M. provided serum sample. M.V. and G.A. wrote the manuscript. M.B. designed experiments and wrote the manuscript.
ACKNOWLEDEGMENT
We thank Marianne Zwicker for production of wild type and mutant RBDs. Open Access Funding provided by Universitat Bern.
Augusto G, Mohsen MO, Zinkhan S, Liu X, Vogel M, Bachmann MF. In vitro data suggest that Indian delta variant B.1.617 of SARS‐CoV‐2 escapes neutralization by both receptor affinity and immune evasion. Allergy. 2022;77:111–117. 10.1111/all.15065
Funding information
The work was supported by Saiba AG and the Swiss National Science Foundation (SNF grants 31003A 149925 and 310030‐179459)
REFERENCES
- 1. Robbiani D, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS‐CoV‐2 infection in convalescent individuals. Nature. 2020;584(7821):437‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu NC, Yuan M, Bangaru S, et al. A natural mutation between SARS‐CoV‐2 and SARS‐CoV determines neutralization by a cross‐reactive antibody. PLoS Pathog 2020;16(12):e1009089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brigger D, Horn MP, Pennington LF, et al. Accuracy of serological testing for SARS‐CoV‐2 antibodies: first results of a large mixed‐method evaluation study. Allergy 2021;76(3):853‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Q, Zhang Y, Wu L, et al. Structural and functional basis of SARS‐CoV‐2 entry by using human ACE2. Cell 2020;181(4):894‐904. e899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jangra S, Ye C, Rathnasinghe R, et al. The E484K mutation in the SARS‐CoV‐2 spike protein reduces but does not abolish neutralizing activity of human convalescent and post‐vaccination sera. medRxiv 2021. [Google Scholar]
- 6. Gómez CE, Perdiguero B, Esteban M. Emerging SARS‐CoV‐2 variants and impact in global vaccination programs against SARS‐CoV‐2/COVID‐19. Vaccines (Basel) 2021;9(3):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xie X, Liu Y, Liu J, et al. Neutralization of SARS‐CoV‐2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine‐elicited sera. Nat Med 2021;27(4):620‐621. [DOI] [PubMed] [Google Scholar]
- 8. Faria NR, Mellan TA, Whittaker C, et al. Genomics and epidemiology of the P.1 SARS‐CoV‐2 lineage in Manaus, Brazil. Science 2021;372(6544):815‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tegally H, Wilkinson E, Giovanetti M, et al. Detection of a SARS‐CoV‐2 variant of concern in South Africa. Nature 2021;592(7854):438‐443. [DOI] [PubMed] [Google Scholar]
- 10. BBC News . Covid Indian variant: where is it, how does it spread and is it more infectious? https://www.bbc.com/news/health‐57157496. Accessed June 7, 2021.
- 11. Petersen RL. Strategies using bio‐layer interferometry biosensor technology for vaccine research and development. Biosensors (Basel) 2017;7(4):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vogel M, Augusto G, Chang X, et al. Molecular definition of SARS‐CoV‐2 RBD mutations: receptor affinity versus neutralization of receptor interaction. Allergy. 2022;77:143‐149. 10.1111/all.15002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang X, Augusto GS, Liu X, et al. BNT162b2 mRNA COVID‐19 vaccine induces antibodies of broader cross‐reactivity than natural infection, but recognition of mutant viruses is up to 10‐fold reduced. Allergy 2021;76:2895‐2998. 10.1111/all.14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaidyanathan G. Coronavirus variants are spreading in India‐What scientists know so far. Nature 2021;593:321. [DOI] [PubMed] [Google Scholar]
- 15. Bakhshandeh B, Jahanafrooz Z, Abbasi A, et al. Mutations in SARS‐CoV‐2; consequences in structure, function, and pathogenicity of the virus. Microb Pathog 2021;154:104831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edara VV, Norwood C, Floyd K, et al. Infection‐ and vaccine‐induced antibody binding and neutralization of the B.1.351 SARS‐CoV‐2 variant. Cell Host Microbe 2021;29(4):516‐521. e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferreira I, Kemp S, Datir R, et al. SARS‐CoV‐2 B.1.617 mutations L452 and E484Q are not synergistic for antibody evasion. J Infect Dis 2021:jiab368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu L, Peng C, Xu Z, Zhu W. Predicting the potential effect of E484K mutation on the binding of 28 antibodies to the spike protein of SARS‐CoV‐2 by molecular dynamics simulation and free energy calculation. ChemRxiv 2021. [Google Scholar]


