Abstract
Aim
We aimed to analyse the influence of the COVID‐19 pandemic on the frequency and clinical presentation of celiac disease.
Methods
The study included the patients with celiac disease since January 2008. They were divided into 2 groups (diagnosed in pre‐pandemic [January 2008 and February 2020] [n = 148] and in pandemic period [March 2020 and June 2021] [n = 47]). Clinical and histological findings were compared between groups. Additionally, data about severe acute respiratory syndrome coronavirus 2 infection were obtained in subgroup patients (n = 22) with celiac disease diagnosed during pandemic period.
Results
The number of patients per year (12.1–37.6) and the percentage of patients who were diagnosed with celiac disease/total endoscopy were increased during the pandemic period (2.2% vs. 10%, p < 0.00001). The association of celiac disease with type 1 diabetes mellitus was significantly high in pandemic period (4% vs. 17%, p = 0.002). Frequency of moderate‐severe mucosal lesions was low in pandemic period (42.4% vs. 81.7%, p = 0.0001). Clinical and laboratory markers for the past severe acute respiratory syndrome coronavirus 2 infection were found in 36.3% of patients diagnosed during the pandemic period.
Conclusion
It seems that the frequency of celiac disease and its association with type 1 diabetes mellitus is increased during the COVID‐19 pandemic in children.
Keywords: celiac disease, SARS‐CoV‐2, type 1 diabetes mellitus
Abbreviations
- ACE
angiotensin‐converting enzyme
- CD
celiac disease
- DM
diabetes mellitus
- EMA‐IgA
IgA‐endomysial antibodies
- MISC
multisystem inflammatory syndrome in children
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- TMPRSS2
transmembrane serine protease 2
- tTG IgA
tissue transglutaminase antibody IgA
Key Notes.
Outbreak of celiac disease may be seen in the forthcoming feature after SARS‐CoV‐2 infection, and we aimed to analyse the influence of the COVID‐19 pandemic on celiac disease.
It seems that both frequency of celiac disease and association of celiac disease with type 1 diabetes mellitus was increased in the pandemic period.
With the prolonged duration of pandemic, it seems that the COVID‐19 pandemic will show us unexpected novel features in near future.
1. INTRODUCTION
Celiac disease (CD) is a chronic immune‐mediated enteropathy of the small intestine that develops in genetically susceptible individuals (HLA‐DQ2 [alleles DQA1*0501 and DQB1*0201] and HLA‐DQ8 [DQA1*0301 and DQB1*0302] haplotypes) by exposure to gluten proteins found in certain cereals. 1
Disruption of the tight junction integrity between enterocytes due to zonulin activation by α gliadin‐CXCR3 pathway increases the paracellular movement of luminal protease‐resistant gluten fragments especially α gliadins. Deamination of gliadin peptides by transglutaminase 2 is the key pathogenic event that increases gliadin immunogenicity in CD. The presentation of deaminated gliadin to CD4+ T cells leads the production of high level Th1 type cytokines, such as interferon‐γ, TNF‐α, IL‐18 and IL‐21. Some gliadin peptides that are not recognised by T lymphocytes activates epithelial cells to produce IL‐15. All inflammatory cytokines induce epithelial damage, fibrocyte activation and releasing of matrix metalloproteinases mediating pathological remodelling of small gut mucosa manifest as villous atrophy and crypt hyperplasia that the characteristic features of CD. Simultaneously, CD4+ T cells cooperate with B cells to differentiate into plasmatic cells and secrete specific antibodies against transglutaminase 2. 2 , 3
Although gluten is the major trigger for CD, some studies suggest that microorganisms may also play a role in the pathogenesis of CD and can influence the disease onset, clinical presentation and progression. Molecular mimicry may play a pathogenic role between antigenic epitopes of viral proteins and gliadins via shared antigenic determinants. It is believed that reoviridae, adenoviruses, the respiratory syncytial virus, herpes simplex type 1, hepatitis C and B viruses, enteroviruses, influenza virus, cytomegalovirus and Epstein‐Barr virus might play role in CD development. 4
In December 2019, a novel outbreak of a new strain of coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS‐CoV‐2]) infection emerged in Wuhan, China. The disease was declared as a pandemic in early March 2020. 5 Initially, it seems that children with COVID‐19 have mild symptoms and require hospitalisation rarely. 6 In April 2020, it was shown that COVID‐19 could lead to autoimmune and autoinflammatory diseases, such as multisystem inflammatory syndrome in children (MISC). 7 Thereafter, COVID‐19 has been implicated in the initiation of chronic inflammatory or autoimmune diseases in adult patients. 8 Additionally, an increased incidence and severe clinical presentation of type 1 diabetes mellitus (DM) have been reported in children during the COVID‐19 pandemic. 9
Although SARS‐CoV‐2 may primarily enter the cells of the lungs, the small bowel may also be an important entry site, as the enterocytes are rich in angiotensin‐converting enzyme (ACE)‐2 and transmembrane serine protease 2 (TMPRSS2) receptors. 10 Post‐mortem animal studies have demonstrated SARS‐CoV‐2 infection causes diffuse mucosal damage and impairs the intestinal barrier resulting in increased intestinal permeability that may allow the passage of gliadin peptides to intestinal lamina easily. 10 , 11 Therefore, an outbreak of CD may be seen in the forthcoming feature after SARS‐CoV‐2 infection. We aimed to analyse the influence of the COVID‐19 pandemic, on the frequency and clinical presentation of CD in our centre.
2. PATIENTS AND METHODS
The study included the patients with CD since January 2008 in our centre (n = 195). They were divided into 2 groups: group 1; patient with CD diagnosed between January 2008 and February 2020 (pre‐pandemic period) (n = 148) and group 2; patients with CD diagnosed between March 2020 and June 2021 (pandemic period) (n = 47). Although histopathological examination is not mandatory according to ESPGHAN 2012 and 2020 guidelines, we made the final diagnosis of CD according to histopathological examination in all patients in pre‐pandemic period, but we could not able to perform histopathological examination in 14 patients in the pandemic period due to strict lockdown guidelines. 12 , 13 Their diagnosis was made by positive results of all three parameters: tissue transglutaminase antibody IgA (tTG IgA)≥10x ULN, positive IgA‐endomysial antibodies (EMA IgA) (>1/5 titter) and HLA DQ2 and/or DQ8 positivity in non‐biopsied patients. 12 Demographic, clinical and histological findings of the patients were recorded from the hospital files and compared between the groups.
Our hospital is a tertiary care hospital and mainly serve patients in 6 cities (Trabzon, Giresun, Rize, Artvin, Gümüşhane and Bayburt). Our unit is the only paediatric gastroenterology centre that all the patients who are suspected or with positive serology are referred to our centre, but the nearest paediatric gastroenterology unit (Erzurum) was closed in the half of 2019, and the patients were also referred to our centre since then.
The clinical spectrum of the patients was divided into two categories according to the main symptoms: (i) typical CD, clinical malabsorption, chronic diarrhoea or failure to thrive; and (ii) atypical CD, abdominal pain, iron deficiency anaemia, chronic hypertransaminasaemia, constipation or screening of risk groups. 14 Nutritional status of patients was classified as obese if body mass index was >95 percentile. Histopathological findings compatible with >Marsh 3a lesions were accepted as ‘moderate‐severe’, and others were accepted as ‘mild’ mucosal lesions (Marsh 2‐3a). 15 We also divided the patients into two groups according to their referral cities (inside patients; Trabzon, Giresun, Rize, Artvin, Gümüşhane and Bayburt, and outside patients; patients from other cities) in order to analyse effect of patients’ referral on to the frequency of new cases during the pandemic.
Additionally, some data about SARS‐CoV‐2 infection (such as antibody positivity, diagnostic PCR positivity or contact history) were obtained in a subgroup patient (n = 22) with CD diagnosed during pandemic period from another ongoing study in our centre.
The study was conducted according to the Helsinki Declarations, with ethical approvals obtained from the Ethics Committee of the KTU Institutional Ethics Committee (study number: 2021/259).
2.1. Statistical analysis
Statistical analysis was performed using the SPSS version 16.0 software (SPSS Inc.). Continuous variables were expressed in mean±standard deviation (SD), and categorical variables were expressed in number and percentage. Comparison of the quantitative data between the groups was performed using Student's t test in the normally distributed variables and Mann‐Whitney test in the non‐normally distributed variables, whereas qualitative data were compared using chi‐square test.
3. RESULTS
Celiac disease was diagnosed in 47 patients (29 between March and December 2020, and 18 between January and June 2021) during pandemic period. There were 148 patients in pre‐pandemic period (between January 2008 and February 2020). The demographic, clinical and histological findings of the patients in two periods are shown in Table 1. The number of patients per year (12.1–37.6) and the percentage of patients who were diagnosed with CD/endoscopy were increased during the pandemic period (2.2% vs. 10%, p < 0.00001, OR: 4.87, 95% CI: 3.28–7.23) (Figure 1). Age of the patients (7.12 ± 4.24 years vs. 8.1 ± 4.9 years), frequency of typical CD (59.4% vs. 72.3%) and obesity (1.3% vs. 2.1%) were increased during the pandemic period but did not reach statistical significance. The number of inside patients in pre‐pandemic and pandemic period was similar (86.3% vs. 83.3%, p = 0.9).
TABLE 1.
Comparison of demographic and clinical parameters of patients with CD diagnosed in pre‐pandemic and pandemic period
| Parameters |
Pre‐pandemic period (January 2008–February 2020) |
Pandemic period (March 2020–June 2021) |
p | OR (95% CI) |
|---|---|---|---|---|
| Number of patients | 148 | 47 | n/a | n/a |
| Number of patients per year | 12.1 | 37.6 | n/a | n/a |
| Number of endoscopies | 6,641 | 330 | n/a | n/a |
| % of CD/endoscopy | 2.2 | 10 a | <0.00001 | 4.87 (3.28–7.23) |
| Inside patients, n (%) | 127 (86.3) | 40 (83.3) | 0.9 | 0.94 (0.37–2.39) |
| Gender, female, n (%) | 99 (66.8) | 32 (68) | 0.879 | 0.95 (0.47–1.91) |
| Age, mean ±SD, years | 7.12 ± 4.24 | 8.1 ± 4.9 | 0.645 | n/a |
| Typical CD, n (%) | 88 (59.4) | 34 (72.3) | 0.111 | 0.6 (0.3–1.22) |
| Obesity, n (%) | 2 (1.3) | 1 (2.1) | 0.706 | 0.63 (0.06–7.11) |
| Type 1 DM, n (%) | 6 (4) | 8 (17) | 0.002 | 4.85 (1.59–14.82) |
| Moderate‐severe mucosal lesion, n (%) | 121/148 (81.7) | 14/33 (42.4) a | 0.0001 | 0.16 (0.07–0.37) |
Abbreviations: CD, celiac disease; DM, diabetes mellitus.
Endoscopic examination could be performed in 33 of 47 patients.
FIGURE 1.
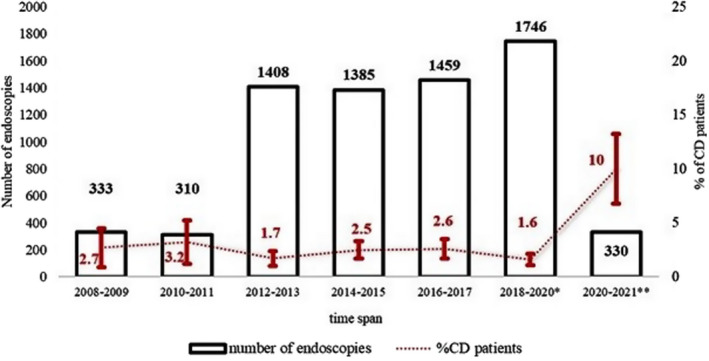
Percentage of patients with CD to endoscopies in 2‐years intervals. Upper and lower bar in percentage line indicates 95% CI. Percentage of patients with CD to endoscopies was higher in 2020–2021 compared with all other time spans (p < 0.05). * Include the patients from January 2018 to February 2020, and ** March 2020 to June 2021
Celiac disease was associated with type 1 DM in 8 patients during the pandemic period, and significantly high compared with pre‐pandemic period (4% vs. 17%, p = 0.002, OR: 4.85, 95% CI: 1.59–14.82). Additionally, 6 of the 8 patients with type 1 DM were diagnosed during the pandemic period. One patient had chronic diarrhoea and failure to thrive during CD screening, whereas others were asymptomatic. Additionally, one of the patients with type 1 DM had autoimmune thyroiditis. Endoscopy could not be performed in two patients with type 1 DM, and the diagnosis of CD was made by tTG IgA ≥10xULN, EMA IgA >1/5 titter and HLA DQ2 positivity in these patients.
Endoscopic examination could be performed in 33 of the 47 patients (70.2%), and we found that frequency of moderate‐severe mucosal lesions was low in pandemic period (42.4% vs. 81.7%, p = 0.0001, OR: 0.16, 95% CI: 0.07–0.37).
Clinical characteristics related to SARS‐CoV‐2 infection in a subgroup of patients with CD diagnosed during pandemic period (n = 22) are shown in Table 2. Diagnostic test for SARS‐CoV‐2 was positive in 1 of 22 patients (4.5%). SARS‐CoV‐2 antibody was found in 5 of 22 patients (22.7%). Totally, clinical history and laboratory markers for the past SARS‐CoV‐2 infection (SARS‐CoV‐2‐positive family number, close contact with SARS‐CoV‐2‐positive one except family member, SARS‐CoV‐2‐positive patients and SARS‐CoV‐2 antibody positivity) were found in 8 of 22 patients (36.3%) diagnosed during the pandemic period.
TABLE 2.
Clinical characteristics related to SARS‐CoV‐2 in a subgroup of patients with CD diagnosed during pandemic period (n = 22)
| Parameters | Number of patients (%) |
|---|---|
| SARS‐CoV−2‐positive family numbers | 4 (18.1) |
| Patients who were admitted to hospital due to SARS‐CoV−2 suspicious | 3 (13.6) |
| Patients close contacted with SARS‐CoV−2‐positive one except family member | 3 (13.6) |
| Patients that were performed SARS‐CoV−2 diagnostic test | 2 (9) |
| SARS‐CoV−2‐positive patients | 1 (4.5) |
| Hospitalised due to SARS‐CoV−2 | 1 (4.5) |
| SARS‐CoV−2 antibody positivity | 5 (22.7) |
| At least one parameter positive a | 8 (36.3) |
Abbreviations: CD, celiac disease; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
SARS‐CoV‐2‐positive family number, contacted with SARS‐CoV‐2‐positive one, SARS‐CoV‐2‐positive patients, SARS‐CoV‐2 antibody positivity.
4. DISCUSSION
In this study, we report the demographic and clinical characteristics of the patients with CD diagnosed during the pandemic period, and we compared them with the patients diagnosed during pre‐pandemic period. We found that (i) the frequency of the patients with CD was increased in pandemic period when considered the number of new patients per year and the percentage of patients diagnosed with CD underwent endoscopic examination, (ii) the association of CD with type 1 DM was increased during the pandemic period, (iii) frequency of moderate‐severe mucosal lesions was low compared with pre‐pandemic period and (iv) SARS‐CoV‐2 association was found in approximately one‐third of the patients who were analysed.
During the COVID‐19 pandemic, the prevalence of SARS‐CoV‐2 infection in patients with CD, novel diagnostic approaches for CD during the pandemic period, worse clinical status due to delay admissions or lower diagnostic rate due to decreased admission have been reported. 16 , 17 , 18 , 19 , 20
It was shown that gastrointestinal tractus serve as a nidus for SARS‐CoV‐2 for the future immune activation after clinical and laboratory resolutions of initial infection. 11 , 21 SARS‐CoV‐2 antigens were detected in the stool and on intestinal biopsies 4 months after acute infection in adults. 22 Prolonged exposure to SARS‐CoV‐2 in the gastrointestinal tract may cause dysbiosis and result in zonulin‐dependent loss of gut integrity. MISC, which is an immune activation syndrome associated with prior SARS‐CoV‐2 infection, was associated with increased circulating zonulin levels. Increased intestinal permeability by zonulin‐dependent pathway allows the passage of large antigens into the bloodstream: the Spike and S1 protein, in MISC and initiates hyperinflammatory response. Blocking intestinal permeability with zonulin inhibitor AT100 (larozitide acetate) significantly reduces the levels of plasma antigens, hyperinflammation and related symptoms in MISC. 11 Similar pathogenesis have been reported in CD, inflammatory bowel disease and Kawasaki disease. 11 , 23 , 24 Increased expression of zonulin has been reported in patients with CD. 25 Loss of gut integrity by SARS‐CoV‐2 may contribute or facilitate the development of CD. In our study, we found that the number of patients per year and the percentage of patients with definite diagnosis of CD per total number of endoscopies were increased during the pandemic period. Similarly, Trovato et al. 26 claimed that an outbreak of CD may be seen following COVD‐19 pandemic in the near future triggered by epithelial damage in patients with previous SARS‐CoV‐2 exposure. On the contrary, Valitutti et al. 20 showed a reduction of 32.4% in new diagnoses of patients with CD compared with the average of previous 5‐year time frame due to postpone of family screenings or decreased admissions of subclinical cases to the hospital in 2020, but they are waiting for an increased number of patients after reopening or normalisation in 2021.
We analyse the association of SARS‐CoV‐2 infection with a subgroup of our patients who were diagnosed during the pandemic period, and we found that approximately one‐third of the patients had direct or indirect exposure. The risk of contracting with COVID‐19 in CD adult and paediatric patients was evaluated, and the studies showed no increased risk of for the contracting, hospitalisation, severity and poor outcome for COVID‐19 in patients with CD, 16 , 17 , 27 but there has been no study that analyse the risk of developing CD after SARS‐CoV‐2 infection.
In addition, we found an increased frequency of type 1 DM in patients with CD during the pandemic period, and additionally, 6 of the 8 patients were diagnosed with type 1 DM during the pandemic period. Similar pathophysiological mechanisms that were defined in CD may be attributed to the development of type 1 DM after the infection or exposure to SARS‐CoV‐2. 28 Altered intestinal permeability and activation of inflammatory cytokines or direct pancreatic β‐cell cytotoxicity by TMPRSS2 receptors may cause pancreatic autoimmunity in SARS‐CoV‐2 infection. 9 Type 1 DM triggered by viral infections was shown in previous pandemics, SARS‐CoV‐2 and influenza pandemics. 29
In our study, we found that mucosal lesions of the patients with CD diagnosed during the pandemic period were milder compared with pre‐pandemic period. It may be related with short time span from the initiation of pandemic to the diagnosis of patients. Additionally, mild mucosal lesions may be a feature of virus‐induced CD.
Limitations of our study are (i) lack of showing direct evidence of SARS‐CoV‐2 infection in patients with CD diagnosed during pandemic period. Expression of SARS‐CoV‐2 related antigens or antibodies may be stained in mucosal biopsies of patients with CD and (ii) the number of new‐onset patients with CD during the pandemic period may be small for to make a generalisable conclusion, but it may be supported by multicentre studies.
In conclusion, we share our experience about the clinical characteristics of children with CD diagnosed during the pandemic period and its association with SARS‐CoV‐2 infection. It seems that the frequency of CD and its association with type 1 DM is increased during the COVID‐19 pandemic. Mucosal lesions are generally mild. With the prolonged duration of pandemic, it seems that the COVID‐19 pandemic will show us unexpected novel features such as outbreak of functional gastrointestinal disease or inflammatory bowel disease in the near future.
CONFLICT OF INTEREST
None.
Cakir M, Guven B, Issi F, Ozkaya E. New‐onset celiac disease in children during COVID‐19 pandemic. Acta Paediatr.2022;111:383–388. 10.1111/apa.16173
Funding information
None
REFERENCES
- 1. Caio G, Volta U, Sapone A, et al. Celiac disease: a comprehensive current review. BMC Med. 2019;17:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharma N, Bhatia S, Chunduri V, et al. Pathogenesis of celiac disease and other gluten related disorders in wheat and strategies for mitigating them. Front Nutr. 2020;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dunne MR, Byrne G, Chirdo FG, et al. Coeliac disease pathogenesis: the uncertainties of a well‐known immune mediated disorder. Front Immunol. 2020;11:1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sánchez D, Hoffmanová I, Szczepanková A, et al. Contribution of infectious agents to the development of celiac disease. Microorganisms. 2021;9:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 6. Ludvigsson JF. Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramcharan T, Nolan O, Lai CY, et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS‐CoV‐2 (PIMS‐TS): cardiac features, management and short‐term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol. 2020;41:1391‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ehrenfeld M, Tincani A, Andreoli L, et al. Covid‐19 and autoimmunity. Autoimmun Rev. 2020;19:102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salmi H, Heinonen S, Hästbacka J, et al. New‐onset type 1 diabetes in finnish children during the COVID‐19 pandemic. Arch Dis Child. 2021;0:1‐6. [DOI] [PubMed] [Google Scholar]
- 10. Sharma L, Riva A. Intestinal barrier function in health and disease‐any role of SARS‐CoV‐2? Microorganisms. 2020;8:1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yonker LM, Gilboa T, Ogata AF, et al. Multisystem inflammatory syndrome in children is driven by zonulin‐dependent loss of gut mucosal barrier. J Clin Invest. 2021;131(14):e149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Husby S, Koletzko S, Korponay‐Szabó IR, et al. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136‐160. [DOI] [PubMed] [Google Scholar]
- 13. Husby S, Koletzko S, Korponay‐Szabó I, et al. European society paediatric gastroenterology, hepatology and nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141‐156. [DOI] [PubMed] [Google Scholar]
- 14. Admou B, Essaadouni L, Krati K, et al. Atypical celiac disease: from recognizing to managing. Gastroenterol Res Pract. 2012;2012:637187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dickson BC, Streutker CJ, Chetty R. Coeliac disease: an update for pathologists. J Clin Pathol. 2006;59:1008‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lionetti E, Fabbrizi A, Catassi C. Prevalence of COVID‐19 in Italian children with celiac disease: a cross‐sectional study. Clin Gastroenterol Hepatol. 2021;19:1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhen J, Stefanolo JP, Temprano MP, et al. The risk of contracting COVID‐19 is not increased in patients with celiac disease. Clin Gastroenterol Hepatol. 2021;19:391‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trovato CM, Montuori M, Pietropaoli N, et al. COVID‐19 and celiac disease: a pathogenetic hypothesis for a celiac outbreak. Int J Clin Pract. 2021;75(9):e14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Catassi GN, Vallorani M, Cerioni F, et al. A negative fallout of COVID‐19 lockdown in Italy: life‐threatening delay in the diagnosis of celiac disease. Dig Liver Dis. 2020;52:1092‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Valitutti F, Troncone R, Pisano P, et al. Where have all the other coeliacs gone in 2020? Road for a 2021 catch‐up with missed diagnoses. Dig Liver Dis. 2021;2021(53):504‐505. [DOI] [PubMed] [Google Scholar]
- 21. Cheung KS, Hung IFN, Chan PPY, et al. Gastrointestinal manifestations of SARS‐CoV‐2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta‐analysis. Gastroenterology. 2020;159:81‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS‐CoV‐2. Gastroenterology. 2020;158:1831‐3.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wyatt J, Vogelsang H, Hübl W, et al. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993;341:1437‐1439. [DOI] [PubMed] [Google Scholar]
- 24. Drago S, El Asmar R, Di Pierro M, et al. Gliadin, zonulin and gut permeability: effects on celiac and non‐celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006;41:408‐419. [DOI] [PubMed] [Google Scholar]
- 25. Fasano A, Not T, Wang W, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;355:1518‐1519. [DOI] [PubMed] [Google Scholar]
- 26. Trovato CM, Montuori M, Cucchiara S, et al. ESPGHAN ‘biopsy‐sparing’ guidelines for celiac disease in children with low antitransglutaminase during COVID‐19. Eur J Gastroenterol Hepatol. 2020;32:1523‐1526. [DOI] [PubMed] [Google Scholar]
- 27. Lebwohl B, Larsson E, Söderling J, et al. Risk of severe covid‐19 in patients with celiac disease: a population‐based cohort study. Clin Epidemiol. 2021;13:121‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peters A, Wekerle H. Autoimmune diabetes mellitus and the leaky gut. Proc Natl Acad Sci USA. 2019;116:14788‐14790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caruso P, Longo M, Esposito K, et al. Type 1 diabetes triggered by covid‐19 pandemic: a potential outbreak? Diabetes Res Clin Pract. 2020;164:108219. [DOI] [PMC free article] [PubMed] [Google Scholar]


