To the Editor:
The COVID‐19 pandemic has posed a unique challenge in the management of rheumatic diseases. Immunosuppressed patients are at an increased risk of developing severe COVID‐19 and may not derive full protection from the vaccine (1, 2, 3, 4, 5). Thus, it is paramount that clinicians develop strategies to protect rheumatic disease patients from infection with SARS–CoV‐2 and its variants.
In this letter, we describe the clinical response in a 74‐year‐old man with seropositive, erosive rheumatoid arthritis (RA) that was initially diagnosed in 1974. The patient is currently receiving 200 mg of hydroxychloroquine daily, 25 mg of etanercept weekly, and 20 mg of leflunomide daily. With this RA treatment regimen, low levels of disease activity have been maintained over the last 5 years.
The patient received 2 doses of the messenger RNA (mRNA) vaccine mRNA‐1273 (Moderna) without interruption of his RA treatment, with the first dose administered January 18, 2021 and the second dose administered February 11, 2021. In mid‐April, a semiquantitative analysis revealed a spike protein receptor‐binding domain (RBD) antibody level of 53.9 units/ml (normal reference range 0–2,500), and the results of a SARS–CoV‐2 anti‐spike (S1/RBD) IgG test were negative. An assay designed to detect blocking of the interaction between the SARS–CoV‐2 spike protein RBD and the human angiotensin‐converting enzyme 2 (ACE‐2) receptor demonstrated <10% blocking activity (6). The results of an interferon‐γ–release assay detecting SARS–CoV‐2–specific T cells were also negative (7). The patient and his care team presumed that his suboptimal response to the vaccine was due to the immunosuppressive medications he was taking at the time of vaccination.
Based on his test results, the patient obtained an additional vaccine dose on his own accord. On June 6, 2021, he received 1 dose of the viral vector SARS–CoV‐2 vaccine Ad26.COV2.S (Johnson & Johnson). No side effects developed. In late June, a repeat semiquantitative analysis revealed a spike protein RBD antibody level of 2,455.0 units/ml, and the results of an S1/RBD IgG test were positive. The ACE‐2 blocking assay demonstrated 90–100% blocking activity (Figure 1). The results of the interferon‐γ release assay remained negative, suggesting that T cell–mediated immunity was not achieved. A blunted T cell response to the SARS–CoV‐2 vaccine has been demonstrated in patients receiving medications such as methotrexate or tacrolimus, but, to our knowledge, it has not been evaluated in patients treated with leflunomide (8, 9).
Figure 1.
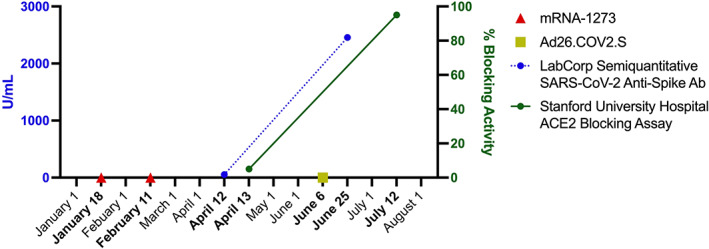
SARS–CoV‐2 anti‐spike IgG and human angiotensin‐converting enzyme 2 (ACE‐2) blocking activity before and after booster vaccination in an immunosuppressed patient with rheumatoid arthritis. Ab = antibody.
In summary, we describe an immunosuppressed patient who experienced an ineffective immune response after 2 doses of an mRNA SARS–CoV‐2 vaccine. The patient subsequently achieved a robust antibody response after a booster vaccination with the Johnson & Johnson SARS–CoV‐2 vaccine, all while continuing treatment with RA medications. Current guidance put forth by the American College of Rheumatology (ACR) does not recommend obtaining antibody testing after vaccination, in part due to a lack of clinically meaningful cutoff values for available antibody tests (10). The US Food and Drug Administration revised the emergency use authorization on August 12, 2021 for the 2 available mRNA SARS–CoV‐2 vaccines to permit a third dose for certain immunocompromised patients, and the ACR does support booster vaccination (11). Our report demonstrates the possibility of achieving humoral immunity against SARS–CoV‐2 after initial failure through the use of a cross‐platform booster vaccination strategy. Prior research has demonstrated that heterologous vaccination strategies may induce a more robust immune response in healthy adults (12, 13, 14). We believe future research is needed to establish relevant antibody reference values to identify patients without adequate protection against COVID‐19 infection, and to understand the role of cross‐platform booster vaccination when primary mRNA vaccination and/or booster vaccination fails to induce a sufficient immune response.
Supporting information
Disclosureform
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002.2Fart.41978&file=art41978‐sup‐0001‐Disclosureform.pdf.
References
- 1. Pablos JL, Galindo M, Carmona L, Lledo A, Retuerto M, Blanco R, et al. Clinical outcomes of hospitalised patients with COVID‐19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis 2020;79:1544–9. [DOI] [PubMed] [Google Scholar]
- 2. D'Silva KM, Serling‐Boyd N, Wallwork R, Hsu T, Fu X, Gravallese EM, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID‐19) and rheumatic disease: a comparative cohort study from a US ‘hot spot.’ Ann Rheum Dis 2020;79:1156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deepak P, Kim W, Paley MA, Yang M, Carvidi AB, El‐Qunni AA, et al. Glucocorticoids and B cell depleting agents substantially impair immunogenicity of mRNA vaccines to SARS‐CoV‐2 [preprint]. medRxiv 2021.
- 4. Mahil SK, Bechman K, Raharja A, Domingo‐Vila C, Baudry D, Brown MA, et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID‐19 vaccine BNT162b2: a cohort study. Lancet Rheumatol 2021;3:e627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simon D, Tascilar K, Schmidt K, Manger B, Weckwerth L, Sokolova M, et al. Humoral and cellular immune responses to SARS–CoV‐2 infection and vaccination in autoimmune disease patients with B cell depletion. Arthritis Rheumatol 2022;74:33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roltgen K, Powell AE, Wirz OF, Stevens BA, Hogan CA, Najeeb J, et al. Defining the features and duration of antibody responses to SARS‐CoV‐2 infection associated with disease severity and outcome. Sci Immunol 2020;5:eabe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murugesan K, Jagannathan P, Pham TD, Pandey S, Bonilla HF, Jacobson K, et al. Interferon‐γ release assay for accurate detection of SARS‐CoV‐2 T cell response. Clin Infect Dis 2021;73:e3130–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hadjadj J, Planas D, Ouedrani A, Buffier S, Delage L, Nguyen Y, et al. Immunogenicity of BNT162b2 vaccine against the alpha and delta variants in immunocompromised patients [preprint]. medRxiv 2021. [DOI] [PubMed] [Google Scholar]
- 9. Prendecki M, Clarke C, Edwards H, McIntyre S, Mortimer P, Gleeson S, et al. Humoral and T‐cell responses to SARS‐CoV‐2 vaccination in patients receiving immunosuppression. Ann Rheum Dis 2021;80:1322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, et al. American College of Rheumatology guidance for COVID‐19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol 2021;73:e60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American College of Rheumatology . COVID‐19 vaccine clinical guidance summary for patients with rheumatic and musculoskeletal diseases: developed by the ACR COVID‐19 Vaccine Clinical Guidance Task Force. October 2021. URL: https://www.rheumatology.org/Portals/0/Files/COVID‐19‐Vaccine‐Clinical‐Guidance‐Rheumatic‐Diseases‐Summary.pdf.
- 12. Schmidt T, Klemis V, Schub D, Mihm J, Hielscher F, Marx S, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV‐19/mRNA vaccination. Nat Med 2021;27:1530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barros‐Martins J, Hammerschmidt SI, Cossmann A, Odak I, Stankov MV, Ramos GM, et al. Immune responses against SARS‐CoV‐2 variants after heterologous and homologous ChAdOx1 nCoV‐19/BNT162b2 vaccination. Nat Med 2021;27:1525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tenbusch M, Schumacher S, Vogel E, Priller A, Held J, Steininger P, et al. Heterologous prime‐boost vaccination with ChAdOx1 nCoV‐19 and BNT162b2 [letter]. Lancet Infect Dis 2021;21:1212–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosureform


