Abstract
Background
Coronavirus disease 2019 (COVID‐19) has had profound effects on population health to date. African American cancer survivors are particularly vulnerable to developing severe consequences; therefore, understanding the impact of the virus on this patient population is critical.
Methods
The Detroit Research on Cancer Survivors cohort is a unique effort to understand the determinants of poor outcomes in African American cancer survivors. To date, more than 4500 cancer survivors and nearly 950 primary caregivers have been enrolled; participation includes a survey and the collection of biospecimens, medical records, and tumor tissue. Beginning in the spring of 2020, a supplemental survey focusing on the impact of COVID‐19 was offered to enrolled participants. The analysis included 890 survivors.
Results
Nearly all survivors (>99%) reported changes in their daily activities in an effort to reduce the risk of infection. More than 40% of the survivors reported some disruption in their access to medical care. A substantial proportion of the survivors (>40%) reported feeling anxious, depressed, and/or isolated during the COVID‐19 pandemic. Approximately 40% of the patients reported changes in health behaviors shown to negatively affect survivorship outcomes (physical inactivity, smoking, and alcohol use) as a result of the pandemic.
Conclusions
The influence of the COVID‐19 pandemic on African American cancer survivors is substantial: it has affected both their physical and mental health. Coupled with changes in health behaviors, these factors will likely affect outcomes in this high‐risk patient population, and this makes further study and interventions necessary to mitigate the long‐term impact of the pandemic on cancer outcomes.
Keywords: African American, coronavirus, epidemiology, outcomes, quality of life
Short abstract
The COVID‐19 pandemic has had a profound impact on the physical and mental health of African American cancer survivors living in Detroit, Michigan. Strategies focusing on health behaviors such as physical activity would improve patient health and quality of life.
Introduction
On March 10, 2020, the first 2 cases of coronavirus disease 2019 (COVID‐19) were confirmed in the state of Michigan. It was on this day that the governor declared a state of emergency and shortly thereafter enacted Executive Order 2020‐21, the first of many, which prohibited in‐person work that was not necessary to protect or sustain life, and Michigan residents were ordered to practice safe, social distancing measures when away from their residence whenever possible. Since then, there have been more than 40 additional orders from the governor's office and the Michigan Department of Health and Human Services issued in an effort to protect Michigan residents, to reduce the COVID‐19 incidence in order to not overwhelm the health care system, and to prevent death. 1 During the earliest months of this pandemic, the state of Michigan was ranked among the highest in the United States in both number of cases and deaths. 2 By the end of 2020, Michigan had documented nearly 500,000 cases and more than 12,000 COVID‐19–related deaths. 1 These are certainly underestimates because it is difficult to determine the number of asymptomatic carriers.
Several factors have been identified that predispose individuals to developing the most severe consequences as the result of infection with COVID‐19. Mortality rates increase with increasing age, with a significant risk of death among those older than 80 years. Risk is also greater among those with 1 or more comorbid conditions, including diabetes, hypertension, obesity, and autoimmune disease. 3 , 4 , 5 It is widely known that African Americans are at increased risk for being diagnosed with most cancers and experience poorer outcomes after diagnosis, including both cancer‐specific and nonspecific mortality. 6 African Americans have also been shown to be disproportionately affected by COVID‐19 for reasons not entirely understood but likely explained in part by the presence of comorbidities and more limited access to medical care. 5 , 7 , 8 Recent studies have shown that persons diagnosed with cancer are at increased risk for COVID‐19 infection. 9 , 10 This risk may be an artifact of increased testing in this population because they are more likely under regular physician care. Increased infection rates among patients with cancer may also be the result of their immune system being compromised on account of their treatment.
Finally, a recently published study out of Detroit, Michigan, reported that increased physical activity and physical fitness were associated with a reduced risk of developing the most severe consequences of COVID‐19. 11 This study of patients who had taken an exercise stress test within the previous 4 years found that those patients in the lowest quartile of physical fitness as measured by this test were 4 times more likely to be hospitalized for COVID‐19. These findings suggest that regular exercise is critically important to protecting the population from COVID‐19 complications and death. The current study focuses on the challenges and impact of the COVID‐19 pandemic as well as the individual actions taken to prevent infection and changes in activities and health in this high‐risk population.
Materials and Methods
The Detroit Research on Cancer Survivors (ROCS) study is a prospective cohort of cancer survivors and their caregivers living in the city of Detroit and the surrounding metropolitan tricounty area (Wayne, Oakland, and Macomb) in Southeast Michigan. Eligible participants for this unique study have been identified through the Metropolitan Detroit Cancer Surveillance System Cancer Registry, a founding member of the National Cancer Institute's Surveillance, Epidemiology, and End Results program. All participants are identified as African Americans who were diagnosed with invasive cancer on or after January 13, 2013, and were 20 to 79 years old at the time of diagnosis. Enrollment for the Detroit ROCS study began in early 2017 and was initially focused on survivors diagnosed with female breast, prostate, colorectal, or lung cancer because these tumors are the most common cancers diagnosed in the catchment area of the registry and also disproportionately affect African Americans to varying degrees in risk or prognosis. In 2019, the criteria were expanded to include both endometrial cancer and any survivor diagnosed with early‐onset disease (age at diagnosis < 50 years), regardless of the tumor type.
All eligible survivors are invited to participate in a baseline survey, which is completed either as a written questionnaire or online and is either self‐administered or taken with the assistance of a trained phone interviewer. Upon completion of the baseline survey, subjects are asked to provide consent for the collection of biospecimens (blood or saliva) for genetic studies, tumor tissue, and medical records; for the sharing of deidentified data in public databases (eg, the Database of Genotypes and Phenotypes); and for future contact for relevant studies. All survivors are asked to provide contact information for a primary caregiver if one is identified during the baseline interview. These caregivers are also invited to participate in a baseline survey. Annual follow‐up surveys are administered to assess changes in medical history, health behaviors, and study outcomes of interest. A detailed description of the study methods has been previously published. 12 To date, more than 4500 cancer survivors and nearly 950 primary caregivers have been enrolled in the Detroit ROCS study.
In 2020, a supplemental online survey to assess the impact of the COVID‐19 virus on individual behaviors and health was sent to 1922 Detroit ROCS cancer survivors who provided an email address. Subsequently, a written version of this survey was sent to the remaining enrolled survivors and to every new survivor enrolled in the study (n = 1368). To date, surveys have been completed and data have been entered for 890 survivors. The survey, approximately 30 minutes in length, gathered information on changes in behavior as a result of COVID‐19, disruptions in medical care, economic challenges, and the effect of the pandemic on patient‐reported quality of life (measured with the Functional Assessment of Cancer Therapy General Survey [FACT‐G]) and anxiety and depression (measured with the Patient‐Reported Outcomes Measurement Information System [PROMIS] survey).
Statistical analyses were performed with either SAS (version 9.4; SAS, Cary, North Carolina) or R (version 3.5.1). Frequency distributions were reported for all categorical and ordinal measures, including scaled responses, and means and standard deviations were reported for all continuous responses. Means and either standard deviations or interquartile ranges were calculated for the FACT‐G, its subscales for individual domains (physical, functional, emotional, and social), and the PROMIS anxiety and depression scales at the last survey (baseline or last follow‐up) and for the COVID‐19 survey. Mean changes in these responses between the last completed survey and the COVID‐19 survey were also calculated for reported changes in physical activity, alcohol consumption, and smoking. Regression models were used to evaluate statistically significant associations between changes in health behaviors and either FACT‐G or PROMIS scores on the COVID survey or changes in FACT‐G or PROMIS scores between the last survey available and the COVID survey. The study protocol was approved by the Wayne State University School of Medicine Institutional Review Board.
Results
Participant Characteristics
Table 1 provides summary characteristics for the first 890 Detroit ROCS survivors responding to the COVID‐19 questionnaire, and the distributions are similar to those for the overall study population (data not shown). The majority of the respondents were diagnosed with either breast (39%) or prostate cancer (37%) and were initially diagnosed with organ‐confined disease (65%). There was an average of approximately 4 years between the date of diagnosis and the time of this survey, with 9 months on average between the last (pre–COVID‐19) survey and the COVID‐19 survey. The majority of the respondents were female (55%), were married or living in a marriage‐like relationship (40%), and were college‐educated (42%), but nearly two‐thirds of the respondents were living in a census tract where ≥20% of the population was living in poverty. Nearly 94% of the survivors reported having 1 or more comorbid conditions, with 49% being obese. At the time of the COVID‐19 survey, 15% of the participants reported currently smoking, and 43% reported consuming alcohol, with 72% participating in any physical activity within the previous month.
TABLE 1.
Description of ROCS Cases Who Completed the COVID Survey (n = 890)
| Variable | No. (%) |
|---|---|
| Cancer site | |
| Breast | 345 (38.8) |
| Colorectal | 85 (9.5) |
| Endometrial | 29 (3.3) |
| Lung | 73 (8.2) |
| Prostate | 333 (37.4) |
| Other a | 25 (2.8) |
| Gender | |
| Male | 399 (44.8) |
| Female | 491 (55.2) |
| Age at diagnosis, mean (SD), y | 60.0 (9.8) |
| Education | |
| <High school | 74 (8.4) |
| High school diploma/GED | 210 (23.8) |
| Some college | 230 (26.0) |
| College degree | 225 (25.5) |
| Graduate/professional degree | 144 (16.3) |
| Missing | 7 |
| Marital status | |
| Married | 329 (37.1) |
| Living with a partner | 29 (3.3) |
| Widowed | 89 (10.0) |
| Divorced/separated | 227 (25.6) |
| Never married | 213 (24.0) |
| Missing | 3 |
| Census‐tract poverty indicator (% living below the poverty level) | |
| <5% | 52 (5.9) |
| 5%‐9.9% | 96 (10.9) |
| 10%‐19.9% | 156 (17.7) |
| ≥20% | 579 (65.5) |
| Missing | 7 |
| Smoking status | |
| Never | 435 (50.8) |
| Former | 296 (34.6) |
| Current | 125 (14.6) |
| Missing | 34 |
| Comorbidities | |
| 0 | 57 (6.5) |
| 1 or 2 | 325 (37.0) |
| 3 or 4 | 328 (37.3) |
| ≥5 | 169 (19.2) |
| Missing | 11 |
| BMI b | |
| Underweight (<18.5 kg/m2) | 9 (1.0) |
| Normal weight (18.5‐24.9 kg/m2) | 150 (17.0) |
| Overweight (25‐29.9 kg/m2) | 295 (33.5) |
| Obese (≥30 kg/m2) | 428 (48.5) |
| Missing | 8 |
| Any physical activity c | |
| Yes | 591 (71.9) |
| No | 231 (28.1) |
| Missing | 68 |
| Alcohol consumption d | |
| Yes | 359 (43.4) |
| No | 468 (56.6) |
| Missing | 63 |
| Time from cancer diagnosis to COVID survey, mean (SD), y | 4.0 (1.9) |
| SEER summary stage | |
| Local | 571 (65.0) |
| Regional | 254 (28.9) |
| Distant | 53 (6.1) |
| Missing/unknown | 12 |
Abbreviations: BMI, body mass index; COVID, coronavirus disease; GED, General Educational Development; ROCS, Detroit Research on Cancer Survivors; SD, standard deviation; SEER, Surveillance, Epidemiology, and End Results.
Other cancer types are included only for those diagnosed at the ages of 20 to 49 years (breast, colorectal, endometrial, lung, and prostate cancers are excluded).
BMI from either the last survey or the enrollment survey (when missing at the last survey).
Participation in physical activity within 4 weeks of the completion of the last survey.
Consumption of wine, liquor, or beer within 1 month of the completion of the last survey.
Risk Mitigation Strategies and Access to Care
Figure 1 summarizes responses related to the adoption of practices by Detroit ROCS survivors to reduce the risk of infection. More than 90% of the participants indicated that they wore a face mask, practiced social distancing, and regularly washed their hands to prevent spread. The vast majority of the survivors also responded that they stayed home and avoided crowded places and/or high‐risk contacts. Approximately half of the respondents indicated that they had cancelled plans for travel and stockpiled food/necessities. Just less than 1% of the participants reported no change in their behavior to prevent infection.
Figure 1.
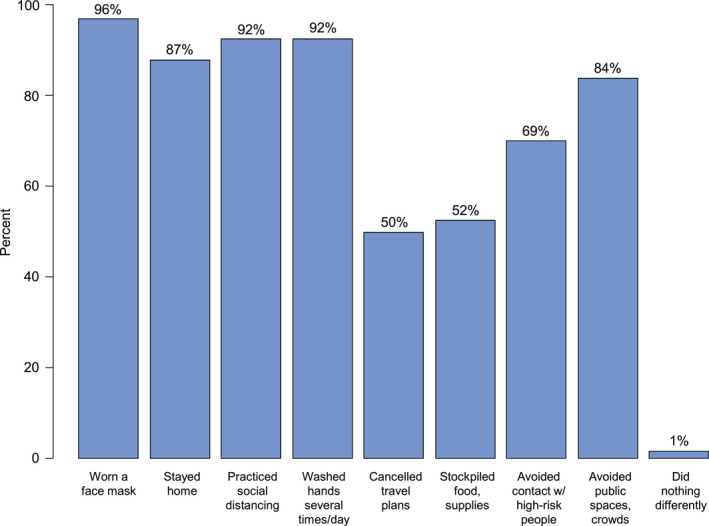
Reported behavior changes to mitigate coronavirus disease 2019 infection since the pandemic began among African American cancer survivors.
At the time of the COVID‐19 survey, 36% of the survivors reported having been tested at least once for COVID‐19, with 83% of those tested reporting a negative result, 14% reporting a positive result, and 3% reporting an inconclusive result (Fig. 2.)
Figure 2.
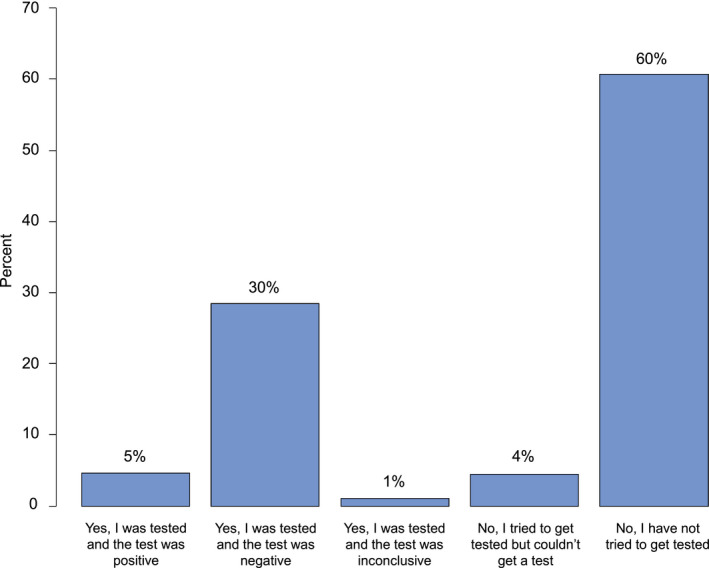
Self‐reported coronavirus disease 2019 testing among African American cancer survivors.
Fifty‐seven percent of the patients reported participating in 1 or more telemedicine visits, with nearly 35% of the participants reported missing 1 or more doctor appointments because of the pandemic, and 14% of the patients reported being unable to fill prescriptions (Table 2). Just 12% of the survivors reported being in treatment at the time of the COVID survey. Of these, 36% reported cancelling or rescheduling at least 1 routine appointment with their oncologist or treating physician, with 8 participants (7%) reporting the same for treatment (surgery, chemotherapy, or radiation; data not shown).
TABLE 2.
Access to Health Care During the COVID Pandemic
| Measure | No. (%) |
|---|---|
| Any canceled doctor's appointments other than cancer doctor | |
| Yes | 508 (59.5) |
| No | 300 (35.1) |
| N/A | 46 (5.4) |
| Missing | 36 |
| Any teleconference doctor's appointments other than cancer doctor | |
| Yes | 486 (56.8) |
| No | 341 (39.9) |
| N/A | 28 (3.3) |
| Missing | 35 |
| Any time needed the following but could not access | |
| Doctor's appointments | 299 (34.7) |
| Prescription medications | 121 (14.0) |
| Over‐the‐counter medications | 35 (4.1) |
| Mental health care or counseling | 43 (5.0) |
| None of the above | 511 (59.2) |
Abbreviations: COVID, coronavirus disease; N/A, not applicable.
Anxiety and Depression
Figure 3 summarizes additional challenges experienced by cancer survivors in this cohort as a result of the pandemic. Eleven percent of the patients reported moderate to severe anxiety, with nearly 60% of the patients reporting feeling anxious about being infected with COVID‐19 (Fig. 4). Nearly 40% of the survivors reported worry over infecting others, and 75% reported worry about a loved one becoming infected. Nearly 7% of the patients reported moderate to severe depressive symptoms and experienced changes in sleep (35%), changes in eating (31%), difficulty with concentrating (23%), and feelings of isolation (39%; Fig. 4).
Figure 3.
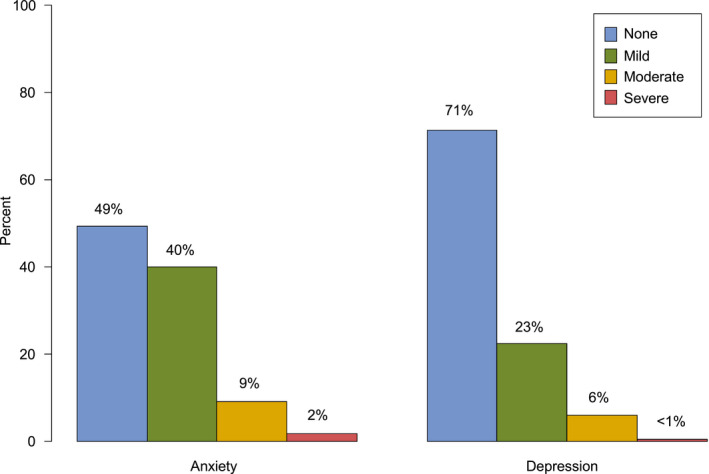
Anxiety and depression as measured by the Patient‐Reported Outcomes Measurement Information System scale among African American cancer survivors.
Figure 4.
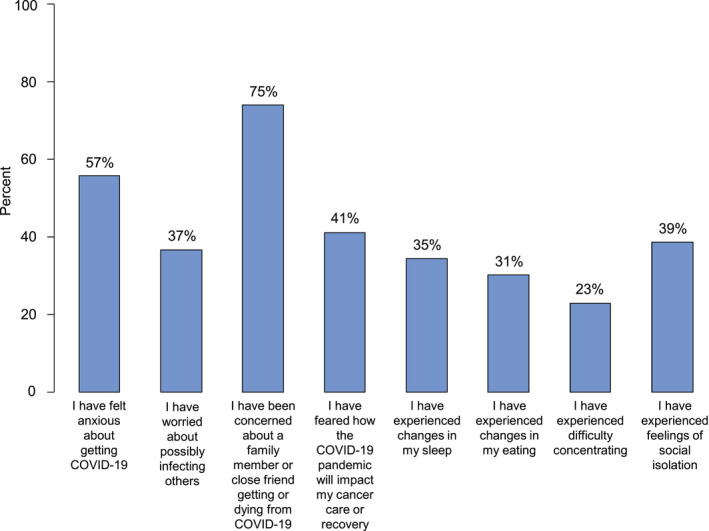
Physical and emotional impact of the COVID‐19 pandemic among African American cancer survivors. Blue bars represent the percentage of respondents who either agreed or strongly agreed with the given statement. COVID‐19 indicates coronavirus disease 2019.
Effect on and of Health Behaviors
Nearly 27% of the active survivors (n = 585) reported declines in their physical activity as a result of the pandemic. Among those who reported being less physically active, self‐reported overall health‐related quality of life (HRQOL) was significantly and meaningfully lower in comparison with survivors who reported either no change or increases in physical activity during the pandemic. Individual measured domains (emotional, social, and functional) were also significantly lower among those with decreases in activity, with marginal differences in physical well‐being (Table 3). Declines in physical activity were also inversely related to reported anxiety and depression (Table 4). Further analyses stratified by gender revealed stronger relationships between physical activity and HRQOL for men than women but similar relationships for survivors diagnosed with earlier onset disease (≤55 years) and survivors diagnosed with later onset disease (>55 years). The relationship between physical activity and anxiety/depression did not differ appreciably by either gender or age (Supporting Table 1).
TABLE 3.
Relationships Between Health‐Related Quality of Life Measures and Changes in Health Behaviors During the COVID‐19 Pandemic
| FACT‐G Total | EWB | FWB | PWB | SWB | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | P | Mean (SD) | P | Mean (SD) | P | Mean (SD) | P | Mean (SD) | P | |
| Change in PA (n = 585) | ||||||||||
| Decrease | 78.6 (18.8) | <.001 | 18.9 (4.7) | <.001 | 16.2 (6.5) | <.001 | 23.4 (5.2) | .062 | 19.7 (7.4) | .012 |
| No change/increase | 84.1 (15.4) | 20.2 (3.8) | 18.4 (6.5) | 24.2 (4.6) | 21.3 (6.5) | |||||
| Change in alcohol consumption (n = 323) | ||||||||||
| No change/decrease | 82.0 (15.2) | .016 | 19.7 (4.0) | .001 | 17.7 (6.3) | .280 | 24.1 (4.6) | .392 | 20.3 (6.6) | .071 |
| Increase | 76.1 (19.5) | 17.5 (5.7) | 16.6 (7.0) | 23.5 (5.1) | 18.4 (6.9) | |||||
| Change in smoking (n = 140) | ||||||||||
| No change/decrease | 76.2 (16.4) | .100 | 18.9 (4.9) | .299 | 15.7 (6.5) | .133 | 22.7 (5.7) | .290 | 19.2 (7.5) | .092 |
| Increase | 70.5 (19.8) | 17.9 (5.0) | 13.8 (7.4) | 21.5 (6.0) | 16.7 (6.9) | |||||
| Change in marijuana use (n = 130) | ||||||||||
| No change/decrease | 77.3 (18.2) | .874 | 19.3 (4.4) | .225 | 16.3 (7.3) | .183 | 23.0 (5.3) | .223 | 18.7 (7.5) | .826 |
| Increase | 76.7 (20.7) | 18.0 (5.6) | 18.3 (6.3) | 21.5 (7.1) | 19.0 (6.0) | |||||
Abbreviations: COVID, coronavirus disease; EWB, emotional well‐being; FACT‐G, Functional Assessment of Cancer Therapy General Survey; FWB, functional well‐being; PA, physical activity; PWB, physical well‐being; SD, standard deviation; SWB, social/family well‐being.
This table examines those who reported engaging in a behavior both before and during the COVID‐19 pandemic. The FACT‐G total and subscale scores are presented as mean (SD).
TABLE 4.
Relationships Between Anxiety and Depression Symptoms (Measured by PROMIS) and Changes in Health Behaviors During the COVID‐19 Pandemic
| PROMIS Anxiety | PROMIS Depression | |||
|---|---|---|---|---|
| Mean (SD) | P | Mean (SD) | P | |
| Change in PA (n = 585) | ||||
| Decrease | 56.1 (9.0) | <.001 | 51.2 (9.6) | <.001 |
| No change/increase | 52.2 (9.1) | 46.9 (8.5) | ||
| Change in alcohol consumption (n = 323) | ||||
| No change/decrease | 54.0 (9.5) | .008 | 48.8 (9.1) | .001 |
| Increase | 57.8 (9.4) | 53.5 (10.5) | ||
| Change in smoking (n = 140) | ||||
| No change/decrease | 52.0 (10.5) | .009 | 48.7 (9.8) | .184 |
| Increase | 57.3 (9.6) | 51.2 (10.1) | ||
| Change in marijuana use (n = 130) | ||||
| No change/decrease | 53.2 (9.8) | .184 | 48.4 (9.2) | .100 |
| Increase | 56.2 (12.1) | 51.9 (12.1) | ||
Abbreviations: COVID, coronavirus disease; PA, physical activity; PROMIS, Patient‐Reported Outcomes Measurement Information System; SD, standard deviation.
This table examines those who reported engaging in a behavior both before and during the COVID‐19 pandemic. PROMIS categories are presented as mean (SD).
Twenty‐nine percent of the patients (41 of 140 current smokers) reported increases in the consumption of cigarettes, 24% (31 of 130 users) reported increases in the use of marijuana, and 17% (55 of 323 drinkers) reported consuming alcohol with greater frequency. We did not observe the same consistent associations between changes in these behaviors due to the pandemic and reported HRQOL as with physical activity (Table 3) with 1 exception: reduced alcohol consumption was associated with greater emotional well‐being. An increase in the use of alcohol and cigarettes was associated with significantly higher levels of reported anxiety, with alcohol use also associated with higher depression scores (Table 4).
Effect on HRQOL
The average change in HRQOL as measured by the FACT‐G between the last survey (before COVID‐19) and the COVID‐19 survey among all respondents was –1.5 (range, –56.0 to 42.0). The average changes in anxiety and depressive symptom scores measured by PROMIS were 4.3 for anxiety (range, –25.0 to 41.3) and 1.0 for depression (range, –29.0 to 29.0) among all survivors. Because of the rapidly evolving nature of the COVID pandemic and documented seasonal fluctuations in infection rates, we evaluated whether the measures used in this analysis differed between time periods of COVID survey completion. Specifically, we dichotomized dates of completion as spring/summer (May to August) or fall/winter (September to early January). We did not find any significant heterogeneity in PROMIS scores of anxiety (P = .247) or depression (P = .582) by period of completion. We did observe a significant difference in the total FACT‐G score (spring/summer, 82; fall/winter, 79; P = .039). However, the change in FACT‐G scores between the last survey and the COVID survey was not significantly different by season (spring/summer, –1.7, fall/winter, –1.0; P = .516). Changes in PROMIS anxiety and depression scores were also not significantly different between the 2 time periods (P = .430 for anxiety score change and P = .301 for depression score change). Health behavior changes during the COVID pandemic did not vary significantly over the time of survey collection (data not shown).
Discussion
Findings from this investigation indicate a profound, negative impact of COVID‐19 on this cohort of African American cancer survivors with the potential to widen current cancer health disparities. Survivors reported fear of infection and the use of strategies to prevent infection that affected their access to proper medical care and negatively influenced health behaviors. They also reported higher levels of anxiety and depression and poorer quality of life at the time of COVID‐19 survey completion. Declines in physical activity in particular were consistently associated with significantly greater anxiety and depression and poorer HRQOL; however, interestingly, similar patterns were not consistently observed with increased use of alcohol, cigarettes, or marijuana. These areas in particular should be considered in the development of strategies to improve quality of life during the pandemic and in the postpandemic era.
It is expected that published literature describing the impact of this pandemic on the US population as a whole and especially among those at greatest risk for developing severe complications and death will increase dramatically over time. However, to date, there are few observational, population‐based studies of COVID‐19, particularly among cancer survivors. As of February 2021, approximately 1 year since the first case was reported in the United States, there have been 27.8 million cases reported and 468,000 deaths. 13 As up to 40% of those infected are predicted to be asymptomatic carriers, it is exceedingly difficult to estimate the true prevalence of infection. Furthermore, current COVID‐19 reporting has obscured mortality rates due to other causes, and these are expected to rise over time as greater numbers of people are exposed or vaccinated. We are only beginning to realize the more long‐term toll of this pandemic on the US population's health, both physical and mental, and longer term investigations of its effect are required.
Kuderer et al, 10 in a large cohort of cancer survivors in the United States, Canada, and Spain, identified cancer survivors from the COVID‐19 and Cancer Consortium database who developed severe acute respiratory distress (ARD) syndrome as a result of infection. Thirteen percent of the patients died within 30 days of the development of ARD. Mortality was higher among patients who were older, were male, were smokers, had 2 or more comorbid conditions, or had an Eastern Cooperative Oncology Group performance status of 2 or higher and among those patients whose cancer was not in remission. Among the few published studies to date addressing the impact of COVID‐19 on cancer survivors, even fewer address the issue of health disparities. In a retrospective study of the risk of infection among patients diagnosed with cancer using electronic medical records from more than 73 million patients, Wang and Zhang 9 reported that a recent cancer diagnosis (within the past year) was associated with a significant increase in the odds of infection (odds ratio, 7.1; 95% confidence interval, 6.9‐7.4), with the highest odds ratios observed for patients diagnosed with leukemia, non‐Hodgkin lymphoma, or lung cancer. Black patients diagnosed with cancer were significantly more likely to be infected with COVID‐19 than Whites, but the mortality rates were similar. Finally, cancer patients infected with COVID‐19 had significantly poorer outcomes (higher rates of hospitalization and death) than patients without cancer. 9
Our study participants reported a number of disruptions to their normal medical care as a result of the pandemic; this is a major concern because delays in detection and treatment would certainly affect cancer survival, particularly in an already underserved population. Moreover, because African American patients with cancer are more likely than their non‐Hispanic White counterparts to suffer multiple comorbidities, these disruptions to primary care would have a profound effect on disease management. 14 Because of the nature of case identification and our enrollment strategy, the vast majority of Detroit ROCS participants reported completing treatment before the start of the pandemic. However, we cannot exclude the possibility of some interference, particularly among those patients on longer term therapies. Studies in Europe have predicted that the pandemic will cause up to a 10% increase in breast cancer mortality, a 15% increase in colorectal cancer mortality, and a 5% to 6% increase in lung and esophageal cancer mortality on account of delays in detection and diagnosis. 15 Others have estimated that even 3‐ to 6‐month delays in cancer surgeries related to COVID‐19 will result in 1 to 2 years of life loss. 16 To compound these problems, vaccine hesitancy in the African American community is likely to further exacerbate the observed racial disparities related to the pandemic. 17 The cause of reduced vaccine uptake in African Americans appears partially rooted in mistrust of the medical system. 18
Detroit ROCS survivors also reported changes in health behaviors that could affect both cancer‐specific and nonspecific outcomes as a direct result of COVID‐19. Most notably, nearly one‐third of African American survivors reported declines in physical activity due to the pandemic, and these declines were associated with greater anxiety and depression and poorer HRQOL. Reducing both cancer‐specific and overall mortality, increased physical activity has been consistently shown to be beneficial to cancer survivors, so much so that the American Cancer Society has recommended that cancer survivors engage in at least 150 minutes per week of moderate to vigorous physical activity coupled with strength training. 19 As previously mentioned, a Michigan study found that measures of physical fitness were inversely associated with the risk of being hospitalized with COVID‐19. 11 A recent review from the University of Virginia reported that regular exercise reduces the risk of developing ARD syndrome, which affects up to 17% of patients with COVID‐19 and is a major cause of death among individuals infected with COVID‐19. Yan and Spaulding 20 suggested that cardiovascular exercise increases the production of extracellular superoxide dismutase, an antioxidant secreted by muscles that protects target tissues from free radical damage. Taken together, these data suggest that finding ways to reduce barriers to regular physical activity will be critical to improving COVID‐19 and cancer‐related outcomes.
This study has numerous strengths. The Detroit ROCS cohort is a one‐of‐a‐kind resource, with investigators focused on understanding the determinants of poorer outcomes among African American cancer survivors. This study is population‐based, and this makes the results generalizable to a broader population. The cohort follows enrolled participants for up to 4 years after their baseline survey, and this allows investigators an opportunity to ask important new research questions, such as the impact of COVID‐19, that are highly relevant to the population. The characteristics of the respondents to this particular survey were similar to those of the cohort as a whole, which currently exceeds 4500 survivors. However, there are also some limitations that require consideration in the interpretation of the findings. Per protocol and even though cases are identified typically 3 to 6 months after the time of diagnosis, the vast majority of the Detroit ROCS participants enrolled more than 6 months after their diagnosis and are not currently undergoing active treatment. Therefore, we will not capture the impact of the pandemic on either delays or disruptions in treatment among patients with newly diagnosed cancer. This survey captures the experience of participants at a finite point in time, with most respondents participating 3 to 5 months after the onset of the pandemic in Michigan, which was a period of decline in infection rates for the state. Therefore, the prevalence, results, and availability of testing reported here do not reflect the experience of the cohort today. We expect that both reports of infection and the availability of testing have increased over time. It will be important for us to continue to follow these respondents over time to measure the true impact of the virus.
In conclusion, the COVID‐19 pandemic has had a profound impact on African Americans diagnosed with cancer in this study. It remains critical to use strategies to prevent infection and repeated infection in light of the introduction of new variants. These strategies include the use of masks, social distancing, sanitization of surface areas, hand‐washing, and quarantine among those exposed. Vaccination is also highly recommended for these patients because of their predisposition to developing the most severe consequences if they are infected. Further investigations will be required to fully understand the long‐term effects of this pandemic on cancer survivors, and the Detroit ROCS cohort will continue to provide data to address this in a vulnerable population.
Funding Support
This work was supported by the National Cancer Institute of the National Institutes of Health (U01 CA199240 and CA199240‐04S1), the Epidemiology Research Core and National Institutes of Health through a grant awarded to the Karmanos Cancer Institute at Wayne State University (P30CA022453), and the Metropolitan Detroit Cancer Surveillance System.
Conflict of Interest Disclosures
Theresa A. Hastert reports a Scholar‐in‐Training Award to attend the 2020 American Association for Cancer Research Science of Cancer Health Disparities Meeting. Hayley S. Thomspon reports grants or contracts from the Patient‐Centered Outcomes Research Institute and the National Institutes of Health and payments or honoraria from the American Cancer Society, Virginia Commonwealth University, Georgetown University, and Campbell Ewald. The other authors made no disclosures.
Author Contributions
Jennifer L. Beebe‐Dimmer: Research design, research implementation, and writing of the manuscript. Christine M. Lusk: Data analysis and writing of the manuscript. Julie J. Ruterbusch: Research implementation, data analysis, and writing of the manuscript. Tara E. Baird: Research implementation and writing of the manuscript. Stephanie S. Pandolfi: Research implementation and writing of the manuscript. Angela S. Wenzlaff: Research implementation and writing of the manuscript. Theresa A. Hastert: Research design and writing of the manuscript. Felicity W. K. Harper: Research design and writing of the manuscript. Hayley S. Thompson: Research design and writing of the manuscript. Ann G. Schwartz: Research design, research implementation, and writing of the manuscript. All coauthors participated in the review and editing of the manuscript.
Supporting information
Table S1
Beebe‐Dimmer JL, Lusk CM, Ruterbusch JJ, Baird TE, Pandolfi SS, Wenzlaff AS, Hastert TA, Harper FWK, Thompson HS, Schwartz AG. The impact of the COVID‐19 pandemic on African American cancer survivors. Cancer.2022. 10.1002/cncr.33987
References
- 1. Coronavirus statistics and response. State of Michigan Department of Community Health. Accessed April 1, 2021. https://www.michigan.gov/coronavirus [Google Scholar]
- 2. CDC COVID‐19 Response Team . Geographic differences in COVID‐19 cases, deaths, and incidence—United States, February 12–April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:465‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gianfrancesco M, Hyrich KL, Al‐Adely S, et al. Characteristics associated with hospitalisation for COVID‐19 in people with rheumatic disease: data from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Ann Rheum Dis. 2020;79:859‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ko JY, Danielson ML, Town M, et al. Risk factors for coronavirus disease 2019 (COVID‐19)–associated hospitalization: COVID‐19–associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin Infect Dis. 2021;72:e695‐e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Millett GA, Jones AT, Benkeser D, et al. Assessing differential impacts of COVID‐19 on Black communities. Ann Epidemiol. 2020;47:37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69:211‐233. [DOI] [PubMed] [Google Scholar]
- 7. Yehia BR, Winegar A, Fogel R, et al. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID‐19) at 92 US hospitals. JAMA Netw Open. 2020;3:e2018039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rentsch CT, Kidwai‐Khan F, Tate JP, et al. Covid‐19 by race and ethnicity: a national cohort study of 6 million United States veterans. medRxiv. Preprint posted online May 18, 2020. doi: 10.1101/2020.05.12.20099135 [DOI] [Google Scholar]
- 9. Wang H, Zhang L. Risk of COVID‐19 for patients with cancer. Lancet Oncol. 2020;21:e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID‐19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907‐1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brawner CA, Ehrman JK, Bole S, et al. Inverse relationship of maximal exercise capacity to hospitalization secondary to coronavirus disease 2019. Mayo Clin Proc. 2021;96:32‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beebe‐Dimmer JL, Ruterbusch JJ, Harper FWK, et al. Physical activity and quality of life in African American cancer survivors: the Detroit Research on Cancer Survivors study. Cancer. 2020;126:1987‐1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. COVID data tracker. Centers for Disease Control and Prevention. Accessed April 1, 2021. https://covid.cdc.gov/covid‐data‐tracker [Google Scholar]
- 14. Beebe‐Dimmer JL, Albrecht TL, Baird TE, et al. The Detroit Research on Cancer Survivors (ROCS) pilot study: a focus on outcomes after cancer in a racially diverse patient population. Cancer Epidemiol Biomarkers Prev. 2019;28:666‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maringe C, Spicer J, Morris M, et al. The impact of the COVID‐19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population‐based, modelling study. Lancet Oncol. 2020;21:1023‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sud A, Jones ME, Broggio J, et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID‐19 pandemic. Ann Oncol. 2020;31:1065‐1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tram KH, Saeed S, Bradley C, et al. Deliberation, dissent, and distrust: understanding distinct drivers of COVID‐19 vaccine hesitancy in the United States. Clin Infect Dis. Published online July 16, 2021. doi: 10.1093/cid/ciab633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thompson HS, Manning M, Mitchell J, et al. Factors associated with racial/ethnic group–based medical mistrust and perspectives on COVID‐19 vaccine trial participation and vaccine uptake in the US. JAMA Netw Open. 2021;4:e2111629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rock CL, Doyle C, Demark‐Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243‐274. [DOI] [PubMed] [Google Scholar]
- 20. Yan Z, Spaulding HR. Extracellular superoxide dismutase, a molecular transducer of health benefits of exercise. Redox Biol. 2020;32:101508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


