Abstract
Excluding pregnant people from Covid‐19 clinical trials may lead to unintended harmful consequences. For this study, an online questionnaire was sent to physicians belonging to Canadian professional medical associations in order to evaluate their perspectives on the participation of pregnant women in Covid‐19 clinical trials. The majority of respondents expressed support for including pregnant women in Covid‐19 trials (119/165; 72%), especially those investigating therapies with a prior safety record in pregnancy (139/164; 85%). The main perceived barriers to inclusion identified were unwillingness of pregnant patients to participate and of treating teams to offer participation, the burden of regulatory approval, and a general “culture of exclusion” of pregnant women from trials. We describe why some physicians may be reluctant to include pregnant individuals in trials, and we identify barriers to the appropriate participation of pregnant people in clinical research.
Keywords: human subjects research, Covid‐19 clinical trials, pregnant research participants, research with pregnant women, maternal‐fetal ethics, inclusion of pregnant women in trials
Despite calls over the past decades for greater inclusion of pregnant people in clinical research, their systematic exclusion remains common practice. 1 Several clinical trials investigating potential therapies for Covid‐19 are underway throughout the world. 2 However, most of these trials (829/1282; 65%) enrolling people of reproductive age exclude pregnant persons or fail to address the issue of pregnancy, 3 even though, for many of the investigational therapies, safety data about their use in pregnant patients is available. 4
The exclusion of pregnant people from Covid‐19 clinical trials may result in several unintended consequences. First, because of a lack of data on the safety and effectiveness of potential therapies during pregnancy, pregnant people may be untreated or inadequately treated for Covid‐19. 5 Second, in the absence of adequate pharmacokinetic data, medications may be over‐ or underdosed for pregnancy. 6 Third, investigational therapies are being used in pregnant patients outside of the purview of a clinical trial setting, 7 and thus pregnant people may be exposed to the risk of adverse events in the absence of demonstrated efficacy.
The Coalition to Advance Maternal Therapeutics, which includes over 20 organizations whose shared goal is to address gaps in research on therapeutics for pregnant women, has urged the U.S. National Institutes of Health and the U.S. Food and Drug Administration to call for inclusion of pregnant women in Covid‐19 trials. 8 However, to successfully broaden the inclusion of pregnant people in research related to Covid‐19 therapeutics, additional barriers may need to be addressed. 9
Physicians belong to one of the several key professional groups with the potential to shape the inclusion of pregnant people in clinical research concerning SARS‐CoV‐2 and future emerging pathogens. Physicians' opinions may affect their motivation to design investigator‐initiated research studies that accommodate pregnant participants. Moreover, pregnant people may look to their physicians for guidance about participation in clinical research, viewing them as trusted sources of information. 10 The beliefs of treating physicians may therefore influence the recruitment of eligible pregnant patients in ongoing trials. A description of individual physician perspectives regarding the inclusion of pregnant people in clinical trials for Covid‐19 is currently lacking in the literature. Thus, we conducted a survey among physicians on their willingness to include pregnant women in Covid‐19 clinical trials. As to terminology, we acknowledge that not all pregnant people may identify as women. However, because “pregnant women” was used in the survey, we use that term when referring to the survey responses. Otherwise, more inclusive language is used.
Study Methods
Survey development
We developed an online questionnaire relevant to the topic of Covid‐19 in pregnancy, including clinicians and researchers specialized in maternal fetal medicine, obstetric medicine, infectious diseases, medical microbiology, neonatology, general internal medicine, and critical care. To define the population of respondents, we collected information on age, gender, medical specialty, years of practice, proportion of practice devoted to research, and research experience with pregnant women, as well as the setting and location of practice. Respondents' experience with Covid‐19 in general and in pregnancy was assessed. The “Perspectives and Opinions” section of the questionnaire included multiple‐choice close‐ended and open‐ended questions addressing (1) physicians' individual beliefs regarding the appropriateness of including pregnant women in Covid‐19 clinical trials (“Do you believe that it would be appropriate for pregnant women to be included in some Covid‐19 clinical trials?” and “Do you believe it would be appropriate to include pregnant women in Covid‐19 clinical trials involving repurposed drugs with a safety track record in pregnancy [e.g. hydroxychloroquine or lopinavir/ritonavir]?”), (2) physicians' individual beliefs regarding the urgency of including pregnant women in such trials (“How urgent is the need to include pregnant women in Covid‐19 clinical trials?”), and (3) physicians' comfort with enrolling pregnant women in such trials (“How comfortable would you be enrolling pregnant women in Covid‐19 clinical trials involving experimental drugs otherwise thought to be safe during pregnancy [e.g. hydroxychloroquine or lopinavir/ritonavir]?”). In the “Final Questions” section of the questionnaire, physicians were asked, “Have you identified any barriers for the inclusion of pregnant women in Covid‐19 clinical trials?,” followed by, “If yes, please elaborate.” Participants were also given the opportunity to provide additional comments in free text sections. Twenty‐two total items were administered over four pages, with none of the questions marked as mandatory to answer. The complete survey can be found in appendix A (available online, along with appendix B and tables 1 and 2; see the “Supporting Information” section below).
Survey validation
The survey was pilot tested among ten physicians from different specialties to verify the clarity of the questions and the appropriateness of the order in which they were asked and to strengthen content‐related validity. A test‐retest strategy was employed to demonstrate reproducibility. Surveys were administered twice to the same physicians two to four weeks apart, and four questions were sampled from the “Perspectives and Opinions” section. In addition, we assessed internal consistency by measuring the correlation between answers provided for two questions within a similar domain (“Do you believe that it would be appropriate for pregnant women to be included in some clinical trials for Covid‐19?” and “Do you believe it would be appropriate to include pregnant women in trials of Covid‐19 looking at repurposed drugs with a safety track record in pregnancy [e.g. hydroxychloroquine or lopinavir/ritonavir]?”).
Survey dissemination
The target population was defined as a diverse group of physicians most likely among types of specialists to design and conduct Covid‐19 clinical trials, treat pregnant patients with Covid‐19, or recruit pregnant patients for Covid‐19 clinical trials. The following Canadian professional associations were approached: the Society of Obstetricians and Gynecologists of Canada, the Canadian Critical Care Society, the Canadian Society of Internal Medicine, and the Association of Medical Microbiology and Infectious Disease of Canada. Each professional association disseminated the electronic letter inviting members to participate according to their respective internal policies. No incentives were offered. Respondents indicated their voluntary agreement to participate by choosing to answer the questionnaire. The questionnaire was administered via LimeSurvey, 11 an internet‐based survey service hosted by McGill University. Respondents were able to review and modify answers prior to submission. The survey was open from May 19, 2020, until October 31, 2020.
Statistical analyses
We measured reproducibility of the survey by assessing Cronbach's alpha coefficients for four questions from two surveys administered within two to four weeks to the same physicians. We measured internal consistency by calculating the intraclass correlation coefficient for responses to two questions within a similar domain. Answers to multiple‐choice questions were described in numbers and percentages and, as appropriate, in means with standard deviations. We performed exploratory analyses whereby the answers to the questions, “Do you believe that it would be appropriate for pregnant women to be included in some Covid‐19 clinical trials?” and, “How comfortable would you be enrolling pregnant women in Covid‐19 clinical trials involving experimental drugs otherwise thought to be safe during pregnancy [e.g. hydroxychloroquine or lopinavir/ritonavir]?” were compared between respondents from different specialties (obstetrics and maternal fetal medicine versus others), with and without research experience with pregnant women and with and without experience caring for patients with Covid‐19, using two‐way chi‐square tests. Qualitative analyses on the free text answers from four open‐ended questions were conducted by two independent investigators (MJT and IM), who used a semi‐inductive approach to extract codes and themes for each question. The codes and themes were periodically cross‐validated during the analysis phase. Quantitative analyses were performed using the Statistical Package for Social Sciences (SPSS, version 27, IBM New York) and LimeSurvey. Qualitative analyses were performed using Dedoose, a qualitative research software (version 8.0.35). 12
Ethical considerations
This study was conducted in accordance with the TCPS 2, Tri‐Council Policy Statement: Ethical Conduct for Research Involving Humans (2018) and was approved by the McGill University Health Centre Research Ethics Board (project #MP‐37‐2021‐6706).
Study Results
Regarding survey validation, the Cronbach's alpha coefficients for the four survey questions were 0.96, 0.85, 0.91, and 0.85, respectively. The intraclass correlation
Most physicians surveyed supported the inclusion of pregnant women in Covid‐19 trials, particularly when using repurposed drugs with existing safety data in pregnancy.
coefficient for responses to two questions within a similar domain was 0.82 (95% confidence interval 0.21‐0.96, p = 0.013). The survey had acceptable reproducibility and internal consistency. 13
In total, 83% (168/202) of respondents who agreed to participate submitted the last page of the questionnaire. For each multiple‐choice question, a maximum of 3% of responses were incomplete. Participant characteristics are summarized in table 1. While most respondents were from obstetrics and gynecology or maternal fetal medicine (58%), other respondents were from varied specialties (family medicine [17%], infectious diseases or microbiology [17%], critical care, respirology, or anesthesiology [9%], obstetric medicine [5%], or general internal medicine [4%]) and from both academic (54%) and community practice settings. Importantly, 50% (82/165) of respondents reported prior involvement in a research study that included pregnant women.
Most respondents (97/165; 59%) reported experience in caring for patients with Covid‐19, including 43% (71/164) with experience in caring specifically for pregnant patients with Covid‐19. Thirty‐five percent (57/164) of respondents reported that their hospital was recruiting participants for enrollment in Covid‐19 clinical trials. Among these, 34% (19/56) reported that pregnant women were eligible for enrollment, including 7% (4/56) in all ongoing trials and 27% (15/56) in some but not all ongoing trials. Four (4/165; 2%) respondents affirmed that pregnant women with Covid‐19 at their hospital had received treatment with experimental therapies (hydroxychloroquine, chloroquine, lopinavir/ritonavir, remdesivir, tocilizumab, or others) outside of a clinical trial setting. Other respondents were either unsure (63/165; 38%) or reported that, to their knowledge, pregnant women had not received experimental therapies for Covid‐19 outside of a clinical trial setting at their hospital (98/165; 59%).
The “Perspectives and Opinions” section of the questionnaire comprised four main questions. The responses to these are shown in figure 1. Extracted themes of free‐text answers to open‐ended questions with exemplary quotations are presented in table 2.
Figure 1.
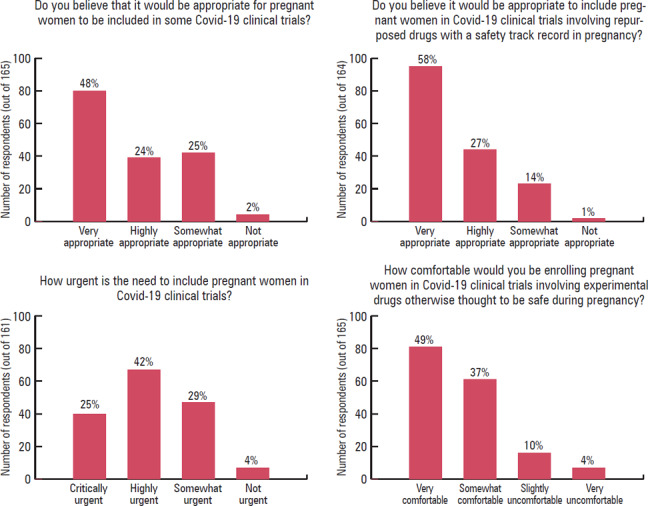
Perspectives and Opinions regarding the Inclusion of Pregnant Women in Covid‐19 Trials
In terms of the appropriateness of including pregnant women in Covid‐19 clinical trials, the majority (119/165; 72%) of respondents answered positively (“very appropriate” or “highly appropriate”). This number was even higher when participants were asked whether it would be appropriate to include pregnant women in Covid‐19 clinical trials involving repurposed drugs with a safety track record in pregnancy; 85% (139/164) of respondents answered positively (“very appropriate” or “highly appropriate”). Only four respondents (4/164; 2%) thought that the inclusion of pregnant women in Covid‐19 trials would be inappropriate. The majority (107/161; 66%) of respondents answered that the inclusion of pregnant women in such trials was urgent (“critically urgent” or “highly urgent”). Most respondents (142/165; 86%) reported being comfortable (“very comfortable” or “somewhat comfortable”) enrolling pregnant women in Covid‐19 clinical trials involving investigational drugs otherwise thought to be safe during pregnancy (e.g., hydroxychloroquine or lopinavir/ritonavir). Alternatively, 14% (23/165) answered that they would be “slightly uncomfortable” or “very uncomfortable” (see figure 1 in connection to the findings reported thus far in this paragraph). There were no substantial differences in physician perspectives based on medical specialty, research experience with pregnant women, or clinical experience caring for patients with Covid‐19 (see appendix B).
Expressions of reluctance
Among respondents who answered that the inclusion of pregnant patients would be “inappropriate,” two provided explanations, both citing fetal safety as their main concern. One respondent specifically cited the example of the drug thalidomide as a reason to exclude pregnant patients from Covid‐19 clinical trials. Among those who answered that the inclusion of pregnant patients in Covid‐19 clinical trials was “non‐urgent,” five provided explanations. The most common theme extracted among these responses was the perception of low risk of severe illness among pregnant patients. Among respondents who answered that they would be uncomfortable enrolling pregnant patients in Covid‐19 drug trials, 12 provided explanations. The following themes were extracted from these responses: potential harm to the fetus (n = 5), lack of demonstrated efficacy of the drugs investigated (n = 5), perception of low risk of severe maternal illness from Covid‐19 (n = 4), fear of side effects for the mother (n = 3), fear of litigation (n = 2), a culture of nonprescription in obstetrics (n = 2), lack of experience with the investigational drugs (n = 2), and lack of understanding of the natural history of Covid‐19 (n = 2).
Barriers to inclusion
Barriers to the inclusion of pregnant women in Covid‐19 trials were identified by 77 out of 164 (47%) respondents, 68 of whom provided comments elaborating on these perceived barriers. The following themes were extracted from the responses: unwillingness of pregnant women to be included in clinical trials (n = 22), unwillingness of other members of the health care team to include pregnant women in clinical trials (n = 14), the burden of regulatory approval to include pregnant women in clinical trials (n = 14), a culture of exclusion of pregnant women (n = 13), the burden of recruitment for this specific population (n = 6), fear of potential participants and their family (n = 5), lack of eligibility among pregnant women (n = 5), liability concerns (n = 2), and lack of infrastructure to include pregnant women in trials (n = 3).
Additional comments
Participants were asked to provide additional comments about the inclusion of pregnant women in Covid‐19 clinical trials or feedback for the study. The following themes were extracted from the expressions of support for the inclusion of pregnant women in Covid‐19 clinical trials: the relevance and feasibility of including pregnant patients in Covid‐19 drug trials (n = 6) and the ethical obligation to include pregnant women in clinical research (n = 3).
Discussion
Most physicians surveyed supported the inclusion of pregnant women in Covid‐19 trials, particularly when using repurposed drugs with existing safety data in pregnancy. Additionally, we gathered insights into the reasons that some physicians may be reluctant to include pregnant women in clinical trials, and we described elements taken into consideration when weighing the risks and benefits of offering trial participation to pregnant women. We identified important barriers to the inclusion of pregnant women in clinical trials, including the perceived unwillingness of pregnant women to participate in them, the perceived unwillingness of other treating team members to enroll pregnant women in trials, the added burden imposed by regulatory authorities, and the lack of infrastructure to facilitate recruitment.
Interpretation
For trials investigating drugs with limited available pregnancy‐specific safety data, concern for fetal safety was a dominant theme. The example of thalidomide has left an important heritage for the culture of obstetrics and for clinical research among pregnant people. 14 Thalidomide was a sedative drug used as an antiemetic in pregnancy in the 1950s with limited prior human safety data. Thalidomide was subsequently associated with severe congenital anomalies in thousands of children exposed in utero. 15 However, the “thalidomide disaster” is not attributable to the participation of pregnant people in clinical trials but, rather, to the inadequate testing of the drug among pregnant people prior to its widespread use in this population. 16 Indeed, had thalidomide been rigorously studied in pregnant people—for example, as part of a carefully designed clinical trial—teratogenic effects would have been reported sooner, and fewer adverse pregnancy outcomes linked to fetal exposure would have occurred. 17
Respondents also expressed concern about potential maternal adverse effects of investigational therapies. Yet excluding pregnant people from clinical trials may induce further maternal harm by preventing the collection of pregnancy‐specific pharmacokinetic data, leading to subsequent under‐ or overtreatment. 18 Furthermore, the absence of pregnancy‐specific drug data can result in reluctance to use potentially beneficial therapies to treat pregnant people, a harmful phenomenon referred to as “reticence.” 19 Approximately 70% of pregnant women take one or more prescription medications during pregnancy. 20 Therefore, despite attempts to limit their exposure to medications, an important proportion of women still require medical treatment during pregnancy. While the vulnerability of pregnant persons has often been cited as a reason for their exclusion from the realm of clinical research, their vulnerability lies in the lack of high‐quality data to inform their care. 21 In an effort to prevent systematic exclusion of pregnant people from clinical research, the American College of Obstetricians and Gynecologists (ACOG) now recommends against the use of the term “vulnerable” to describe pregnant people. 22 Rather, ACOG describes pregnant people as “scientifically complex,” a term that refers to physiological complexity without implying an inability to protect one's own interests. 23
An estimation of risks and benefits to including pregnant women in clinical trials was described by some respondents, who weighed the underlying severity (or lack thereof) of Covid‐19 against the potential harm of investigational therapies. Pregnant women's complex reasoning surrounding the decision to participate in clinical trials has previously been described. 24 Factors taken into consideration include the availability of evidence in human pregnancy, potential risks perceived as being most important, and trust in providers and public health authorities. 25 Given that such nuanced decisions are inherently personal and value laden 26 and that pregnant persons do not lack the capacity to make informed decisions, pregnant women are best positioned to decide whether to participate in clinical trials. Thus, a greater emphasis should be placed on the values, experiences, and agency of potential pregnant participants when designing nonobstetrical clinical trials.
The unwillingness of pregnant individuals to enroll in clinical trials was perceived as a potential barrier to trial inclusion. However, it has repeatedly been demonstrated that some pregnant people are willing to participate in research studies, even in the absence of direct benefit for themselves. 27 Reasons for agreeing to participate include obtaining treatment for their disease, enhanced monitoring, and altruism. 28 A culture of exclusion of pregnant women from clinical trials also emerged as an important theme. Despite calls to “[move] from a presumption of exclusion to one of inclusion,” 29 a change in practice has yet to occur.
In line with previous reports, regulatory approval was found to be an important barrier to the appropriate inclusion of pregnant women in clinical research. 30 According to Article 4.3 of the Tri‐Council Policy Statement on the Ethical Conduct for Research Involving Humans (TCPS2 2018), “Women shall not be inappropriately excluded from research solely on the basis of their reproductive capacity, or because they are pregnant or breastfeeding.” 31 Nevertheless, common misconceptions among research ethics boards may lead to the unjust exclusion of pregnant people from trials. 32 The creation of committees specializing in clinical research among pregnant people and enhanced training for ethics review board members on the topic may help to overcome this barrier. 33
Fear of litigation was consistently highlighted. Indeed, liability concerns have previously been identified as a barrier to the inclusion of pregnant people in clinical trials. 34 However, the major risk posed to individual providers lies in the fact that medications used have not been tested in pregnancy. 35 Hence, inclusion of pregnant people in clinical trials may contribute to alleviating the overall litigation potential in obstetrics. The potential for litigation in the context of appropriate consent procedures needs to be clarified.
Finally, respondents expressed that, as a result of the culture of exclusion, the research infrastructure—for example, time and research personnel—necessary to facilitate inclusion and recruitment of pregnant women in clinical trials is lacking. Whether this represents a true or simply a perceived barrier among physicians is not known. Nonetheless, normalizing the inclusion of pregnant people in clinical trials could help ensure that the required resources are put in place to facilitate participation of pregnant people in both obstetrical and nonobstetrical trials.
Limitations and future directions
Although our survey was specific to Covid‐19 trials, insights provided could be applied to clinical trials of interventions for nonobstetric conditions in general. Our results exposed a need for increased continued medical education about the potential benefits of pregnant people's participation in clinical trials, the harms of their systematic exclusion, and the importance of self‐determination for potential pregnant participants. Such educational activities may contribute to enforcing the cultural change that must occur. To facilitate the inclusion of pregnant people in Covid‐19 and non‐Covid‐19 clinical trials, barriers identified in this study will need to be addressed. While we surveyed only physicians, future research is required to better understand the perspectives of other health care providers and stakeholder groups, including nurses, midwives, industry partners, manufacturers, and sponsoring institutions, about the inclusion of pregnant individuals in clinical trials. Unless clear, strong policies are developed and supported at all levels, pregnant people will continue to be underrepresented in clinical research. Given the design and voluntary nature of the survey, results may not be generalizable to the entire population of physicians. Physicians who self‐selected to respond may express stronger feelings, either in favor of or against the inclusion of pregnant patients in Covid‐19 trials, representing a potential self‐selection bias. 36 Furthermore, only those members of professional societies who previously agreed to respond to surveys were contacted. Quantitative findings should thus be interpreted with caution. Another limitation is that the questionnaire was completed by different groups of physicians at different moments of the pandemic. Therefore, as more information on the possible increased risk of severe manifestations of Covid‐19 in pregnant patients became available, 37 physician attitudes may have evolved. Despite these limitations, we were able to survey a broad, diverse sample of physicians, representative of the physicians who are most likely to care for and enroll pregnant patients with Covid‐19 in clinical trials. While the majority of respondents were obstetricians, this professional group would undoubtedly be involved in the study design and conduct of clinical trials for Covid‐19 involving pregnant participants. In addition, the large number of free‐text answers to open‐ended questions provided valuable insights, which allowed us to identify barriers to the inclusion of pregnant people in clinical trials.
Our results may be used by researchers to inform clinical trial design and strategies to facilitate the enrollment of eligible pregnant people in Covid‐19 research and other emerging pathogens. Ultimately, reducing the exclusion of pregnant people from clinical trials and tailoring trials for pregnant people will lead to better and greater representation of this population in clinical research.
Acknowledgments
We would like to acknowledge Eva Suarthana, from the Department of Obstetrics and Gynecology at McGill University, for her support with the survey validation. We would also like to thank the survey respondents for their time and insights.
Supporting information
The appendices and tables are available in the “Supporting Information” section for the online version of this article and via Ethics & Human Research's “Supporting Information” page: https://www.thehastingscenter.org/supporting-information-ehr/.
Supporting Information
References
- 1. Committee on Ethics of the American College of Obstetricians and Gynecologists , “Ethical Considerations for Including Women as Research Participants. Committee Opinion No. 646,” Obstetrics & Gynecology 126 (2015): 1127–28; Shields, K. E., and A. D. Lyerly, “Exclusion of Pregnant Women from Industry‐Sponsored Clinical Trials,” Obstetrics & Gynecology 122 (2013): 1077–81. [DOI] [PubMed] [Google Scholar]
- 2. Cheng, M. P. , et al., “Generating Randomized Trial Evidence to Optimize Treatment in the COVID‐19 Pandemic,” CMAJ 192, no. 15 (2020): E405–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pastick, K. A. , et al., “A Systematic Review of Treatment and Outcomes of Pregnant Women with COVID‐19—a Call for Clinical Trials,” Open Forum Infectious Diseases 7, no. 9 (2020): doi: 10.1093/ofid/ofaa350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malhamé, I. , D'Souza R., and Cheng M. P., “The Moral Imperative to Include Pregnant Women in Clinical Trials of Interventions for COVID‐19,” Annals of Internal Medicine (2020): doi.org/10.7326/M20-3106. [DOI] [PMC free article] [PubMed]
- 5. Baylis, F. , and Halperin S., “Research Involving Pregnant Women: Trials and Tribulations,” Clinical Investigation 2, no. 2 (2012): 139–46. [Google Scholar]
- 6. Ibid.
- 7. Delahoy, M. J. , et al., “Characteristics and Maternal and Birth Outcomes of Hospitalized Pregnant Women with Laboratory‐Confirmed COVID‐19—COVID‐NET, 13 States, March 1‐August 22, 2020,” Morbidity and Mortality Weekly Report 69 (2020): 1347–54; Hirshberg, A., et al., “Care of Critically Ill Pregnant Patients with COVID‐19: A Case Series,” American Journal of Obstetrics & Gynecology 223, no. 2 (2020): 286–90; Hantoushzadeh, S., et al., “Maternal Death Due to COVID‐19 Disease,” American Journal of Obstetrics & Gynecology 223, no. 1 (2020): doi: 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Members of the Coalition to Advance Maternal Therapeutics to Francis Collins, director, National Institutes of Health and Stephen M. Hahn, commissioner, U.S. Food and Drug Administration , “Re: COVID‐19 and Pregnant Women and Lactating Women,” March 18, 2020, https://s3.amazonaws.com/cdn.smfm.org/media/2268/Final_CAMT_COVID_Letter_March_2020.pdf.
- 9. Van der Zande, I. S. E. , et al., “Fair Inclusion of Pregnant Women in Clinical Research: A Systematic Review of Reported Reasons for Exclusion,” in Clinical Research Involving Pregnant Women, ed. Baylis, F. , and Ballantyne A. (Cham, Switzerland: Springer, 2016), 65–94. [Google Scholar]
- 10. Jaffe, E. , Lyerly A. D., and Goldfarb I. T., “Pregnant Women's Perceptions of Risks and Benefits When Considering Participation in Vaccine Trials,” Vaccine 38 (2020): 6922–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.“LimeSurvey,” LimeSurvey, accessed May 4, 2020, http://www.limesurvey.org.
- 12.“Dedoose Version 8.0.35,” Dedoose, accessed November 5, 2020, at https://www.dedoose.com.
- 13. Cortina, J. M. , “What Is Coefficient Alpha? An Examination of Theory and Applications,” Journal of Applied Psychology 78, no. 1 (1993): 98–104; Taber, K. S., “The Use of Cronbach's Alpha When Developing and Reporting Research Instruments in Science Education,” Research in Science Education 48 (2018): 1273–96. [Google Scholar]
- 14. Committee on Ethics of the American College of Obstetricians and Gynecologists , “Ethical Considerations for Including Women as Research Participants.”
- 15. Ibid.
- 16. Lyerly, A. D. , Little M. O., and Faden R., “The Second Wave: Toward Responsible Inclusion of Pregnant Women in Research,” International Journal of Feminist Approaches to Bioethics 1, no. 2 (2008): 5–22, at 5; see also Committee on Ethics of the American College of Obstetricians and Gynecologists, “Ethical Considerations for Including Women as Research Participants.” [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Committee on Ethics of the American College of Obstetricians and Gynecologists , “Ethical Considerations for Including Women as Research Participants”; Lyerly, Little, and Faden, “The Second Wave.”
- 18. Malhamé, D'Souza, and Cheng, “The Moral Imperative to Include Pregnant Women.” [DOI] [PMC free article] [PubMed]
- 19. Lyerly, Little, and Faden, “The Second Wave.”
- 20.“Prescription Medicine during Pregnancy,” March of Dimes, accessed November 2020, https://www.marchofdimes.org/pregnancy/prescription-medicine-during-pregnancy.aspx#.
- 21. Van der Zande, I. S. E. , et al., “Vulnerability of Pregnant Women in Clinical Research,” Journal of Medical Ethics 43 (2017): 657–63. [DOI] [PubMed] [Google Scholar]
- 22. Committee on Ethics of the American College of Obstetricians and Gynecologists , “Ethical Considerations for Including Women as Research Participants.”
- 23. Ibid.
- 24. Jaffe, Lyerly, and Goldfarb, “Pregnant Women's Perceptions.”
- 25.Ibid.; Lyerly, A. D. , et al., “Women's Views about Participating in Research While Pregnant,” IRB: Ethics & Human Research 34, no. 4 (2012): 1–8. [PubMed] [Google Scholar]
- 26. Jaffe, Lyerly, and Goldfarb, “Pregnant Women's Perceptions”; Lyerly et al., “Women's Views about Participating in Research.”
- 27. Ibid.
- 28. Lyerly et al., “Women's Views about Participating in Research.” [PubMed]
- 29. Blehar, M. C. , et al. “Enrolling Pregnant Women: Issues in Clinical Research,” Women's Health Issues 23, no. 1 (2013): e39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van der Zande et al., “Fair Inclusion of Pregnant Women in Clinical Research”; Blehar et al., “Enrolling Pregnant Women.”
- 31. Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and Social Sciences and Humanities Research Council , Tri‐Council Policy Statement: Ethical Conduct for Research Involving Humans—TCPS (2018), December 2018, https://ethics.gc.ca/eng/tcps2-eptc2_2018_chapter4-chapitre4.html.
- 32. Van der Zande et al., “Fair Inclusion of Pregnant Women in Clinical Research”; Blehar et al., “Enrolling Pregnant Women.”
- 33. Blehar et al., “Enrolling Pregnant Women.”
- 34. Van der Zande et al., “Fair Inclusion of Pregnant Women in Clinical Research”; Mastroianni, A. C., et al., “Research with Pregnant Women: New Insights on Legal Decision‐Making,” Hastings Center Report 47, no. 3 (2017): 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mastroianni et al., “Research with Pregnant Women.”
- 36. Eysenbach, G. , “Improving the Quality of Web Surveys: The Checklist for Reporting Results of Internet E‐Surveys (CHERRIES),” Journal of Medical Internet Research 6, no. 3 (2004): e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ellington, S. , et al., “Characteristics of Women of Reproductive Age with Laboratory‐Confirmed SARS‐CoV‐2 Infection by Pregnancy Status—United States, January 22‐June 7, 2020,” Morbidity and Mortality Weekly Report 69 (2020): 769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The appendices and tables are available in the “Supporting Information” section for the online version of this article and via Ethics & Human Research's “Supporting Information” page: https://www.thehastingscenter.org/supporting-information-ehr/.
Supporting Information


