INTRODUCTION
Mucormycosis, also known as zygomycosis or “black fungus,” is a highly invasive fungal infection that impacts immunocompromised individuals. 1 Its prevalence in developing countries, including but not limited to Iran, Turkey, Chile, and India, 2 , 3 during the coronavirus disease 2019 (COVID‐19) pandemic has been of immense interest. Diabetes and corticosteroid use are known to severely impact outcomes in COVID‐19 patients with mucormycosis. 1 In the COVID‐19 era, the high prevalence of diabetes in countries such as India, poor infection control at the hospital treating COVID‐19 patients, and clandestine use of high‐dose corticosteroids are attributed as possible factors linked with the increasing prevalence of mucormycosis and associated morbidity in developing countries. 4 COVID‐19 patients in the setting of mucormycosis are associated with increased hospital utilization, intensive care unit (ICU) stay, and need for invasive surgical procedures, as well as increased mortality. As such, the prevalence of mucormycosis in COVID‐19 patients is of clinical and public health interest. The objective of this study is to estimate the prevalence of mucormycosis amongst hospitalized COVID‐19, and COVID‐19 among hospitalized mucormycosis patients by performing a systematic review and meta‐analysis.
Our underlying question is as follows: What are the in‐hospital prevalence of mucormycosis in COVID‐19 and mucormycosis in COVID‐19 patients?
MATERIALS AND METHODS
Relevant search terms were explored on PubMed/Medline and Embase. Studies were included based on the following inclusion criteria: (a) patients aged ≥18 years; (b) case series of hospitalized, COVID‐19–positive patients; and (c) groupwise data on COVID‐19 patients with and without mucormycosis or mucormyosis with and without COVID‐19. Relevant data regarding clinical characteristics, management, and treatment were collated. The Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) flowchart in Figure 1 provides details regarding studies included in the systematic review and meta‐analysis. The pooled prevalence of mucormycosis in hospitalized COVID‐19 patients, as well as of COVID‐19 in hospitalized mucormycosis patients were estimated by performing a random‐effects meta‐analysis of proportions obtained from individual studies. Detailed methodology is provided in the Supporting Information. The protocol in this study adheres to the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) Checklist (Supplemental Table 4) and PRISMA‐2020 guidelines (Supplemental Table 5).
FIGURE 1.
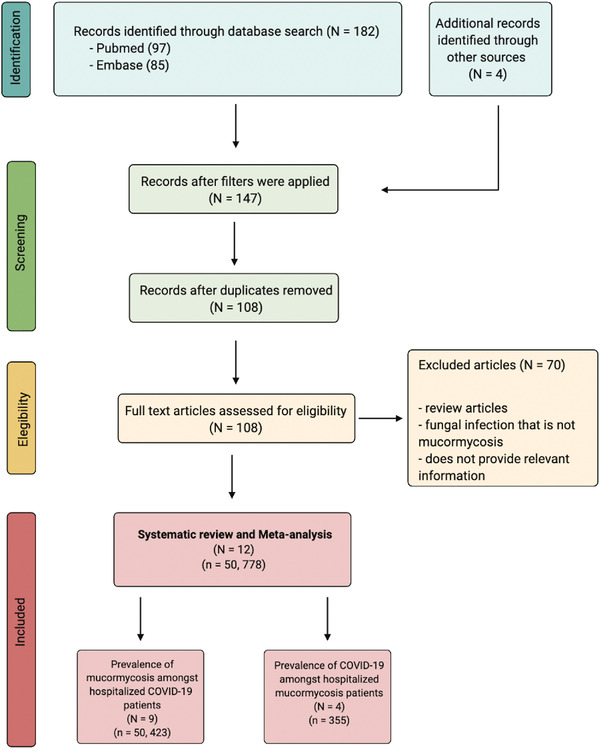
PRISMA flowchart for included studies. Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
RESULTS
Description of included studies
Overall, 12 studies with a cumulative cohort of 50,778 were included in the systematic review and meta‐analysis. Key clinical characteristics and outcomes are summarized (Supplemental Table 1). Of all patients included in the meta‐analysis, 516 were mucormycosis positive and the mean age (reported for 36,759 patients) was 71.2 ± 10.2 years (mean ± SD). The crude prevalence is identical to the estimated pooled prevalence (Figure 2A,B and Supplemental Table 2). The methodological quality assessment and bias analyses are also provided (Supplemental Table 3).
FIGURE 2.
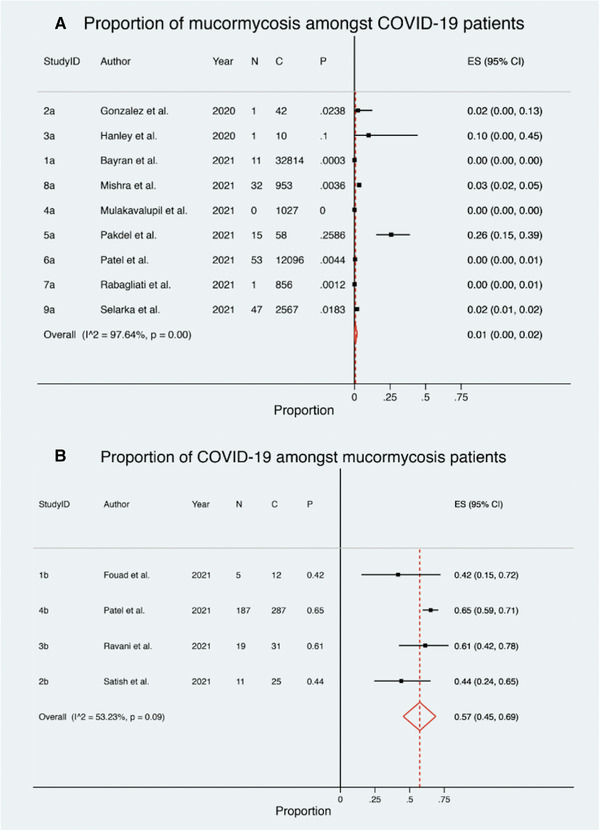
Estimated proportion of (A) mucormycosis among hospitalized COVID‐19 patients and (B) COVID‐19 among hospitalized mucormycosis patients. Abbreviation: COVID‐19, coronavirus disease 2019
Estimated pooled prevalence of mucormycosis among COVID‐19 patients
A total of nine studies, with a cumulative cohort of 50,423 patients, assessed the estimated pooled prevalence of mucormycosis among hospitalized COVID‐19 patients (Figure 2A). The pooled prevalence of mucormycosis among COVID‐19 patients was low and statistically significant (1%; 95% confidence interval [CI], 0% to 2%; p = 0.01). There is considerable heterogeneity among the included case series (I 2 = 97.64%; p ≤ 0.0001).
Estimated pooled prevalence of COVID‐19 among mucormycosis patients
Four case series, with a cumulative cohort of 355 patients, assessed the estimated pooled prevalence of COVID‐19 among hospitalized mucormycosis patients (Figure 2B). The pooled prevalence was high, with over half of the mucormycosis patients being COVID‐19 positive, and statistically significant (57%; 95% CI, 45% to 69%; p ≤ 0.0001). There is moderate to substantial heterogeneity among the included case series (I 2 = 53.2%; p = 0.09).
DISCUSSION
This meta‐analysis demonstrated that there is a low estimated pooled prevalence of mucormycosis among hospitalized COVID‐19 patients (1%). The reports on the incidence of mucormycosis have been of significant interest during the COVID‐19 pandemic compared to previous years. This can be seen in a comparison made by Bayram et al. 5 wherein there were six cases of rhino‐orbital mucormycosis between 2013 and 2016 compared to 11 cases between March and December of 2020. This trend is also supported by results from this meta‐analysis wherein over half of the included patients with mucormycosis were COVID‐19 positive (57%). Previous studies focused on the clinical characteristics of mucormycosis in COVID‐19 patients 2 , 3 ; however, our study reports, to the best of our knowledge presumably for the first time, on the pooled in‐hospital prevalence estimates of mucormycosis in COVID‐19 and COVID‐19 in mucormycosis patients. The story of mucormycosis during the COVID‐19 pandemic is still evolving; this report presents an interim analysis that is currently of significance; however, as evidence accumulates, we postulate this may become more apparent over the coming years once the acute phase of the pandemic has passed through all continents.
The prevalence of mucormycosis is extremely high in developing countries, specifically India, even prior to the COVID‐19 pandemic. 6 , 7 This may be primarily due to the high prevalence of uncontrolled diabetes. 8 , 9 , 10 The results from this systematic review and meta‐analysis must be interpreted with caution. Studies included are of retrospective design and hence subject to high levels of selection bias. There is a high level of heterogeneity amongst the included case series. Further limitation includes the small sample sizes of some of the included studies as well as the varying treatment protocols across different centers. However, mathematical modeling was used to contain the effects of these limitations. The prevalence of and mortality due to mucormycosis in hospitals may not accurately represent cases in the general population as several cases may go undiagnosed and death due to mucormycosis‐related complications after discharge may not be accounted for. This limits the extrapolation of our findings to the general population. Despite the limitations, such meta‐analyses must be performed due to the urgency of the COVID‐19 pandemic and the need for evidence‐based information. For the benefit of readers, clinical characteristics of individual case reports on mucormycosis in COVID‐19 are summarized in Supplemental Table 6.
In conclusion, this study shows that is a low prevalence of mucormycosis among COVID‐19 hospitalized patients whereas over half of the included mucormycosis patients are COVID‐19 positive. COVID‐19 patients are at a high risk of developing a mucormycosis infection. Further studies on the association between COVID‐19 and mucormycosis are warranted.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICS APPROVAL STATEMENT
All analyses were based on previously published studies; thus, no ethical approval and patient consent are required.
AUTHOR CONTRIBUTIONS
Sonu M.M. Bhaskar conceived the study, contributed to the planning, draft, and revision of the manuscript; supervision of the student. Sonu M.M. Bhaskar encouraged Akansha Sinha to investigate and supervised the findings of this work. Akansha Sinha and Sonu M.M. Bhaskar wrote the first draft of this paper. All authors contributed to the revision of the manuscript. All authors approved the final draft of the manuscript.
Supporting information
Supporting Material
ACKNOWLEDGEMENTS
Funding for the NSW Brain Clot Bank (Chief Investigator: Sonu M.M. Bhaskar) from the NSW Ministry of Health (2019‐2022) is acknowledged. The support of the administrative staff and our NSW‐wide partnering clinicians and investigators are also acknowledged. The funding body has no role in the study design, data collection, analysis, interpretation of findings, and manuscript preparation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the affiliated/funding organization/s.
Sinha A, Bhaskar SMM. In‐hospital prevalence of mucormycosis among coronavirus disease 2019 (COVID‐19) patients and COVID‐19 in mucormycosis: a systematic review and meta‐analysis. Int Forum Allergy Rhinol. 2022;12:313–317. 10.1002/alr.22906
Funding information
This work was funded by the NSW Ministry of Health (2019‐2022) to Sonu M.M. Bhaskar.
DATA AVAILABILITY STATEMENT
The original contributions presented in the study are included in the article and Supporting Information; further inquiries can be directed to the corresponding author.
REFERENCES
- 1. Jose A, Singh S, Roychoudhury A, Kholakiya Y, Arya S, Roychoudhury S. Current understanding in the pathophysiology of SARS‐CoV‐2‐associated rhino‐orbito‐cerebral mucormycosis: a comprehensive review. J Maxillofac Oral Surg. 2021;20(3):373–380. 10.1007/s12663-021-01604-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID‐19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15(4):102146. 10.1016/j.dsx.2021.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhattacharyya A, Sarma P, Sharma DJ, et al. Rhino‐orbital‐cerebral‐mucormycosis in COVID‐19: a systematic review. Indian J Pharmacol. 2021;53(4):317–327. 10.4103/ijp.ijp_419_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moorthy A, Gaikwad R, Krishna S, et al. SARS‐CoV‐2, uncontrolled diabetes and corticosteroids‐an unholy trinity in invasive fungal infections of the maxillofacial region? a retrospective, multi‐centric analysis. J Maxillofac Oral Surg. 2021;20(3):1–8. 10.1007/s12663-021-01532-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bayram N, Ozsaygili C, Sav H, et al. Susceptibility of severe COVID‐19 patients to rhino‐orbital mucormycosis fungal infection in different clinical manifestations. Jpn J Ophthalmol. 2021;65(4):515–525. 10.1007/s10384-021-00845-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prakash H, Ghosh AK, Rudramurthy SM, et al. A prospective multicenter study on mucormycosis in India: epidemiology, diagnosis, and treatment. Med Mycol. 2019;57(4):395–402. 10.1093/mmy/myy060 [DOI] [PubMed] [Google Scholar]
- 7. Stone N, Gupta N, Schwartz I. Mucormycosis: time to address this deadly fungal infection. Lancet Microbe. 2021;2(8):e343–e344. 10.1016/s2666-5247 [DOI] [PubMed] [Google Scholar]
- 8. Tandon N, Anjana RM, Mohan V, et al. The increasing burden of diabetes and variations among the states of India: the Global Burden of Disease Study 1990–2016. Lancet Glob Health. 2018;6(12):e1352–e1362. 10.1016/s2214-109x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chakrabarti A, Das A, Sharma A, et al. Ten years' experience in zygomycosis at a tertiary care centre in India. J Infect. 2001;42(4):261–266. 10.1053/jinf.2001.0831 [DOI] [PubMed] [Google Scholar]
- 10. Bala K, Chander J, Handa U, Punia RS, Attri AK. A prospective study of mucormycosis in north India: experience from a tertiary care hospital. Med Mycol. 2015;53(3):248–257. 10.1093/mmy/myu086 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Material
Data Availability Statement
The original contributions presented in the study are included in the article and Supporting Information; further inquiries can be directed to the corresponding author.


