Abstract
Background
Coronavirus disease‐19 (COVID‐19) ranges from asymptomatic infection to severe cases requiring admission to the intensive care unit. Together with supportive therapies (ventilation in particular), the suppression of the pro‐inflammatory state has been a hypothesized target. Pharmacological therapies with corticosteroids and interleukin‐6 (IL‐6) receptor antagonists have reduced mortality. The use of extracorporeal cytokine removal, also known as hemoperfusion (HP), could be a promising non‐pharmacological approach to decrease the pro‐inflammatory state in COVID‐19.
Methods
We conducted a systematic review of PubMed and EMBASE databases in order to summarize the evidence regarding HP therapy in COVID‐19. We included original studies and case series enrolling at least five patients.
Results
We included 11 articles and describe the characteristics of the populations studied from both clinical and biological perspectives. The methodological quality of the included studies was generally low. Only two studies had a control group, one of which included 101 patients in total. The remaining studies had a range between 10 and 50 patients included. There was large variability in the HP techniques implemented and in clinical and biological outcomes reported. Most studies described decreasing levels of IL‐6 after HP treatment.
Conclusion
Our review does not support strong conclusions regarding the role of HP in COVID‐19. Considering the very low level of clinical evidence detected, starting HP therapies in COVID‐19 patients does not seem supported outside of clinical trials. Prospective randomized data are needed.
Keywords: artificial liver, blood purification, Cytosorb, hemoadsorption, inflammation, interleukin‐6, mortality, oXiris, Toraymyxin
Rationale and results of a systematic review on hemoperfusion and blood purification strategies in patients with COVID‐19.

1. INTRODUCTION
Since December 2019, the virus identified as SARS‐CoV‐2 has caused the pandemic of coronavirus disease 2019 (COVID‐19), which has spread worldwide in a sequence of following waves. According to data from Johns Hopkins University, as of September 17th, 2021, there have been over 227 million cases worldwide and over 4.6 million deaths. 1
COVID‐19 ranges from asymptomatic infection to extremely severe cases requiring hospitalization and possibly admission to the intensive care unit (ICU). The most frequent expression of severe COVID‐19 is the occurrence of acute respiratory distress syndrome (ARDS), 2 but the SARS‐CoV‐2 has shown the ability to cause cardiovascular 3 and, eventually, multi‐organ dysfunction. 4 COVID‐19 generates a pro‐inflammatory state with hypothesized cytokine storm, and high levels of interleukin 6 (IL‐6) have been repeatedly observed.
Therefore, together with the attempt to control viral replication, and to provide supportive therapies (with invasive or non‐invasive mechanical ventilation—IMV or NIV, respectively), and eventually with extracorporeal support, 5 the suppression of the pro‐inflammatory state has been a target. 6 , 7 From pharmacological perspectives, the use of corticosteroids and more recently of IL‐6 receptor antagonists (i.e., tocilizumab) has shown improvement of prognosis for patients experiencing severe COVID‐19. 8 , 9 A possible non‐pharmacological approach to limit the pro‐inflammatory state induced by severe COVID‐19 is the use of extracorporeal cytokine removal, also known as hemoperfusion (HP). Extracorporeal approaches include plasma exchange, direct HP on a polymyxin B‐immobilized fiber column (PMX‐DHP), continuous hemodiafiltration with Cytosorb adsorber, and several other methods. These strategies have been previously investigated in other critical illnesses, such as septic shock, ARDS, and also for cases of Middle East Respiratory Syndrome due to coronavirus infection; however, there is no evidence of beneficial effects in these settings.
The use of HP in patients with severe COVID‐19 is pathophysiologically sounded 10 and aims at interrupting the vicious pro‐inflammatory circle and the associated coagulopathy, endothelial damage, and organ failure. There have been increasing reports of the beneficial effects of such treatment among ICU patients with severe COVID‐19, but different methods have been used, 11 , 12 and several platforms/consoles have been modified to host these HP filters. 13
In order to summarize the evidence regarding the use of HP strategies for cytokine removal, we systematically reviewed the existing literature that evaluates the application of different HP strategies in patients with COVID‐19. Our aim was to gather information on biochemical and clinical outcomes described by the authors. From the overview of these outcomes it might be possible to acquire information that could be considered for future prospective studies.
2. METHODS
2.1. Registration, search strategy, and criteria
We undertook a systematic Web‐based advanced literature search through the NHS Library Evidence tool on studies reporting the use of HP in patients with COVID‐19. We followed the approach suggested by the PRISMA statement for reporting systematic reviews and meta‐analyses, and a PRISMA checklist is provided separately (Supporting Digital Content 1). The protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) with assigned number CRD42021253676. Our core search was structured by combining the findings from two groups of terms. The first group included the following: “blood purification” OR “Cytosorb” OR “Cytokine adsor*” OR “Toraymyxin” OR “endotoxin” OR “polymyxin” OR “hemoperfusion”; the second group contained the terms “covid” OR “COVID‐19”.
An initial computerized search of PubMed and EMBASE databases was conducted from inception until February 4th, 2021, to identify the relevant articles, and a draft of results was written. The update of these systematic searches was performed on May 11th, 2021. Two further searches were performed manually and independently by four authors (GM, CP, GC, GDM), also exploring the list of references of the findings of the systematic search.
Inclusion criteria were pre‐specified according to the PICOS approach (Table 1). We excluded experimental and animal studies, reviews, editorials, and letters to editor. Case series were included if involving at least 5 patients. We preventively decided to describe as Supporting Information case series with fewer than 5 patients, and case reports. Language restrictions were applied: we read the full manuscript only for articles published in English.
TABLE 1.
PICOS criteria
| PICOS criteria | |
|---|---|
| Population | Patients with COVID‐19 |
| Intervention | Patients treated with HP strategy for cytokine removal |
| Comparison | None or treated with standard of care but not receiving HP |
| Outcome(s) | Clinical and biological outcomes |
| Study design | Prospective and retrospective studies; case series reporting at least 5 patients |
Abbreviations: COVID‐19, coronavirus disease 19; HP, hemoperfusion.
2.2. Study screening and selection
Study screening for determining the eligibility for inclusion in the systematic review and data extraction were performed independently by four reviewers (FS, GC, GDM, CP). Discordances were resolved involving two senior authors (AA, MA).
Despite an expected significant heterogeneity in the techniques of blood purification, as per protocol registration on PROSPERO, we decided to include in our systematic review all the HP strategies adopted to reduce cytokines and pro‐inflammatory molecules in the context of COVID‐19. In particular, we included artificial liver support (ALS, already applied in cases of influenza A, H7N9 14 ), CytoSorb and HA 440/380/280/230 (which can be used as standalone or placed in series with extracorporeal membrane oxygenation and hemodialysis circuit), Toraymyxin, oXiris, and plasmapheresis. Other “standard” blood purification strategies, such as hemodialysis and continuous renal replacement therapy were not included as our focus was to investigate blood purification strategies aimed at reducing cytokine levels.
Data were inserted in a password protected database on Excel by three authors (GC, GDM, CP) and cross‐checked by other three authors (FS, GM, LLV).
2.3. Analysis of clinical outcomes
From a clinical standpoint, we focused on the description of population characteristics for each study, reporting their hemodynamic support (dose and/or percentage of patients receiving vasopressors), the respiratory variables (oxygenation parameters as well as use of IMV or NIV), the use of other extracorporeal techniques and, finally, the admission to the ICU, the length of stay, and mortality. Whenever possible, information is provided with separation of data before and after the HP treatment.
2.4. Biological variables
Regarding the effects from biological perspectives, we reported data on inflammatory markers and on cytokines levels. As for clinical variables, information is provided with separation of data before and after the HP treatment. We also added information on drug therapies with particular reference to the use of steroids, IL‐6 receptor antagonists, and antibiotics.
3. RESULTS
Our systematic search identified 292 total findings between Pubmed and EMBASE. No further findings were retrieved manually. As shown in the PRISMA flow diagram (Figure 1), after the evaluation of all abstracts, 11 full‐text articles 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 were included as matching the PICOS criteria, and their characteristics from clinical and biological perspectives are reported in Tables 2 and 3, respectively.
FIGURE 1.
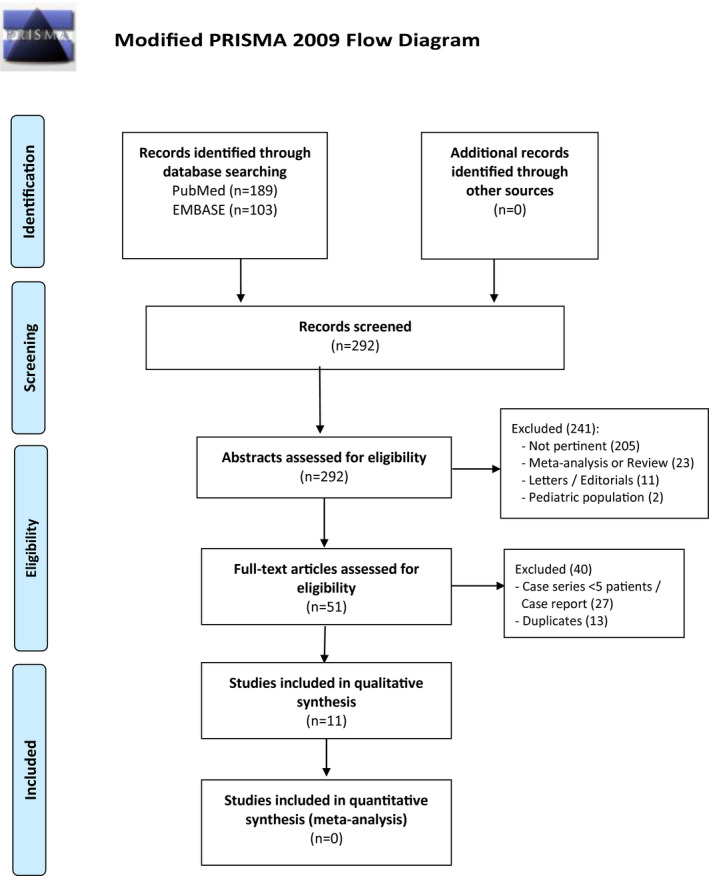
PRISMA flow diagram
TABLE 2.
Clinical data of the studies included in the systematic review
| First author, Journal | Design | M/F ratio | RRT | Vasopressor dose | PaO2/FiO2 | ICU admission |
|---|---|---|---|---|---|---|
| HP strategy (n=) | Age | ECMO | Ventilation strategy | Length of stay (days) | ||
| Mortality (timing) | ||||||
| Villa et al, Critical Care 2020 | Prospective non randomized | 31/6 | 100% | Pre: Vasoactive 65% VIS 10 (22) | PaO2/FiO2 Pre 119 ± 42 at 72 h 168 ± 47 | 100% |
| OXIRIS (37) | 59 ± 9 y | – | Post 72 h: Vasoactive 23% VIS 0 | – | – | |
| 56% (n/r) | ||||||
| Ugurov et al, Braz J Cardiovasc Surg 2020 | Case series | 13/2 | 100% | – | – | 100% |
| OXIRIS (15) | 60 ± 13 y | – | Pre: MV 7% | ICU: 10 + 2 | ||
| Post: MV 13% | 36% (n/r) | |||||
| Dai et al, Artif Organs 2020 | Case‐control multicenter, prospective | ALS:40/10 | – | – | – | – |
| ALS (50 treated + 51 controls) | 60 ± 13 y | – | – | – | ||
| C: 35/16 | ALS 16%; Controls 51% (28 days) | |||||
| 60 ± 15 y | Subgroup of patients (30) in early cytokine storm: ALS: 15/15 improved (discharged without MV); controls 6/15 progressed to severe disease and died | |||||
| Guo et al, Frontiers Immunology 2020 | Case series | 10/2 | – | Pre: Vasoactive 42% (dose not specified) | PaO2/FiO2 Pre 197 ± 51 | 100% |
| ALS (12) | 62 ± 14 y | 25% | Post 230 ± 88 | – | ||
| MV 58% | – | |||||
| Alharty et al, Artif Organs 2021 | Case series | 39/11 | 100% | NE infusion baseline 0.9 ± 0.2 μg/kg/min | PaO2/FiO2 survivors | 100% |
| CytoSorb (50) | 50 ± 9 y | 8% | 35 survivors: after 2 ± 1 sessions of HP, vasopressors were off | Pre: 113 ± 34, post 303 ± 37 | ICU: 21 ± 9 | |
| PaO2/FiO2 non‐survivor | 30% (28 days) | |||||
| Pre: 127 ± 41, post 83 ± 21 | ||||||
| MV 100% (duration MV 17 ± 7 days) | ||||||
| Rampino et al, Blood Purif 2020 | Case‐control | Treated | – | – | PaO2/FiO2 | 100% |
| Cytosorb (5 treated + 4 controls) | 5/0 | – | Pre: Treated 260 ± 52 Controls 165 ± 15 | – | ||
| 58 ± 4 y | Post PaO2/FiO2 improved in treated | Treated 20% Controls 100% (2 months; all survivors were discharged from hospital) | ||||
| Controls | Pre: NIV 100% | |||||
| 3/1 | Post: MV Treated 40% Controls 100% | |||||
| 66 ± 2 y | ||||||
| Hashemian et al, Pulmonology 2020 | Case series | 9/6 | – | – | PaO2/FiO2 Pre 184 ± 56 | 100% |
| Case series | 58 ± 12 y | – | Post 224 ± 57 | ICU: 10 ± 2 | ||
| Pre: MV 26% NIV 74% | 40% (n/r) | |||||
| Post: MV 40% NIV 60% | ||||||
| De Rosa et al, Artif Organs 2020 | Case series (EUPHAS II) | 9/3 | 75% | VDI baseline: 29 (15) | – | 100% |
| Toramixyn (12) | 60 ± 10 y | – | VDI 120 h: 0 (4) | MV 100% | ICU: 26 Hospital: 31 | |
| 50% (28 days); 58% (hospital discharge) | ||||||
| Katagiri et al, Clin Apher 2020 | Case series | 9/3 | 8% | – | PaO2/FiO2 Pre 154 | – |
| Toramixyn (12) | 66 (47) y | 16% | At 96h 214 | – | ||
| MV 42% | 25% (n/r) | |||||
| Hashemian et al, Tanaffos. 2020 Dec | Case‐control | HA380: 2/0 | 83% | NE infusion (mcg/kg/min) | – | 100% |
| HA380 (2) | 54 ± 23 y | – | HA380 pre 2.5 ± 0.1 post 1.5 ± 1.5 | MV 100% | ICU: HA380 7 ± 2; HA ± RRT 8 ± 2; RRT 7 ± 1; 66% (n/r; 50% HA380, 50% HA + RRT, 100% RRT) | |
| HA380+RRT (6) RRT (4) | HA+RRT: 5/1 | HA+RRT pre 2.5 ± 0.0 post 1.0 ± 0.8 | ||||
| 54 ± 12 y | RRT pre 2.4 ± 0.1 post 2.6 ± 0.3 | |||||
| RRT: 2/2 | ||||||
| 48 ± 5 y | ||||||
| Asgharpour et al, BMC Nephrology 2020 | Case series | 5/5 | 100% | – | – | 100% |
| HA280 (5) + HA230 (5) | 57 ± 18 y | – | – | – | ||
| – | 40% (n/r) |
Abbreviations: ALS, artificial‐liver blood‐purification system; ECMO, extracorporeal membrane oxygenation; HA, hemoadsorption; HP, hemoperfusion; ICU, intensive care unit; M/F, male/female; MV, mechanical ventilation; NE, norepinephrine; NIV, non‐invasive ventilation; RRT, renal replacement therapy; VDI, vasopressor dependency index; VIS, Vasoactive Inotropic Score.
TABLE 3.
Biochemical data of the studies included in the systematic review
| First author, Journal | N | Drug therapy (%) | Inflammatory markers | Cytokines data |
|---|---|---|---|---|
| Villa et al, Critical Care 2020 | 37 | Antibiotics (100%), HCQ (100%), antivirals (24%), anti IL6 receptor antagonist (40%) | CRP (mg/L) pre 200 (176) at 72 h 128 (122) | IL‐6 (ng/L) pre 1230 (895) at 72 h 160 (141) |
| OXIRIS | Ferritin (ng/ml) pre 12 ± 6 at 72 h 2033 ± 2855 | Reduction in IL‐6 correlated with decrease in SOFA score | ||
| Ugurov et al, Braz J Cardiovasc Surg. 2020 | 15 | Antibiotics (100%), anti IL6 receptor antagonist (6%) | CRP (mg/L) pre 109 (73) | Decreasing levels of IL‐6, IL‐8 and TNF‐α |
| OXIRIS | ||||
| Dai et al, Artif Organs 2020 | 101 | – | – | IL‐6 (pg/ml) ALS pre 120 post 20 |
| 50 ALS +51 Control | Control pre 40 post 51 | |||
| Guo et al, Frontiers in Immunology 2020 | 12 | Steroids (100%), antibiotics (92%), anti IL‐6 receptor antagonist (87%) | – | 32 cytokines (including IL‐6 and TNF‐α) out of 34 dosed were significantly decreased after each ALS course |
| ALS | ||||
| Alharthy et al, Artif Organs 2021 | 50 | Antibiotics (100%), steroids (100%), antivirals (100%), interferon (100%) | CRP (mg/L) | IL6 (pg/ml) |
| Survivors: pre 145 ± 98 post 44 ± 26 | Survivors: pre 613 ± 186 post 170 ± 78 | |||
| Non‐survivors: pre 128 ± 69 post 144 ± 98 | Non survivors: pre 722 ± 507 post 1253 ± 859 | |||
| Cytosorb | Ferritin (ng/ml) | |||
| Survivors: pre602±142 post 296 ± 63 | ||||
| Non‐survivors: pre 358 ± 175 post 729 ± 163 | ||||
| Rampino et al, Blood Purif 2020 | 9 | Steroids (88%), antibiotics (77%), HCQ (77%), antiviral (55%), anti IL6 receptor antagonist (11%) | CRP decreased in Cytosorb series (not specified) | IL6, IL8 TNFa reduced in Cyto Sorb group (not specified) |
| 5 Cytosorb | ||||
| 4 Control | ||||
| Hashemian SM et al, Pulmonology 2020 | 15 | Antivirals, antibiotics | CRP (mg/dl) pre 47 ± 18 post 28 ± 20 | IL‐6 (pg/ml) pre 8 ± 2 post 6 ± 1 |
| Plasmapheresis | Ferritin (ng/ml) pre 1027 ± 397 post 654 ± 320 | TNF‐α (pg/ml) pre 9 ± 4 post 4 ± 1 | ||
| De Rosa et al, Artif Organs 2020 | 12 | Antivirals (100%), steroids (100%), anti‐IL6 receptor antagonist (50%), HCQ (42%) | – | Data on endotoxin activity |
| Toramixyn | Pre: 0.8 [0.7‐0.9] at 120 h: 0.6 [0.4‐0.7] | |||
| Katagiri et al, Clin Apher. 2020 | 12 | Antivirals, steroids | – | Except for one non‐survivor, IL‐8, IL‐10, and IL‐17 levels remained almost unchanged or trended downward |
| Toramixyn | ||||
| Hashemian et al, Tanaffos 2020 Dec | 12 | Antivirals (100%), HCQ (100%), anti IL6 receptor antagonist (patients with direct kidney involvement and a high level of IL‐6) | IL‐1, pg/ml | |
| HA380 (2) | HA380: pre 75 ± 14 post 40 ± 6 | |||
| HA380+RRT (6) RRT (4) | RRT : pre 70 ± 8 post 44 ± 6 | |||
| HA380+RRT: pre 58 ± 9 post 36 ± 4 | ||||
| IL‐6, pg/ml | ||||
| HA380 : pre 245 ± 92 post 160 ± 70 | ||||
| RRT : pre 229 ± 24 post 164 ± 29 | ||||
| HA380+RRT: pre 248 ± 58 post 154 ± 31 | ||||
| IL‐8, pg/Ml (HP, CRRT, HP+CRRT) | ||||
| HA380: pre 742 ± 11 post 383 ± 61 | ||||
| RRT : pre 830 ± 126 post 489 ± 57 | ||||
| HA380+RRT: pre 805 ± 72 post 431 ± 56 | ||||
| Asgharpour et al, BMC Nephrology 2020 | 10 | Antibiotics (100%), antivirals (100%), HCQ (90%) | CRP (mg/L) pre 136 ± 84 post 78 ± 39 | IL‐6 (ng/ml) pre 140 ± 106 post 72 ± 66 |
| 5 HA280 + 5 HA230 |
Abbreviations: ALS, artificial‐liver blood‐purification system; CRP, reactive protein; HA, hemoadsorption; HCQ, hydroxychloroquine; HP, hemoperfusion; IL, interleukin; RRT, renal replacement therapy; SOFA, Sequential Organ Failure Assessment; TNF, tumor necrosis factor.
As shown in Tables 2 and 3, we found that several HP techniques have been implemented, and that in most cases authors have reported large case series, while we found only three studies with a control group and no prospective randomized study; therefore, any chance to perform a meta‐analysis was deemed not feasible.
In total, 226 patients were identified by our systematic review, while another 59 patients functioned as controls in some of the included studies. As shown in Tables 2 and 3, different HP strategies were implemented. Of the patients receiving HP therapy, the most frequently used approaches were ALS (n = 62), CytoSorb (n = 55) and oXiris (n = 52), followed by Toraymyxin (n = 24), HA 380/280/230 (n = 18) and plasmapheresis (n = 15).
With regard to other non‐pharmacological extracorporeal therapies implemented in this population of patients treated with HP, we found that continuous renal replacement therapy was used in 128 patients (57%), while extracorporeal membrane oxygenation in only 9 patients (4%). The mortality in the included studies varied from 16% 17 to 58%. 18
The largest study with a control group included 101 patients, 50 of whom were treated with HP strategy with ALS, the others functioning as controls. None of the included patients needed continuous renal replacement therapy. 19 The second one was a three‐arm study, with patients receiving HP, continuous renal replacement therapy, or both; in this study the use of HP (±RRT; n = 8) was effective in reducing the norepinephrine infusion compared to the group of patients receiving only RRT (n = 4). Moreover, mortality was halved in those receiving HP versus continuous renal replacement therapy only. 20 The last study was a case‐control study of nine patients, 5 treated with Cytosorb HP (survival 80%), and 4 serving as controls (no survivors); none of these patients received continuous renal replacement therapy. 23 The other included studies reported a variable number of patients treated with any HP strategy (range 10 to 50 patients).
From biological perspectives, most studies found decreasing levels of IL‐6. Considering the studies with a control group, Dai et al 26 found that IL‐6 levels decreased significantly in the ALS groups, while this was not the case in controls. Interestingly, the authors analyzed two subgroups of 15 patients each who were deemed in the early stage of “cytokine storm”. In the early sub‐group treated with ALS, all patients improved and were discharged without need for intubation; conversely, 40% (n = 6/15) of patients in the control group at the early stage of “cytokine storm” progressed to critical illness, and died. The small case‐control series was produced by Rampino et al, 23 and from biological perspectives the authors showed that IL‐6, and IL‐8 TNF‐a were reduced in the Cytosorb group. However, it is difficult to draw conclusions not only for the small number of patients, but also because patients in the Cytosorb group were on average 8 years younger.
Further 27 findings were identified as small case series (2–4 patients, n = 6) and case reports (n = 21), and are reported in Supporting Digital Content 1 for their clinical and biological data.
4. DISCUSSION
The potential usefulness of cytokine adsorption therapies for patients with COVID‐19 has been hypothesized because of the dysregulated systemic immune over‐activation. 27 Initially, scientists suspected that Covid cytokine storm in patients with COVID‐19 was stronger than in other conditions determining critical illness, though this hypothesis has not been subsequently confirmed 28 ; moreover, studies on sub‐phenotypes have shown that the systemic concentrations of inflammatory cytokines typical of the “cytokine storms” are lower in COVID‐19 than in patients with other causes of ARDS. 29 Nonetheless, it should be noted that the only drugs decreasing mortality in COVID‐19 patients act with properties of immune‐modulation (corticosteroids and IL‐6 receptor antagonists). 8 , 30 At the beginning of the pandemic a comprehensive review highlighted the potential importance of HP techniques and the lack of evidence in support of this approach. One year later, the scenario has not improved, but these therapeutic options are used in daily practice. 31 The aim of our systematic review was to pool data on HP therapies in patients with COVID‐19, trying to gather information on their clinical and immune‐modulator effects. The evaluation of a complex therapy (HP) in a disease with a very variable clinical presentation (COVID‐19) certainly warrants strict control for confounding factors. However, our systematic review identified only studies with low methodological quality (no randomized study was found); we summarized the most adopted biological outcomes tested in the current literature with the hope this could be useful for future application (i.e., design of randomized studies).
In general, we found large heterogeneity among the studies in clinical and biological outcomes evaluated. From clinical perspectives, information on cardiovascular pharmacological support was not uniformly reported, while more data were provided on oxygenation and on use of IMV or NIV. For instance, Villa et al 25 and Guo et al 19 reported an improvement of the PaO2/FiO2 ratio, but the lack of a control group hampers any discussion. Regarding the biological outcomes, surely the most reported one was the IL‐6 concentration. 32 Most studies reported decreasing levels of IL‐6 after HP treatment, but it should be noted that the values reported were very different, possibly as a result of variable laboratory methods and techniques, as well as timing in the course of the disease. Moreover, when evaluating a decrease in cytokine concentration, one should note that removal has to be contextualized to the initial cytokine concentration. Indeed, cytokine removal is concentration‐dependent, and the baseline values influences the performance of the HP method.
The cut‐off to define high level of circulating cytokines is not well‐defined, but it is reasonable that the higher the level, the greater the impact (hopefully positive) of the HP therapy. 33 Moreover, the HP methods not only eliminate the cytokines responsible for the hyper‐inflammatory state, but HP will likely remove anti‐inflammatory mediators and many other biological substances as well (up to 55 kDa). The latter are probably not innocent bystanders, but may be crucial for the recovery of the patient. Therefore, focusing only on one or few cytokines may be a misleading and myopic approach. Future studies should also consider the removal of anti‐inflammatory and other biologically relevant molecules eliminated by such “filters.” To add more complexity, knowledge of pharmacokinetics during HP is still on the way, and a potential issue could be the reduction in plasmatic concentration of circulating drugs like corticosteroids and other immune‐modulators, as well as a decrease in the concentration of antibiotics. 34 Moreover, COVID‐19 has shown a tendency to cause coagulation disorders with a pro‐thrombotic state and an associated risk of pulmonary embolism. Whether HP therapies influence the pharmacokinetics of drugs regulating the coagulation cascade in patients with COVID‐19 remains to be determined. This should certainly be considered in the context of a higher risk of pulmonary embolism in these patients. 35 , 36
All these open questions and the absence of good‐quality data make the evaluation of HP usefulness in COVID‐19 patients challenging. For all these reasons, the Extracorporeal Life Support Organization's COVID‐19 guidelines do not currently recommend extracorporeal cytokine HP outside the context of clinical trials. 37 It is worth mentioning that after our screening process one important study was published. This small single‐center randomized study enrolled 34 COVID‐19 patients on extracorporeal membrane oxygenation and randomized them to HP with Cytosorb. Interestingly, the concentration of IL‐6 decreased in both groups (HP and controls) to a similar extent, and the 30‐day mortality was significantly higher in the group receiving HP (18% vs. 76% in those not receiving HP, p = 0.002). 22 These results need external validation by ongoing trials, and it should be noted that patients receiving extracorporeal membrane oxygenation are at very high risk of death, and that HP may not be beneficial in these cases where a very advanced stage of organ dysfunction has already taken place. Therefore, the latter results do not exclude a beneficial effect of HP strategies in severe COVID‐19 patient not requiring extracorporeal membrane oxygenation.
It is also worth noting the recent results of another interesting study (not on COVID‐19 patients) in which the authors studied patients with severe refractory septic shock undergoing cytokine removal with CytoSorb, with ongoing continuous renal replacement therapy, and matched them with a historical cohort. The authors found that IL‐6 levels and vasopressor requirements were not reduced in the treatment group. Importantly, patients treated with HP had an increased risk of death. The authors concluded that their results were consonant with recent evidence that suggests avoidance of indiscriminate use of cytokine adsorption outside of investigational trials. 38
5. CONCLUSION
Our systematic review identified several studies that evaluate the role of different HP strategies in COVID‐19. However, all these studies were of low methodological quality, and only a few had a control group. Considering the very low level of clinical evidence reported so far, starting HP therapies in COVID‐19 patients does not seem to be supported by hard evidence. Prospective randomized data are needed to establish the role of HP in COVID‐19 patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Concept/design: Filippo Sanfilippo, Antonio Arcadipane. Data analysis/interpretation: Carla Pulizzi and Filippo Sanfilippo. Drafting article: Gennaro Martucci. Critical revision of article: Luigi La Via and Gennaro Martucci. Approval of article: Marinella Astuto. Data collection: Giorgio Dimarco and Giuseppe Cuttone.
Supporting information
Supplementary Material
ACKNOWLEDGMENT
We thank Mr. Warren Blumberg for the English editing on our manuscript.
Sanfilippo F, Martucci G, La Via L, Cuttone G, Dimarco G, Pulizzi C, et al. Hemoperfusion and blood purification strategies in patients with COVID‐19: A systematic review. Artif. Organs. 2021;45:1466–1476. 10.1111/aor.14078
Filippo Sanfilippo and Gennaro Martucci contributed equally to this study.
REFERENCES
- 1. Center for Systems Science and Engineering at Johns Hopkins University . COVID‐19 map. [cited 2021 Sep 17]. Available from: https://coronavirus.jhu.edu/map.html [Google Scholar]
- 2. Grasselli G, Tonetti T, Protti A, Langer T, Girardis M, Bellani G, et al. Pathophysiology of COVID‐19‐associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020;8:1201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Messina A, Sanfilippo F, Milani A, Calabrò L, Negri K, Monge García MI, et al. COVID‐19‐related echocardiographic patterns of cardiovascular dysfunction in critically ill patients: a systematic review of the current literature. J Crit Care. 2021;65:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kowalewski M, Fina D, Słomka A, Raffa GM, Martucci G, Lo Coco V, et al. COVID‐19 and ECMO: the interplay between coagulation and inflammation‐a narrative review. Crit Care. 2020;24:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID‐19: a retrospective cohort study. Lancet Respir Med. 2020;8:1121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L. Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. RECOVERY Collaborative Group . Tocilizumab in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. Lancet. 2021;397:1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bozzi G, Mangioni D, Minoia F, Aliberti S, Grasselli G, Barbetta L, et al. Anakinra combined with methylprednisolone in patients with severe COVID‐19 pneumonia and hyperinflammation: an observational cohort study. J Allergy Clin Immunol. 2021;147:561–6.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gruda MC, Ruggeberg K‐G, O’Sullivan P, Guliashvili T, Scheirer AR, Golobish TD, et al. Broad adsorption of sepsis‐related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb® sorbent porous polymer beads. PLoS ONE. 2018;13:e0191676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brouwer WP, Duran S, Kuijper M, Ince C. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28‐day all‐cause mortality in ICU patients with septic shock: a propensity‐score‐weighted retrospective study. Crit Care. 2019;23:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tachikawa R, Tomii K, Murase K, Ueda H, Harada Y, Kida Y, et al. Therapeutic effect of direct hemoperfusion with a polymyxin B‐immobilized fiber column in the treatment of HIV‐negative severe pneumocystis pneumonia. Respiration. 2011;81:318–24. [DOI] [PubMed] [Google Scholar]
- 14. Yu WQ, Ding MD, Dai GH, Gu CJ, Xue L, Chen Y, et al. Analysis of 15 cases of avian influenza virus (H7N9) infection. Intensive Care Med. 2018;41:534–8. [DOI] [PubMed] [Google Scholar]
- 15. Alharthy A, Faqihi F, Memish ZA, Balhamar A, Nasim N, Shahzad A, et al. Continuous renal replacement therapy with the addition of CytoSorb cartridge in critically ill patients with COVID‐19 plus acute kidney injury: a case‐series. Artif Organs. 2021;45:E101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Asgharpour M, Mehdinezhad H, Bayani M, Zavareh MSH, Hamidi SH, Akbari R, et al. Effectiveness of extracorporeal blood purification (hemoadsorption) in patients with severe coronavirus disease 2019 (COVID‐19). BMC Nephrol. 2020;21:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dai X, Zhang Y, Yu L, Jiang YA, Chen L, Chen Y, et al. Effect of artificial liver blood purification treatment on the survival of critical ill COVID‐19 patients. Artif Organs. 2021;45:762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Rosa S, Cutuli SL, Ferrer R, Antonelli M, Ronco C. Polymyxin B hemoperfusion in coronavirus disease 2019 patients with endotoxic shock: case series from EUPHAS2 registry. Artif Organs. 2021;45:E187–94. [DOI] [PubMed] [Google Scholar]
- 19. Guo J, Xia H, Wang S, Yu L, Zhang H, Chen J, et al. The artificial‐liver blood‐purification system can effectively improve hypercytokinemia for COVID‐19. Artif Organs. 2020;11:586073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hashemian SM, Shafigh N, Afzal G, Jamaati H, Mortaz E, Tabarsi P, et al. Blood purification techniques, inflammatory mediators and mortality in COVID‐19 patients. Tanaffos. 2020;19:291–9. [PMC free article] [PubMed] [Google Scholar]
- 21. Hashemian SM, Shafigh N, Afzal G, Jamaati H, Tabarsi P, Marjani M, et al. Plasmapheresis reduces cytokine and immune cell levels in COVID‐19 patients with acute respiratory distress syndrome (ARDS). Pulmonology. 2020;20:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Katagiri D, Ishikane M, Asai Y, Izumi S, Takasaki J, Katsuoka H, et al. Direct hemoperfusion using a polymyxin B‐immobilized polystyrene column for COVID‐19. J Clin Apheresis. 2021;36:313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rampino T, Gregorini M, Perotti L, Ferrari F, Pattonieri EF, Grignano MA, et al. Hemoperfusion with CytoSorb as adjuvant therapy in critically ill patients with SARS‐CoV2 pneumonia. Artif Organs. 2021;50:566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ugurov P, Popevski D, Gramosli T, Neziri D, Vuckova D, Gjorgon M, et al. Early initiation of extracorporeal blood purification using the AN69ST (oXiris®) hemofilter as a treatment modality for COVID‐19 patients: a single‐centre case series. Braz J Cardiovasc Surg. 2020;1:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Villa G, Romagnoli S, De Rosa S, Greco M, Resta M, Pomarè Montin D, et al. Blood purification therapy with a hemodiafilter featuring enhanced adsorptive properties for cytokine removal in patients presenting COVID‐19: a pilot study. Crit Care. 2020;24:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dai X, Zhang Y, Yu L, Jiang Y‐A, Chen L, Chen YE, et al. Effect of artificial liver blood purification treatment on the survival of critical ill COVID‐19 patients. Artif Organs. 2021;45:762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383:2255–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, Calfee CS, et al. Cytokine elevation in severe and critical COVID‐19: a rapid systematic review, meta‐analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8:1233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sinha P, Calfee CS, Cherian S, Brealey D, Cutler S, King C, et al. Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID‐19: a prospective observational study. Lancet Respir Med. 2020;8:1209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gordon AC, Mouncey PR, Al‐Beidh F, Rowan KM, Nichol AD, Arabi YM, et al. Interleukin‐6 receptor antagonists in critically ill patients with Covid‐19. N Engl J Med. 2021;384:1491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Swol J, Lorusso R. Additive treatment considerations in COVID‐19‐the clinician's perspective on extracorporeal adjunctive purification techniques. Artif Organs. 2020;44:918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Supady A, Weber E, Rieder M, Lother A, Niklaus T, Zahn T, et al. Cytokine adsorption in patients with severe COVID‐19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open‐label, randomised, controlled trial. Lancet Respir Med. 2021;9:755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Friesecke S, Träger K, Schittek GA, Molnar Z, Bach F, Kogelmann K, et al. International registry on the use of the CytoSorb® adsorber in ICU patients: study protocol and preliminary results. Med Klin Intensivmed Notfallmed. 2019;114:699–707. [DOI] [PubMed] [Google Scholar]
- 34. Zoller M, Döbbeler G, Maier B, Vogeser M, Frey L, Zander J. Can cytokine adsorber treatment affect antibiotic concentrations? A case report. J Antimicrob Chemother. 2015;70:2169–71. [DOI] [PubMed] [Google Scholar]
- 35. Sanfilippo F, La Rosa V, Astuto M. Micro‐thrombosis, perfusion defects, and worsening oxygenation in COVID‐19 patients: a word of caution on the use of convalescent plasma. Mayo Clin Proc. 2021;96:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sanfilippo F, La Rosa V, Oliveri F, Astuto M. Convalescent plasma for COVID‐19: the risk of pulmonary embolism should not be underestimated! Crit Care. 2020;24:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Badulak J, Antonini MV, Stead CM, Shekerdemian L, Raman L, Paden ML, et al. Extracorporeal membrane oxygenation for COVID‐19: updated 2021 guidelines from the extracorporeal life support organization. ASAIO J. 2021;67:485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wendel Garcia PD, Hilty MP, Held U, Kleinert E‐M, Maggiorini M. Cytokine adsorption in severe, refractory septic shock. Artif Organs. 2021;1:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


