Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is the virus responsible for coronavirus disease 2019 (COVID‐19), which manifests as a flu‐like respiratory infection affecting multiple organ systems, including the gastrointestinal system, central nervous system, cardiovascular system, skin, and mucosa. In this review, we investigated the literature on specific manifestations of COVID‐19 in the oral mucosa. An online literature search in PubMed, Scopus, Google Scholar, and Medline was conducted to retrieve relevant studies on confirmed COVID‐19 patients with oral mucosa findings published between December 31, 2019, and April 07, 2021. After an independent review by two authors, 39 articles considering 59 laboratory‐confirmed cases of SARS‐CoV‐2 infection were included in the final analysis. The most common finding, reported in 29 patients (43.9%), was Kawasaki‐like syndrome. In addition, oral ulcers including aphthous, hemorrhagic, and necrotic ulcers were reported in 24 patients (36.3%). Other lesions reported included pustules, macules, bullae, maculopapular enanthema, and erythema multiforme‐like lesions. Concomitant skin lesions were present in 60.6% of patients. Fever was reported in 86.2% of patients. Forty‐eight patients (76.1%) were hospitalized. Loss of taste and smell was present in 30.8% of the patients. A comprehensive understanding of the dermatologic manifestations of COVID‐19 can improve and facilitate patient management and referrals.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a novel virus that was observed initially as a cluster of cases with pneumonia in December 2019 in Wuhan, China. 1 The fatality rate is reported as between 2% and 4% in all age groups, but it increases with advanced age and the presence of comorbid conditions. 2 Erythema with vesicles or pustules (pseudo‐chilblain) in acral areas, varicella‐like vesicular eruptions, urticaria, maculopapular eruptions, livedo, and necrosis are among the skin manifestations seen in coronavirus disease 2019 (COVID‐19) cases. 3 , 4 The pseudo‐chilblain was usually associated with milder disease, whereas livedo and necrosis were associated with severe disease. 5 In children, SARS‐CoV‐2 infection usually has a benign course; however, Kawasaki‐like multisystem inflammatory syndrome may develop, with associated skin findings, in a subset of children.
Enanthema and oral lesions are among the typical manifestations of many viral diseases. When the diagnosis is uncertain, the presence of enanthema in oral mucosa assists in distinguishing the type of viral exanthema. Recently, we observed four confirmed COVID‐19 patients with oral mucosa findings: three patients with erythema multiforme and accompanying oral ulcers and cracked lips, and one patient with a swollen red tongue (Figure 1). The erythema multiforme in these cases developed presumably due to a reactive response to COVID‐19.
FIGURE 1.
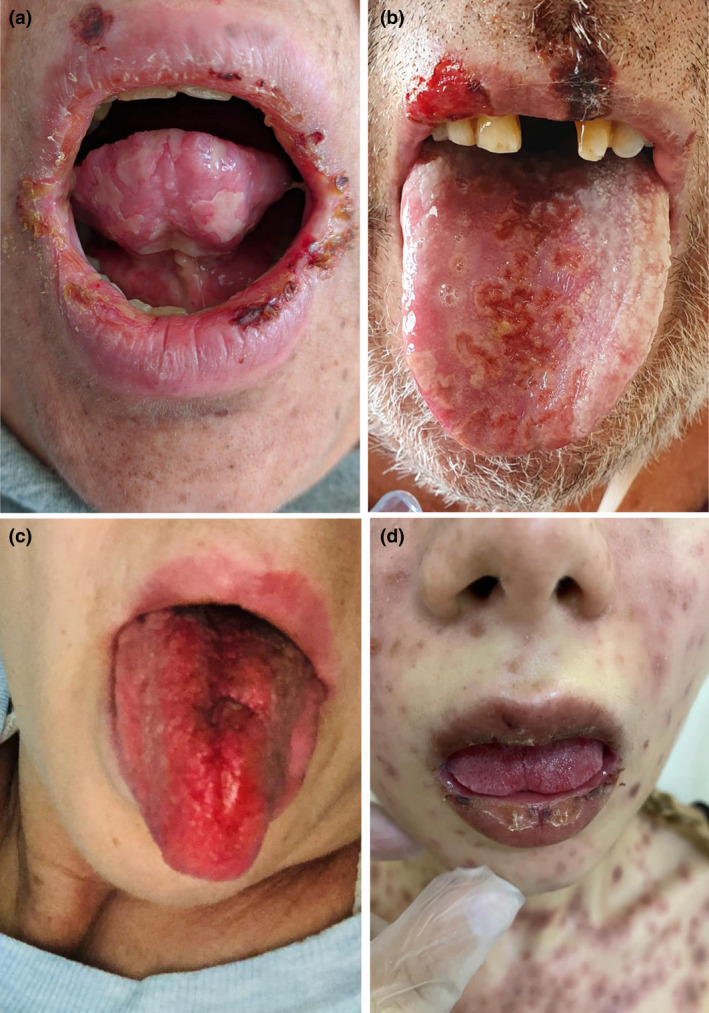
SARS‐CoV‐2 confirmed cases with oral mucosa findings. (a) A 47‐year‐old woman with a maculopapular rash on admission developed erosions under the tongue and ulcers in the oral mucosa 3 days after diagnosing COVID‐19. (b) A 78‐year‐old man developed herpetiform ulcers unresponsive to valacyclovir on the tongue 10 days after diagnosis. Herpes simplex type I IgM was negative, and IgG was positive with a low titer, and herpes simplex type II IgM and IgG were negative (c). A 53‐year‐old woman developed a red, edematous painful tongue 4 days after PCR and chest CT confirmation of COVID‐19. (d) A 25‐year‐old woman with fever and a maculopapular rash developed cracked lips and erosions on the buccal mucosa. Herpes simplex type I and II antibodies were negative, and repeated SARS‐CoV‐2 PCR was positive on the 7th day of admission
High infectivity and fatality rates restricted oral cavity examinations for COVID‐19 patients owing to safety concerns. Still, numerous published case reports and case series show that skin changes in oral mucosa may precede or accompany the disease. Furthermore, many publications have reported on the effect of altered health status in oral mucosa, including the effects of concurrent infections and related conditions, without focusing on the direct impact of viral infection. The concurrent infections considered included herpes simplex virus, candida, and mucormycosis, and concurrent non‐infection conditions considered included drug use (antiplatelet, antibacterial drugs) in addition to other related factors. 6 In this review, we searched the literature in detail for specific manifestations of COVID‐19 in the oral mucosa to promote a comprehensive understanding of possible patterns and to provide up‐to‐date information for clinical practice.
Materials and Methods
This review was planned and conducted based on PRISMA guidelines. The inclusion criteria consisted of case reports and case series that reported the co‐occurrence of COVID‐19 and oral mucosal lesions. We performed a systematic literature search without language, publication time, or patient age, sex, or ethnicity restrictions in PubMed, Scopus, Google Scholar, and Medline for eligible records until April 07, 2021. We searched the electronic databases for relevant articles with the keywords “oral mucosa,” “oral lesions,” “mucocutaneous,” “gingiva,” “tongue,” “Kawasaki‐like,” AND “SARS‐CoV‐2” or “Covid‐19” or “Coronavirus 19.” An additional search across reference lists of included studies was performed. EndNote X9 was used to collect references and remove duplicates. 7
Patients with molecular confirmation of SARS‐CoV‐2 with either reverse transcriptase‐polymerase chain reaction or serologic confirmation of IgG/IgM antibodies against the virus were included. Oral mucosa lesions associated with the cases with concomitant infections (HSV, Candida albicans, or bacterial infections), reports related to mechanical trauma (intubation), reports related to possible drug reactions, studies only reporting taste disorders without mucocutaneous findings, and reports with inadequate investigations were excluded.
For the selection of studies, a two‐phase process was applied. At first, two authors (NE and GE) independently screened the articles based on the titles and abstracts to select those appearing to meet the inclusion criteria. In the second phase, the full text of the articles retrieved in the initial literature research was reviewed by the same two authors independently. The third author (AB) was involved in resolving conflict and making the final decision. All papers selected for inclusion in the systematic review were subjected to critical appraisal using the Joanna Briggs Institute Critical Appraisal Tools for case reports and case series. 8 , 9
The risk of bias in the studies was assessed independently by three authors (GE, NE, and AB) using the appropriate checklist. Information for each included patient regarding gender, age, laboratory testing for SARS‐CoV‐2, medical history, concomitant symptoms including skin findings, histopathology, onset, treatment, and resolution time were collected and summarized. After collecting the initial data, results were recruited into one of three categories: ulcers, Kawasaki‐like syndrome‐associated mucosal lesions, and miscellaneous lesions. Statistical analysis was performed with SPSS version 26. Group comparisons were evaluated with Kruskal–Wallis, Pearson chi‐square test, and Fisher’s exact test.
Results
Study selection
The initial search yielded 5685 references (last updated on April 7, 2021). After removing duplicates, 2072 citations remained. Following title and abstract screening, 1944 reports were considered irrelevant since they did not meet our inclusion criteria. Consequently, 128 articles underwent a complete review. The full‐text review resulted in the exclusion of 89 studies according to the predetermined eligibility criteria, and 39 articles remained for final analysis. A flowchart representing this process is presented in Figure 2.
FIGURE 2.
Article selection flow chart according to PRISMA guidelines
Summary of findings
The epidemiologic and clinical manifestations of the patients reported in the literature are summarized in Table 1. In the 39 studies reviewed, 59 eligible cases were reported, including 24 female and 35 male patients. Overall, patients were aged between 4 months and 83 years. The median age was 28. Among these 59 patients, 48 were diagnosed by SARS‐CoV‐2 RT‐PCR, two had both SARS‐CoV‐2 IgM and IgG positivity, and nine had SARS‐CoV‐2 IgG positivity after the disease. Demographics of patients are summarized in Table 2.
TABLE 1.
Characteristics of included studies
| Study | Design | Sample, n | Gender | Age, years | Risk of bias a | |
|---|---|---|---|---|---|---|
| 1 | Akca et al. 10 | CS | 1, 3/4 cases excluded | M | 7 | Low |
| 2 | Alnashri et al. 11 | CR | 1 | M | 16 | Low |
| 3 | Ansari et al. 12 | CR | 2 | F/M:1/1 | 56;75 | Low |
| 4 | Bahrami et al. 13 | CR | 1 | F | 5 | Low |
| 5 | Balasubramanian et al. 14 | CR | 1 | M | 8 | Low |
| 6 | Bhaswati et al. 15 | CR | 1 | M | 4 months | Low |
| 7 | Blondiaux et al. 16 | CS | 2, 2/4 cases excluded due to the absence of oral mucosal findings | F/M:1/1 | 8;6 | Low |
| 8 | Brandao et al. 17 | CS | 8 | F/M:3/5 | 81;71;83;72;32;35;29;28 | Low |
| 9 | Chaux‐Bodard et al. 18 | CR | 1 | F | 45 | Moderate |
| 10 | Chérif et al. 19 | CR | 1 | F | 35 | Low |
| 11 | Chiotos et al. 20 | CS | 3, 3/6 cases excluded due to the absence of oral mucosal findings | F/M:2/1 | 12;9;5 | Low |
| 12 | Chiu et al. 21 | CR | 1 | M | 10 | Low |
| 13 | Ciccarese et al. 22 | CR | 1 | F | 19 | Low |
| 14 | Cruz Tapia et al. 23 | CS | 4 | F/M:3/1 | 41;51;55;42 | Low |
| 15 | Demirbaş et al. 24 | CR | 1 | F | 37 | Low |
| 16 | De Paulis et al. 25 | CR | 1 | F | 4 | Low |
| 17 | Dominguez‐Santas et al. 26 | CS | 4 | F/M:1/3 | 43;33;37;19 | Low |
| 18 | Gabusi et al. 27 | CR | 1 | M | 78 | Moderate |
| 19 | Haena et al. 28 | CR | 1 | M | 11 | Low |
| 20 | Holcomb et al. 29 | CR | 1 | M | 17 | Moderate |
| 21 | Jones et al. 30 | CR | 1 | F | 6 months | Low |
| 22 | Cebeci‐Kahraman et al. 31 | CR | 1 | M | 51 | Low |
| 23 | Lidder et al. 32 | CR | 1 | M | 45 | Low |
| 24 | Labé et al. 33 | CR | 1 | M | 6 | Moderate |
| 25 | McGoldrick et al. 34 | CR | 1, 1/2 case excluded due to the absence of COVID‐19 confirmation | M | 53 | Moderate |
| 26 | Ng et al. 35 | CS | 1, 2/3 cases excluded due to the absence of oral mucosal findings | M | 17 | Low |
| 27 | Peterson et al. 36 | CR | 1 | F | 2 | Low |
| 28 | Rafiei Tabatabaei et al. 37 | CR | 1 | M | 11 | Low |
| 29 | Renganathan et al. 38 | CR | 1 | M | 10 | |
| 30 | Rivera‐Figueroa et al. 39 | CR | 1 | M | 5 | Low |
| 31 | Rodriguez et al. 40 | CS | 1, 2/3 cases excluded as they were related to candidal infection | F | 43 | Moderate |
| 32 | Shaigany et al. 41 | CR | 1 | M | 45 | Low |
| 33 | Soares et al. 42 | CR | 1 | M | 42 | Low |
| 34 | Soares et al. 43 | CR | 1 | F | 23 | Low |
| 35 | Sokolovsky et al. 44 | CR | 1 | F | 36 | Low |
| 36 | Spencer et al. 45 | CR | 2 | F/M:1/1 | 11; 7 | Low |
| 37 | Taşkın et al. 46 | CR | 1 | F | 61 | Low |
| 38 | Tomo et al. 47 | CR | 1 | F | 37 | Low |
| 39 | Waltuch et al. 48 | CS | 2, 1/3 case excluded due to the absence of oral mucosal findings | M:2 | 5; 13 | Low |
CR, case report, CS, case series, F, female, M, male, n, number of cases.
Risk of bias of each case is assessed by the Joanna Briggs Institute critical appraisal tools for case reports and prevalence studies.
TABLE 2.
Characteristics of COVID‐19 patients with oral mucosa findings
| Age (Median) | 28 (4 months–83 years) |
|---|---|
| Sex | |
| Female | 40.7% (n = 24) |
| Male | 59.3% (n = 35) |
| SARS‐CoV‐2 detection | |
| SARS‐CoV‐2 RT‐PCR (+) | 81.4% (n = 48) |
| SARS‐CoV‐2 IgM (+) IgG (+) | 3.4% (n = 2) |
| SARS‐CoV‐2 IgG (+) | 15.2% (n = 9) |
| Oral lesion type | |
| Cheilitis/cracked lips | 43.9% (n = 29) |
| Oral ulcer | 36.3% (n = 24) |
| Miscellaneous | 19.6% (n = 13) |
| Skin lesions | 60.6% (n = 40) |
| Dysgeusia | 30.8% (n = 20) |
| Fever | 86.2% (n = 56) |
| Onset of oral lesions in relation to other symptoms | |
| Before | 10.1% (n = 6) |
| Simultaneously | 25.4% (n = 15) |
| After | 64.4% (n = 38) |
The onset of oral lesions was synchronous with other symptoms and diagnosis in 15 patients (25.4%). Anosmia/dysgeusia accompanied oral mucosa lesions in 20 patients (30.8%). Additional skin lesions were detected in 40 patients (60.6%). Lesions developed after SARS‐CoV‐2 detection in 38 patients (64.4%). In six (10.1%) patients, oral lesions were present before molecular confirmation and the onset of other symptoms. The cases in the reports consisted of 48 hospitalized patients with severe disease and 11 patients with mild‐moderate symptoms. The mean age was lower in patients with accompanying skin lesions (mean: 25 vs. 43; P = 0.001). Among hospitalized patients, the mean age was 31.1 (P = 0.068).
We categorized the reported oral mucosa findings into three subgroups:
KWL: Kawasaki‐like syndrome‐associated oral mucosa findings (cracked lips, dry lips, cheilitis with/without erythema of oral mucosa; Table 3). 11 , 13 , 14 , 15 , 16 , 19 , 20 , 21 , 25 , 28 , 30 , 32 , 35 , 36 , 37 , 38 , 39 , 41 , 44 , 45 , 48 , 49
OU: Ulcers in the oral mucosa (aphthous, herpetiform, multiple, single, necrotizing; Table 4). 17 , 18 , 22 , 24 , 26 , 27 , 29 , 33 , 40 , 42 , 43 , 46 , 49 , 50
M: Miscellaneous group (macular, papular, pustular, bullous, and overlapping cases; Table 5). 22 , 23 , 24 , 31 , 33 , 34 , 42 , 43 , 47 , 51 Seven patients in this group had accompanying oral ulcers to other mucosal findings and are also included in Table 4. 22 , 24 , 33 , 40 , 42 , 43 , 49
TABLE 3.
Characteristics of SARS‐CoV‐2 (+) Kawasaki‐like systemic disease patients with oral mucosa findings
| Reference | Type of KD | Sex | Age | Systemic manifestions | Skin | Oral lesions | Onset | Hospitalization | Treatment of oral lesions |
|---|---|---|---|---|---|---|---|---|---|
| Akca et al. | IC | M | 7 | Fever, respiratory symptoms | Yes | Erosive hyperemia of oral mucosa | N/A | + | IVIG, azithromycin, hydroxochloroquine, ritonavir, lopinavir, tocilizumab, mesenchymal stem cell treatment |
| Alnashri et al. | KL‐MISC | M | 16 | Fever | Yes | Fissured lips | N/A | + | IVIG, tocilizumab |
| Bahrami et al. | KL‐MISC | F | 5 | Fever | Yes | Swelling and congestion of lips | After SS | + | IVIG, acetylsalicylic acid |
| Balasubramanian et al. | KL‐MISC | M | 8 | Fever, respiratory symptoms | Yes | Cracked lips, strawberry tongue | After SS | + | IVIG, aspirin, tocilizumab |
| Bhaswati et al. | C‐KD | M | 4 months | Fever | Yes | Red lips, red congested throat | N/A | + | Aspirin, IVIG |
| Blondiaux et al. | KL‐MISC | F | 8 | Fever | Yes | Cheilitis | With SS | + | IVIG, prednisolone, aspirin |
| Blondiaux et al. | KL‐MISC | F | 6 | Fever | Yes | Cheilitis | With SS | + | IVIG, prednisolone, aspirin |
| Chérif et al. | C‐KD | F | 35 | Fever, respiratory symptoms, hypogeusia | Yes | Chapped lips, with ulceration above the upper lip, lingual enanthema characterized by a reddish and swollen tongue | After SS | Hydroxychloroquine, azithromycin, cefuroxime | |
| Chiotos et al. | KL‐MISC | M | 12 | Fever, respiratory symptoms | N/A | Fissured lips | N/A | + | Methylprednisolone, IVIG |
| Chiotos et al. | KL‐MISC | F | 9 | Fever | No | Fissured lips, strawberry tongue | After SS | + | IVIG, methylprednisolone, aspirin |
| Chiotos et al. | KL‐MISC | F | 5 | Fever | Yes | Fissured lips | With SS | + | IVIG, methylprednisolone, anakinra |
| Chiu et al. | KL‐MISC | M | 10 | Fever | Yes | Cracked lips | After SS | + | Ibuprofen, dopamine |
| De Paulis et al. | KL‐MISC | F | 4 | Fever, respiratory symptoms | Yes | Cracked lips | After SS | + | Acyclovir, antibiotics, dobutamine, IVIG |
| Haena et al. | C+MIS‐C | M | 11 | Fever | Yes | Cracked lips, strawberry tongue | After SS | + | Aspirin, IVIG |
| Jones et al. | C‐KD | F | 6 months | Fever | Yes | Dry, cracked lips, prominent tongue papilla | After SS | + | IVIG, acetylsalicylic acid |
| Lidder et al. | IC | M | 45 | Fever, respiratory symptoms | Yes | Cheilitis, cracked lips | N/A | + | IVIG, tocilizumab, triamcinolone |
| Ng et al. | C+MIS‐C | M | 17 | Fever, respiratory symptoms | Yes | Cracked lips | N/A | + | IVIG, aspirin, ceftriaxone, clindamycin |
| Peterson et al. | C‐KD | F | 2 | Fever | Yes | Dry, cracked lips, strawberry tongue | After SS | + | IVIG, acetylsalicylic acid |
| Rafiei Tabatabaei S et al. | C‐KD | M | 11 | Fever, diarrhea, respiratory symptoms | Yes | Strawberry tongue | After SS | Yes | IVIG, ASA |
| Renganathan et al. | KL‐MISC | M | 10 | Fever, headache, irritability, disoriented speech | Yes | Dry cracked lips | N/A | + | IVIG, methylprednisolone |
| Rivera‐Figueroa et al. | IC | M | 5 | Fever | No | Dry, cracked, erythematous lips | N/A | + | IVIG, diphenhydramine, methylprednisolone, aspirin |
| Shaigany et al. | KL‐MISC | M | 45 | Fever, respiratory symptoms | Yes | Cracked lips | After SS | + | Heparin, IVIG, tocilizumab |
| Sokolovsky et al. | C‐KD | F | 36 | Fever, respiratory symptoms | Yes | Cracked lips | After SS | + | Aspirin, IVIG, methylprednisolone |
| Spencer et al. | IC | M | 11 | Fever, respiratory symptoms | Yes | Red, swollen lips | After SS | + | IVIG, corticosteroids |
| Spencer et al. | KL‐MISC | F | 7 | Fever, respiratory symptoms | Yes | Cracked lip, strawberry tongue | N/A | + | Corticosteroids. |
| Waltuch et al. | IC | M | 13 | Fever, respiratory symptoms | Yes | Erythematous tongue and oropharynx | After SS | + | Enoxaparin, antibiotics, IVIG, tocilizumab, anakinra |
| Waltuch et al. | IC | M | 5 | Fever, respiratory symptoms | Yes | Dry, cracked lip, mildly erythematous posterior oropharynx | N/A | + | Ceftriaxone, clindamycin, enoxaparin, IVIG, tocilizumab |
TABLE 4.
Characteristics of oral ulcers reported in COVID‐19 patients
| Reference | Sex | Age | Systemic manifestions | Skin lesions | Oral lesions | Ulcer localization | Onset | Hospitalization | Treatment of oral lesions |
|---|---|---|---|---|---|---|---|---|---|
| Akca et al. a | M | 7 | Fever, respiratory symptoms | Yes | Erosive hyperemia | N/A | N/A | + | IVIG, azithromycin, hydroxochloroquine, ritonavir, lopinavir, tocilizumab, mesenchymal stem cell treatment |
| Ansari et al. | F | 56 | Fever, respiratory symptoms | No | Ulcers | The hard palate | After S | + | Remdesivir, azithromycin, magic mouthwash |
| Ansari et al. | M | 75 | Respiratory symptoms | No | Ulcers | The anterior part of the tongue | After S | + | Azithromycin, magic mouthwash |
| Brandao et al. | M | 81 | Fever, respiratory symptoms, dysgeusia | No | Multiple shallow aphthous‐like ulcers of varying sizes and irregular margins covered with mucopurulent membrane | The upper and lower lip mucosa and anterior dorsal tongue | After S | + |
Azithromycin, ceftriaxone, acyclovir, photobiomodulation therapy (PBMT) Unresponsive to acyclovir |
| Brandao et al. | F | 71 | Respiratory symptoms, dysgeusia | No | Hemorrhagic ulcers, focal areas of shallow necrosis on the anterior tongue | The upper and lower lip | After S | + |
Acyclovir, PBMT Unresponsive to acyclovir |
| Brandao et al. | F | 83 | Respiratory symptoms | No | Aphthous‐like ulcers, petechia, shallow necrosis | The lateral border of the tongue | After S | + | PBMT |
| Brandao et al. | M | 72 | Fever, respiratory symptoms | No | Hemorrhagic ulcers, necrotic ulcers, aphthous‐like ulcers | The upper and lower lips, vermilion border, and lower lip mucosa | After S | + | Acyclovir, PBMT |
| Brandao et al. | F | 32 | Fever, respiratory symptoms | No | Aphthous‐like ulcers | The apex and lateral borders of the tongue | After S | − | N/A |
| Brandao et al. | M | 35 | Fever, respiratory symptoms, hyposmia, ageusia | No | Aphthous‐like ulcers | Peritonsillar and lateral border of tongue | After S | − | N/A |
| Brandao et al. | M | 29 | Fever, respiratory symptoms, anosmia ageusia | No | Aphthous‐like ulcers | The ventral portion of the tongue | After S | − | No |
| Brandao et al. | M | 28 | Fever, respiratory symptoms, anosmia, ageusia | No | Aphthous‐like ulcers | The upper and lower labial mucosae, on the border of the tongue | After S | − | 0,12% chlorhexidine mouthwash |
| Chaux‐Bodard et al. | F | 45 | Asthenia | Painful erythematous plane lesion on the toe | Irregular ulcer on the dorsal side of the tongue | The dorsal side of the tongue | Before | N/A | N/A |
| Ciccarese et al. a | F | 19 | Fever, respiratory symptoms, hyposmia | Yes | Erosions, ulcers, blood crust, petechial enanthema | Inner surface of the lower lip | After S | + | I.V. immune globulins, methylprednisolone |
| Demirbaş et al. a | F | 37 | Respiratory symptoms | Yes | Ulcers | Lower lip, tongue, palate |
After S 5th day of treatment |
+ | Methylprednisolone, anesthetic, and antiseptic mouthwashes |
| Dominguez‐Santas et al. | F | 43 | Fever, respiratory symptoms, anosmia | N/A | Aphthous‐like ulcers | Buccal mucosa | After S | N/A | N/A |
| Dominguez‐Santas et al. | M | 33 | Fever, respiratory symptoms, anosmia | N/A | Aphthous‐like ulcers | Mucogingival junction | After S | N/A | N/A |
| Dominguez‐Santas et al. | M | 37 | Fever, respiratory symptoms, anosmia | N/A | Aphthous‐like ulcers | Tongue | After S | N/A | N/A |
| Dominguez‐Santas et al. | M | 19 | Fever, respiratory symptoms, anosmia | N/A | Aphthous‐like ulcers | Labial mucosa | With S | N/A | N/A |
| Gabusi et al. | M | 78 | Respiratory symptoms | No | Painful ulcerated plaque | Tongue, both lips, soft palate | After S | Yes | Topical betamethasone, chlorhexidine gel, topical lidocaine |
| Holcomb et al. | M | 17 | Anosmia, ageusia | Yes | Mucositis, shallow erosions | Lips and hard palate | Without S | N/A |
Betamethasone, intraoral dexamethasone solution, viscous lidocaine, acetaminophen, ibuprofen |
| Labé et al. a | M | 6 | Asymptomatic | Yes | Erosive cheilitis, gingival erosion, thick hemorrhagic crust | Gingiva | N/A | + | N/A |
| Rodriguez et al. a | F | 43 | Fever, respiratory symptoms, anosmia, dysgeusia | N/A | Aphthous‐like ulcers, tongue depapillation | Tongue | After S | − | Triamcinolone acetonide 0.05% |
| Soares et al. a | F | 23 | Fever, respiratory symptoms | Yes | Vesicobullous lesions | Outer surface of the lips | After S | N/A | Systemic dexamethasone |
| Soares et al. a | M | 42 | Fever, cough, shortness of breath | Petechia‐like small vesiculobullous lesions | Ulcer, maculopapular enanthema | Buccal mucosa | After S | N/A | Dexamethasone, dipyrone |
| Taskin et al. | F | 61 | Fever, fatigue, arthralgia, myalgia, respiratory symptoms | Sweet’s syndrome | Aphthous ulcers on the hard palate and buccal mucosa | The palate and buccal mucosa | With S | + |
Tocilizumab, Favipiravir 0.12% chlorhexidine mouthwash |
| Tomo et al. a | F | 37 | Fever, asthenia, dysgeusia, anosmia | No | Mucositis, diffuse bilateral erythema, petechia, depapillation of tongue | Generalized |
After S 9th day |
− |
Chlorhexidine 0.12% mouthwash Dexamethasone, metimazole |
TABLE 5.
Characteristics of the patients with miscellaneous lesions
| Reference | Sex | Age | Systemic manifestions | Skin lesions | Oral lesions | Location/type | Onset | Hospitalization | Treatment of oral lesions |
|---|---|---|---|---|---|---|---|---|---|
| Ciccarese et al. | F | 19 | Fever, respiratory symptoms, hyposmia | Yes | Erosions, ulcers, blood crust, petechial enanthema | Overlap (ulcer, maculopapular enanthema) | After S | + | I.V. immune globulins, methylprednisolone |
| Cruz Tapia et al. | F | 51 | Fever | No | Macule (12 mm), papule‐plaque (8 mm) | Maculopapular enanthema | N/A | + | Dexamethasone, azithromycin, indomethacin |
| Cruz Tapia et al. | M | 42 | Fever, dysgeusia | No | Macules (3–4 mm), mucositis | Maculopapular enanthema |
After S Persistant after resolution |
N/A | Clorhexidine 0.12%, topical mometasone |
| Cruz Tapia et al. | F | 41 |
Fever, hyposmia, Myalgia, dysphagia |
No | Angina bullosa‐like hemorrhagic lesion (6 mm) on palate | Bullous | After S | − | N/A |
| Cruz Tapia et al. | F | 55 | Fever, headache, nasal congestion | No |
Purple bulla (8 mm) On hard palate |
Bullous | After S | − | N/A |
| Demirbaş et al. | F | 37 | Respiratory symptoms | Yes | Ulcers on lower lip tongue palate | Erythema multiforme |
After S 5th day of treatment |
+ | Methylprednisolone, anesthetic, and antiseptic mouthwashes |
| Holcomb et al. | M | 17 | Anosmia, ageusia | Yes | Mucositis, shallow erosions | Erythema multiforme | Without SS | − | Betamethasone, dexamethasone solution, viscous lidocaine, acetaminophen, ibuprofen |
| Kahraman et al. | M | 51 | Sore throat, fever, fatigue, dry cough, inability to taste or smell | No | Erythemathous surface in the oropharynx and in the hard palate, petechiae in the midline and numerous pustular enanthema near the soft palate border (1–3 mm in diameter) | Generalized |
After S 10th day |
− | Clarithromycin 500 mg b.i.d. PO |
| Labé et al. | M | 6 | Asymptomatic | Yes | Erosive cheilitis, gingival erosion, thick hemorrhagic crust |
Generalized Erythema multiforme |
N/A | + | N/A |
| McGoldrick et al. | M | 53 | No | No | Tongue and mouth swelling | Nonspecific | Before SS | + | IV steroid |
| Rodriguez et al. | F | 43 | Fever, respiratory symptoms, anosmia, dysgeusia | N/A | Aphthous‐like ulcer, tongue depapillation | Overlap | After S | − | Triamcinolone acetonide 0.05% |
| Soares et al.a | F | 23 | Fever, respiratory symptoms | Yes | Intact vesicobullous lesions on the vermillion border and hemorrhagic crusts on the outer surface of the lip | Bullous | After SS | N/A | Systemic dexamethasone |
| Soares et al. | M | 42 | Fever, cough, shortness of breath | Petechia‐like small vesiculobullous lesions | Ulcer, maculopapular enanthema | Overlap (ulcer, maculopapular enanthema) | After S | N/A | Dexamethasone, dipyrone |
| Tomo et al. | F | 37 | Fever, asthenia, dysgeusia, anosmia | No | Mucositis, diffuse bilateral erythema, petechia, depapillation of tongue | Generalized |
After S 9th day |
− |
Chlorhexidine 0.12% mouthwash Dexamethasone, metimazole |
Kawasaki‐like multisystem inflammatory disorder associated with oral mucosa symptoms was reported in 29 patients. Male patients constituted 62.1% (18/29) of this group. The reported dermatological manifestations were cracked lips, cheilitis, chapped lips, dry red lips, swollen red lips, strawberry tongue, and hemorrhagic crusts on the tongue. The median age in this group was 9 years (4 months to 45 years). Twenty‐eight patients were reported to have fever (96.1%). Skin lesions were present in 92% of the patients (n = 27). Seventeen patients had respiratory system involvement (58.6%). Anosmia and dysgeusia were reported in one patient (3.4%). The age of patients in the KWL group was lower than that in both the OU and the M groups (P = 0.001).
Oral ulcers were reported in 24 patients. The median age of patients with oral ulcers was 39 years (range: 6–83). In 17 patients (70.8%), multiple lesions were reported. The majority of ulcers (58.3%, 14/24) were defined as aphthous ulcers, in which the lesion is surrounded by an erythematous halo due to dilated blood vessels and the ulcer bed is covered with a yellowish pseudomembrane. Necrotizing or ischemic ulcers were present in 12.5% (n = 3) of the patients. Shallow ulcers with irregular borders were observed in five patients (16.6%). All patients with ulcers reported pain. The tongue was the most common location for ulcers (54.1%), followed by the lips, buccal mucosa, and the palate. In the oral ulcer group, accompanying skin lesions were reported in seven patients (29.7%). Respiratory system involvement was present in 91.7% of the patients. Twelve patients reported dysgeusia and/or anosmia (50%).
The miscellaneous lesions are summarized in Table 5. Various lesions in this group included overlapping lesions such as macular enanthema and oral ulcers. Four patients had maculopapular enanthema, and two of them had erythema multiforme, major type. Angina‐bullosa‐like lesions were reported in two patients (n = 2). In two patients, tongue depapillation was described.
There were more hospitalized patients and patients with systemic symptoms and fever in the KWL group versus the OU group (P = 0.004, 0.016, 0.049, respectively). Skin lesions were increased in the KWL group (P = 0.001). Dysgeusia and ageusia were reported more commonly among patients with oral ulcers (P = 0.001).
Histopathology was available for five lesions. The biopsy specimens from reddish skin areas and the ischemic ulcer, as the authors describe, on the buccal mucosa and the hard palate showed vacuolization and hemorrhage in the lamina propria, CD34+ thrombi and endothelial cells, CD3+ inflammatory cell infiltration, and cytotoxic T cells. 42 Ulcers from two patients revealed edema, mucosal desquamation, granulation, ulceration under the mucosa, invasion of mononuclear cells, and neutrophilic infiltration. 50 Histopathological evaluation of multiple red macules on the hard palate was consistent with stratified squamous epithelium with paranuclear keratinocytes, vacuolization focally in the spinous layer, perivascular lymphocytic proliferation in the lamina propria, and subepithelial tissue with marked vascular congestion, hemorrhage, and lymphatic vessel ectasia mixed with fibrin and cellular debris. 23 Lip biopsy from a COVID‐19 patient revealed moderate lymphocytic infiltrate and microvascular thrombosis. In this case, SARS‐CoV‐2 spike protein was shown by immunohistochemistry in inflammatory endothelial cells, keratinocytes, and acinar and ductal cells of the minor salivary glands. 43
Fever was reported in 86.2% of cases (51/59). Respiratory or gastrointestinal symptoms were present in 69.4% (n = 41) of the cases with oral mucosa findings. One patient with asthenia as the only symptom of COVID‐19 infection later developed painful tongue papillae and an irregular oral ulcer accompanied by an erythematous macule on the left toe. 18
Certainty of evidence
In accordance with the types of studies (cases and case series) collected and publication bias, the evidence obtained is considered to be low grade.
Discussion
The present systemic review was designed to collect and review reports of oral mucosa lesions in patients with COVID‐19. Aphthous ulcers and Kawasaki‐associated enanthema were reported multiple times, which enabled us to subcategorize the oral mucosa findings. Oral ulcers and cracked lips with erythema were among the most common findings in the oral mucosa.
Although symptoms and signs of infection including dysgeusia and mucosal lesions were reported, the involvement of the oral cavity in COVID‐19 has not yet been clarified. The underlying event causing the oral mucosa lesions is unclear; however, multiple etiologic factors may have a role. The lesions may be a direct result of the SARS‐CoV‐2 virus infection, or they could be related to stress, drugs used for COVID‐19 treatment, or the general immunosuppressive status brought about by prolonged disease and hospitalization. 52 Kaya et al. reviewed the skin lesions associated with COVID‐19 and reported that the frequency of such lesions varied between 1.8 and 20.4%. 53 Sousa et al. suggested that the frequency of oral mucosa lesions is probably comparable to the frequency of skin lesions, supposing that both have similar underlying pathology. 54 Among the cases included in this review, 60% had accompanying skin lesions. The rate was higher, however, in the KWL group. The presence of skin lesions may indicate the possibility of accompanying oral lesions; when evaluating such patients, physicians may consider asking about oral mucosa symptoms during the examination.
Moreover, it is possible to encounter a COVID‐19 patient with isolated oral mucosa lesions. SARS‐CoV‐2 infection was recently shown in the oral mucosa and glands. Virus shedding was confirmed in patients' saliva. 55 Asymptomatic transmission of SARS‐CoV‐2 is a potential mechanism for virus spread in this pandemic; therefore, necessary precautions must be taken when examining patients in outpatient clinics with lesions in the oral mucosa.
Angiotensin‐converting enzyme II (ACE2) stands in the foreground of the portrait of cellular entry of SARS‐CoV‐2. 56 ACE2 receptors are distributed diffusely on the mucous membrane of the whole oral cavity, especially on the tongue. 57 The high expression of ACE2 receptors in the oral mucosa may contribute to the onset of oral mucosa lesions by causing a hyperinflammatory state in the mouth upon infection with the SARS‐CoV‐2 virus. In the COVID‐19 cases reviewed here, the tongue was also the most common location for aphthous ulcers. Many etiological factors have been associated with aphthous lesions, including stress, viral and bacterial infections, inflammatory conditions (Behçet’s disease, inflammatory bowel disease), neutropenia, and mechanical trauma. COVID‐19 can cause an exaggerated immune response, and this response may be critical in the formation of oral mucosa lesions. In aphthous lesions, for instance, T helper 1 proinflammatory cytokine expression is dominant. Moreover, heat‐shock protein expression decreases, and neutrophils exhibit a hyperactive state. T regulatory cells and CD4+ T cells are decreased in the area of inflammation. 58 Jouan et al. have shown that mucosal‐associated invariant T (MAIT) cells are decreased in the peripheral blood of severe COVID‐19 patients. In contrast, their numbers were significantly increased in the mucosal surfaces. 59 Whether this recruitment of T cells has a functional relevance related to oral lesions is not clear. The COVID‐19 disease state may prepare a suitable environment for aphthous lesions through cytokine storm and upregulated T helper 17 response. COVID‐19‐associated coagulation (CAC) is provoked by endothelial injury, immobilization, and increased circulating prothrombotic factors. 60 CAC and immune attacks may contribute to the formation of necrotic ulcers in the oral mucosa.
In addition to the direct effect of the infection, reactivation of herpes gingivostomatitis and candidal superinfection in COVID‐19 cases was reported in the recent literature. 6 , 17 , 61 According to Dziedzic et al., 62 oral lesions could be related to a weakened immune system or multi‐drug treatment. Recurrent HSV‐1 infections typically accompany an impaired immune system, and fungal infections commonly stem from dysbiosis after antibacterial therapies. The findings could also be rooted in a complex relationship between COVID‐19 and the microbiome. Physicians should be aware of potential secondary infections in severe COVID‐19 patients receiving multi‐drug regimens, including antibiotics.
Our review has several limitations. COVID‐19 is a relatively new disease, and the published information in the literature is limited. Even though we included cases with detailed medical reports and laboratory testing, we cannot entirely rule out the possibility of a secondary cause. Two authors searched independently for cases, and we repeated our search multiple times until submission, but we cannot guarantee that the latest publications are included. In addition, due to limited publications, we had a small sample size, which might pose a risk of bias. Last, we included nine patients with IgG positivity. Although the majority of the patients were diagnosed before July 2020, which makes the possibility of a past infection unlikely due to the timeline and none of them were vaccinated, we cannot completely rule out the possibility of a previous COVID‐19 infection.
This review illustrates that oral mucosa findings in COVID‐19 patients may be heterogeneous. However, ulcers, enanthema, and cheilitis comprise most of the lesions. The patients with oral lesions demonstrated a wide range of clinical phenotypes, including severe and asymptomatic cases. According to our review, oral mucosa lesions in Kawasaki‐like multisystem inflammatory syndrome (cracked lips, cheilitis, strawberry tongue) correlates with more severe disease and hospitalization.
Although COVID‐19 cases around the world had increased to 174 million by June 2021, low numbers of patients with specific oral mucosa findings have been reported in the literature. The SARS‐CoV‐2 virus may infect oral mucosa; however, this tissue may be resistant to the direct effect of the virus due to the protection afforded by innate immune barriers and rich vasculature. Teledermatology is an effective method to diagnose and treat oral and skin symptoms of COVID‐19 without increasing the risk of infection through a doctor’s visit. 63 In light of safety concerns, teleconsultation or self‐photography may help monitor signs and symptoms in the oral mucosa and may aid in identifying more cases.
Supporting information
Table S1. The studies that were excluded after critical reading.
Conflict of interest: None.
Funding source: None.
References
- 1. Guo Y‐R, Cao Q‐D, Hong Z‐S, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak – an update on the status. Mil Med Res 2020; 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Biomed 2020; 91: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marzano AV, Genovese G, Moltrasio C, et al. The clinical spectrum of COVID‐19‐associated cutaneous manifestations: an Italian multicenter study of 200 adult patients. J Am Acad Dermatol 2021; 84: 1356–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galvan Casas C, Catala A, Carretero Hernandez G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020; 183: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gisondi P, PIaserico S, Bordin C, et al. Cutaneous manifestations of SARS‐CoV‐2 infection: a clinical update. J Eur Acad Dermatol Venereol 2020; 34: 2499–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amorim dos Santos J, Normando A, Carvalho da Silva RL, et al. Oral manifestations in patients with COVID‐19: a living systematic review. J Dent Res 2021; 100: 141–154. [DOI] [PubMed] [Google Scholar]
- 7. Bramer WM, Giustini D, de Jonge GB, et al. De‐duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc 2016; 104: 240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. <JBI_Critical_Appraisal‐Checklist_for_Case_Reports2017_0.pdf>. https://jbi.global/sites/default/files/2019‐05/JBI_Critical_Appraisal‐Checklist_for_Case_Reports2017_0.pdf
- 9. <JBI_Critical_Appraisal‐Checklist_for_Case_Series2017_0.pdf>. https://jbi.global/sites/default/files/2019‐05/JBI_Critical_Appraisal‐Checklist_for_Case_Series2017_0.pdf
- 10. Accardo‐Palumbo A, Ferrante A, Cadelo M, et al. The level of soluble Granzyme A is elevated in the plasma and in the Vgamma9/Vdelta2 T cell culture supernatants of patients with active Behçet's disease. Clin Exp Rheumatol 2004; 22: S45–S49. [PubMed] [Google Scholar]
- 11. Alnashri H, Aljohani N, Tayeb S, et al. A challenging case of multisystem inflammatory syndrome in children related to coronavirus disease‐19 hospitalized under adult medical service. IDCases 2020; 22: e00957. 10.1016/j.idcr.2020.e00957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ansari R, Gheitani M, Heidari F, et al. Oral cavity lesions as a manifestation of the novel virus (COVID‐19). Oral Dis 2021; 27: 771–772. [DOI] [PubMed] [Google Scholar]
- 13. Bahrami A, Vafapour M, Moazzami B, et al. Hyperinflammatory shock related to COVID‐19 in a patient presenting with multisystem inflammatory syndrome in children: first case from Iran. J Paediatr Child Health 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Balasubramanian S, Nagendran T, Ramachandran B, et al. Hyper‐inflammatory syndrome in a child with COVID‐19 treated successfully with intravenous immunoglobulin and tocilizumab. Indian Pediatr 2020; 57: 681–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhaswati CA, Saumyabrata A, Dhritabrata D. Research Square; 2020. [Google Scholar]
- 16. Blondiaux E, Parisot P, Redheuil A, et al. Cardiac MRI of children with multisystem inflammatory syndrome (MIS‐C) associated with COVID‐19: case series. Radiology 2020; 202–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brandão TB, Gueiros LA, Melo TS, et al. Oral lesions in patients with SARS‐CoV‐2 infection: could the oral cavity be a target organ? Oral Surg Oral Med Oral Pathol Oral Radiol 2021; 131: e45–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaux‐Bodard A‐G, Deneuve S, Desoutter A. Oral manifestation of Covid‐19 as an inaugural symptom? J Oral Med Oral Surg 2020; 26: 18. [Google Scholar]
- 19. Chérif MY, de Filette JM, André S, et al. Coronavirus disease 2019–related Kawasaki‐like disease in an adult: a case report. JAAD Case Rep 2020; 6: 780–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chiotos K, Bassiri H, Behrens EM, et al. Multisystem inflammatory syndrome in children during the Coronavirus 2019 pandemic: a case series. J Pediatric Infect Dis Soc 2020; 9: 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiu JS, Lahoud‐Rahme M, Schaffer D, et al. Kawasaki disease features and myocarditis in a patient with COVID‐19. Pediatr Cardiol 2020; 41: 1526–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ciccarese G, Drago F, Boatti M, et al. Oral erosions and petechiae during SARS‐CoV‐2 infection. J Med Virol 2021; 93: 129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cruz Tapia RO, Peraza Labrador AJ, Guimaraes DM, et al. Oral mucosal lesions in patients with SARS‐CoV‐2 infection. Report of four cases. Are they a true sign of COVID‐19 disease? Spec Care Dentist 2020; 40: 555–560. [DOI] [PubMed] [Google Scholar]
- 24. Demirbaş A, Elmas ÖF, Atasoy M, et al. A case of erythema multiforme major in a patient with COVID 19: the role of corticosteroid treatment. Dermatol Ther 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Paulis M, Oliveira DBL, Vieira RP, et al. Multisystem inflammatory syndrome associated with COVID‐19 with neurologic manifestations in a child: a brief report. Pediatr Infect Dis J 2020; 39: e321–e324. [DOI] [PubMed] [Google Scholar]
- 26. Dominguez‐Santas M, Diaz‐Guimaraens B, Fernandez‐Nieto D, et al. Minor aphthae associated with SARS‐CoV‐2 infection. Int J Dermatol 2020; 59: 1022–1023. 10.1111/ijd.15004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gabusi A, Gissi DB, Rossi R, et al. Persistent lesions in oral cavity after SARS‐CoV‐2 infection. Oral Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haena K, Jung Yeon S, Jae‐Hoon K, et al. Research Square; 2020. [Google Scholar]
- 29. Holcomb ZE, Hussain S, Huang JT, et al. Reactive infectious mucocutaneous eruption associated with SARS‐CoV‐2 infection. JAMA Dermatol 2021; 157: 603–605. [DOI] [PubMed] [Google Scholar]
- 30. Jones VG, Mills M, Suarez D, et al. COVID‐19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr 2020; 10: 537–540. [DOI] [PubMed] [Google Scholar]
- 31. Cebeci Kahraman F, ÇaŞkurlu H. Mucosal involvement in a COVID‐19‐positive patient: a case report. Dermatol Ther 2020; e13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lidder AK, Pandit SA, Lazzaro DR. An adult with COVID‐19 kawasaki‐like syndrome and ocular manifestations. Am J Ophthalmol Case Rep 2020; 20: 100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Labé P, Ly A, Sin C, et al. Erythema multiforme and Kawasaki disease associated with COVID‐19 infection in children. J Eur Acad Dermatol Venereol 2020; 34: e539–e541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McGoldrick DM, Sarai R, Green J. Tongue and floor of mouth swelling: a potential rare manifestation of COVID‐19. Br J Oral Maxillofac Surg 2021; 59: 500–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ng KF, Kothari T, Bandi S, et al. COVID‐19 multisystem inflammatory syndrome in three teenagers with confirmed SARS‐CoV‐2 infection. J Med Virol 2020; 92: 2880–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peterson N, Sagdeo K, Tyungu D, et al. Discovering associations: kawasaki disease and COVID‐19. Case Reports Pediat 2020; 2020: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rafiei Tabatabaei S, Karimi A, Rahimi H, et al. Three probable pediatrics COVID‐19 cases with kawasaki‐like disease: a case series with fallow‐up. Arch Ped Infect Dis 2021; 9. [Google Scholar]
- 38. Renganathan A, Garg A, Chowdhary S, et al. SARS‐CoV‐2 infection triggering recurrence of Kawasaki disease in a 10‐year‐old child. BMJ Case Rep 2021; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rivera‐Figueroa EI, Santos R, Simpson S, et al. Incomplete kawasaki disease in a child with Covid‐19. Indian Pediatr 2020; 57: 680–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rodriguez MD, Romera AJ, Villarroel M. Oral manifestations associated with COVID‐19. Oral Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shaigany S, Gnirke M, Guttmann A, et al. An adult with Kawasaki‐like multisystem inflammatory syndrome associated with COVID‐19. Lancet 2020; 396: e8–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Soares CD, Carvalho RA, Carvalho KA, et al. Letter to editor: oral lesions in a patient with Covid‐19. Med Oral Patol Oral Cir Bucal 2020; 25: e563–e564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Soares CD, Mosqueda‐Taylor A, de Carvalho MGF, et al. Oral vesiculobullous lesions as an early sign of COVID‐19: immunohistochemical detection of SARS‐CoV‐2 spike protein. Br J Dermatol 2021; 184: e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sokolovsky S, Soni P, Hoffman T, et al. COVID‐19 associated Kawasaki‐like multisystem inflammatory disease in an adult. Am J Emerg Med 2021; 39: 253.e1–253.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spencer R, Closson RC, Gorelik M, et al. COVID‐19 inflammatory syndrome with clinical features resembling kawasaki disease. Pediatrics 2020; 146. [DOI] [PubMed] [Google Scholar]
- 46. Taşkın B, Vural S, Altuğ E, et al. COVID‐19 presenting with atypical sweet’s syndrome. J Eur Acad Dermatol Venereol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tomo S, Miyahara GI, Simonato LE. Oral mucositis in a SARS‐CoV‐2‐infected patient: secondary or truly associated condition? Oral Dis 2020. [DOI] [PubMed] [Google Scholar]
- 48. Waltuch T, Gill P, Zinns LE, et al. Features of COVID‐19 post‐infectious cytokine release syndrome in children presenting to the emergency department. Am J Emerg Med 2020; 38: 2246.e3–2246.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Akca UK, Kesici S, Ozsurekci Y, et al. Kawasaki‐like disease in children with COVID‐19. Rheumatol Int 2020; 40: 2105–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ansari R, Gheitani M, Heidari F, et al. Oral cavity lesions as a manifestation of the novel virus (COVID‐19). Oral Dis 2021; 27(Suppl 3): 771–772. [DOI] [PubMed] [Google Scholar]
- 51. Diaz Rodriguez M, Jimenez Romera A, Villarroel M. Oral manifestations associated with COVID‐19. Oral Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Novak N, Peng W, Naegeli MC, et al. SARS‐CoV‐2, COVID‐19, skin and immunology – what do we know so far? Allergy 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kaya G, Kaya A, Saurat JH. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID‐19: review of the literature. Dermatopathology 2020; 7: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de Sousa FACG, Paradella TC. Considerations on oral manifestations of COVID‐19. J Med Virol 2021; 93: 667–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang NI, Pérez P, Kato T, et al. SARS‐CoV‐2 infection of the oral cavity and saliva. Nat Med 2021; 27: 892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579: 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 2020; 12: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Slebioda Z, Szponar E, Kowalska A. Etiopathogenesis of recurrent aphthous stomatitis and the role of immunologic aspects: literature review. Arch Immunol Ther Exp 2014; 62: 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jouan Y, Guillon A, Gonzalez L, et al. Phenotypical and functional alteration of unconventional T cells in severe COVID‐19 patients. J Exp Med 2020; 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Singhania N, Bansal S, Nimmatoori DP, et al. Current overview on hypercoagulability in COVID‐19. Am J Cardiovasc Drugs 2020;20:393–403. 10.1007/s40256-020-00431-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Riad A, Gad A, Hockova B, et al. Oral candidiasis in non‐severe COVID‐19 patients: call for antibiotic stewardship. Oral Surg 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dziedzic A, Wojtyczka R. The impact of coronavirus infectious disease 19 (COVID‐19) on oral health. Oral Dis 2021; 27(S3): 703–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fahmy DH, El‐Amawy HS, El‐Samongy MA, et al. COVID‐19 and dermatology: a comprehensive guide for dermatologists. J Eur Acad Dermatol Venereol 2020; 34: 1388–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The studies that were excluded after critical reading.


