Abstract
Biological adjuvants that target the gut immune system are being developed for modulating the immune system. Hyperimmune bovine colostrum (HBC), produced by harvesting the bovine colostrum of dairy cows immunized to exogenous antigens, has been shown to modulate the immune responses and alleviate immune‐mediated organ damages. The aim of the present study was to determine the ability of HBC to promote antiviral interferonγ (IFNγ) T cell responses. In a preclinical study, mice were orally administered with HBC for 5 days and tested for the number of T cell clones secreting IFNγ in response to viral antigens of the swine flu, New Caledonia influenza, and cytomegalovirus. In a phase I/IIa clinical trial, five healthy volunteers were treated for 5 days with HBC followed by testing the anti‐coronavirus disease (COVID‐19) immunity. In the preclinical study, oral administration of HBC augmented the number of T cell clones secreting IFNγ in response to viral antigens. In the clinical trial, oral administration of HBC to healthy males significantly increased the number of anti‐COVID‐19 spike protein IFNγ positive T cell clones. Oral administration of HBC provides a novel method for augmenting antiviral responses. Its high‐safety profile makes it ideal for all disease stages and for pre‐emptive therapy among medical personnel and other workers who are at a high risk of exposure to infections. The relatively low cost of HBC is expected to minimize care provider burdens, costs, and enable its global application.
Keywords: antiviral treatment, COVID‐19, prevention, T cell immunity
Abbreviations
- CMV
cytomegalovirus
- ELISpot
the enzyme‐linked immune absorbent spot
- HBC
hyperimmune bovine colostrum
- SFC
spot forming colonies
1. INTRODUCTION
The ongoing pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the causative agent of coronavirus disease (COVID‐19), is associated with high morbidity and mortality rates worldwide (Omer et al., 2020; Yang et al., 2020). An effective innate and adaptive immune response to the virus can clear the virus and virus‐infected cells. In COVID‐19, the virus escapes the immune system and induces severe inflammation that leads to acute and chronic lung injury. This immune response may also mediate injury in the heart, intestine, and the nervous system (Gelman et al., 2020; Ishay, Kessler, et al., 2020).
The development of resistance to antiviral agents that attempt to block viral entry or replication and immunomodulatory agents is common in all RNA respiratory viruses, which may become a major obstacle for effective therapies against COVID‐19 (Mason et al., 2018). Prevention of transmission among household contacts and in populated areas is paramount for the control of respiratory infections.
Current therapies for COVID‐19 are far from addressing this unmet need. While antibody‐based therapies are being developed (Ishay, Kessler, et al., 2020), augmenting the antiviral cellular responses may provide improved protection. A combination of both types of therapies may be a superior therapeutic strategy against COVID‐19.
There is also a need for safe, affordable, and effective therapeutic methods that can overcome viral resistance while augmenting effective antiviral immunity and viral clearance, and reducing severe inflammation associated with multiorgan damage. Overcoming the COVID‐19 pandemic requires therapies that can reach the market rapidly for the prevention of viral transmission, while minimizing care provider burden and costs.
Hyperimmune bovine colostrum (HBC) is produced by immunizing cows with antigens during pregnancy, resulting in a high level of antigen‐specific immunoglobulin (Ig) G. A balancing effect on the immune system has been described for hyperimmune colostrum. Treatment with HBC was shown to be beneficial in treating enteric pathogens without disrupting the normal microbiota (Otto et al., 2011; Sponseller et al., 2015). In addition to antibodies, HBC contains several sphingolipids, which have emerged as major bioactive molecules and potent immune mediators (Silva et al., 2019). IMM‐124E is an over‐the‐counter medication (Travelan©, Immuron Ltd.) comprised of anti‐LPS‐enriched HBC. The aim of the present study was to determine the effectiveness of IMM‐124E in augmenting antiviral T cell responses and to assess its application for the prevention and treatment of COVID‐19.
2. MATERIALS AND METHODS
2.1. Preclinical studies
2.1.1. Animals
Ten‐week‐old male C57BL/6 mice were purchased from Harlan Laboratories (Jerusalem, Israel). Mice were maintained in the animal core facility of the Hadassah‐Hebrew University Medical School. All mice were fed with standard laboratory chow and water ad libitum and maintained in a 12‐h light/dark cycle. The animal experiments were carried out according to the guidelines of the Hebrew University‐Hadassah Institutional Committee for Care and Use of Laboratory Animals under the committee's ethics approval.
2.1.2. Oral administration of bovine colostrum
Mice were orally administered with 200 μg of bovine colostrum dissolved in water or water only (negative control) for 5 days.
2.1.3. Preparation of cells
Ficoll gradient separation was performed on 20 ml blood samples collected in acid citrate dextrose tubes within 1 h of collection to isolate peripheral blood mononuclear cells (PBMCs). PBMCs were washed twice with RPMI‐1640 with 10% fetal calf serum (FCS) and cultured in 96‐well plates (1 x 105 cells/well) in RPMI 1640 with 10% FCS (Ilan et al., 2016).
2.1.4. Enzyme‐linked immunosorbent assay
Interferon γ (IFNγ) spot‐forming cells (SFC) were determined using an antigen‐specific enzyme‐linked immune absorbent spot (ELISpot) assay (Mabtech, Nacka, Sweden) with the following modifications (Israeli et al., 2004). In brief, 96‐well filtration plates coated with a high‐protein binding hydrophobic polyvinylidene disulfide (PVDF) membrane were used (Millipore Corp., Bedford, MA, USA). Plates were coated with 1‐D1K anti‐IFNγ antibody (15 mg/ml, Mabtech, Nacka, Sweden) for 24 h at 40°C.
2.1.5. Viral‐antigens' stimulation of lymphocytes
Triplicates were prepared either without antigens or with the following three completely inactivated viruses: the swine flu, New Caledonia flu, cytomegalovirus (CMV). Plates were incubated for 48 h at 37°C and 5% CO2. Following washing, biotinylated antibodies (7‐B6‐1‐biotin, Mabtech, Nacka, Sweden) diluted in filtered PBS with 0.5% FCS to a final concentration of 1 μg/ml were added for a total volume of 100 μl/well. Plates were incubated for 3 h at room temperature. Following washing, 100 ml of streptavidin‐alkaline phosphatase was added, and the plates were incubated for 90 min at room temperature. After washing, a substrate was added (BCIP/NBT, BioRad, Richmond, USA) for 30 min until dark red purple spots emerged, indicating IFNγ‐secreting clones. Two independent investigators counted the dark spots under a dissection microscope. Results were expressed as means of IFNγ‐secreting cells per 105 PBMCs in triplicates in comparison to the number of cells without viral antigen treatment.
2.2. Phase I/IIa clinical trial
2.2.1. Study design
The clinical trial was a phase I/IIa open‐label study to examine the safety and efficacy of IMM124E oral administration in healthy volunteers. The subjects were enrolled in a single‐center trial at the Hadassah‐Hebrew University Medical Center in Jerusalem, Israel. The trial was registered at ClinicalTrials.gov (NCT04643561).
2.2.2. Study population
Eligible subjects were healthy males between 18 and 45 years with no acute chronic disorders and did not receive any immunomodulatory medications. Exclusion criteria included any acute illness resulting in emergency department (ED) evaluation or hospitalization in the last 6 months or any acute illness in the month before enrollment. Subjects were excluded if they had any evidence of chronic or acute infections, malignant, autoimmune, hepatic, renal, or other systemic diseases. Subjects were excluded if they ever used chronic drug therapy, took over the counter medication 4 weeks prior to enrollment, had an allergy to milk, or had substance abuse for at least a year prior to enrollment. Volunteers were also excluded if they participated in any other clinical trial within 30 days prior to the intervention. All subjects provided written informed consent.
2.2.3. Efficacy and safety assessment
The primary endpoint was the efficacy and safety of IMM124E in enhancing the immune response to SARS‐CoV‐2 spike proteins. Efficacy was demonstrated by measuring the IFNγ response after the exposure to SARS‐CoV‐2 proteins using an ELISPOT assay before and after the treatment with IMM124E in healthy volunteers. Safety was assessed by monitoring adverse events (AEs) throughout the intervention phase. AEs were defined as any unfavorable and unintended signs, symptoms, or disease that appeared during the study period, regardless of whether they were related to the study drug, accidental injuries, changes in medications, reasons for admission to the hospital, reasons for surgical procedures, and any laboratory abnormality.
2.2.4. Intervention
Five healthy volunteers meeting the inclusion criteria were recruited and received IMM‐124E at a daily dose of 600 mg for four consecutive days and 1200 mg for an additional day. Blood was collected in lithium‐heparin tubes before and after IMM‐124E treatment. Ficoll gradient separation was performed on 15 mL blood samples within 1 h of collection to isolate PBMCs.
2.2.5. ELISPOT assay using SARS‐Cov‐2 spike protein
IFNγ SFC were determined using an IFNγ Human ELISPOT kit (Abcam, UK). In brief, 96‐well PVDF bottomed plates precoated by the manufacturer with human anti‐IFNγ antibodies were used as described above.
Triplicates of 1.5 × 105 PBMCs from each subject were treated with SARS‐CoV‐2 spike protein (10 μg/ml, S1 + S2 ECD, His tag, Sino Biological, China), Hepatitis B (1 μg/ml, surface antigen EngerixTM, GSK), or RPMI without antigen at a total volume of 100 μl/well. Plates were incubated for 20 h at 37°C and 5% CO2. Following washing, dilute biotinylated antibodies were added to the plates, which were incubated for 1.5 h at room temperature. Following washing, 100 ml of streptavidin‐alkaline phosphatase was added, and the plates were incubated for 1 h at room temperature. After washing, a substrate was added (BCIP/NBT, BioRad, Richmond, USA) for 15 min until dark red purple spots emerged, indicating IFNγ‐secreting clones. Dark spots were counted using a dissection microscope. Results are expressed as percentage mean increase in SFC after a 5‐day treatment of IFNγ‐secreting cells per 1.5 × 105 PBMCs in comparison to that the cell numbers without viral antigen treatment.
2.2.6. Statistical analysis
Statistical analysis was performed using the Student's t‐test. Statistical significance was set at p < .05.
3. RESULTS
IFN induction plays a key role in the defense against coronavirus infections (Nezhad et al., 2020; Yoshikawa et al., 2010). We have assessed the effect of HBC oral administration on the generation of antiviral specific IFNγ producing clones in animal models, and against SARS‐CoV‐2 spike proteins in healthy humans. The aim was to determine the ability to activate a systemic antiviral immunity by targeting the gut immune system.
3.1. Preclinical study
In naïve mice, 5 days of HBC oral administration augmented the number of IFNγ‐positive viral‐specific T cell clones as compared to that in the control mice (Figure 1).
Figure 1.
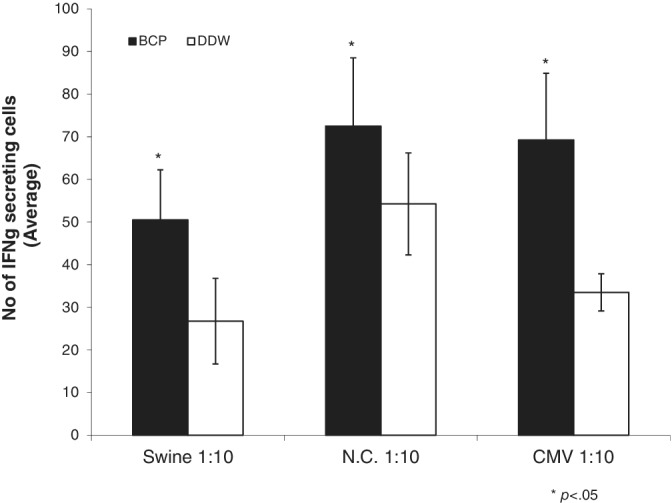
The effect of oral administration of IMM124E on antiviral T cells immunity. Mice were fed with HBC for 5 days, followed by an ELISPOT assay to assess the number of T cell clones secreting IFNγ in response to two strains of influenza and CMV viral proteins. Data are presented as the average of triplicates of the number of T cell clones compared to those in mice fed with water. CMV, cytomegalovirus; ELISPOT, enzyme‐linked immune absorbent spot; IFNγ, interferonγ
3.2. Clinical trial
Among the five healthy volunteers who received IMM124E HBC orally, mild side effects such as tiredness, abdominal discomfort, and increased acne were noted in three subjects. One of the volunteers developed flu‐like symptoms on the final day of HBC administration and thus was unable to complete the trial.
In the four subjects that completed the trial, a marked increase in the number of IFNγ‐secreting T cell clones in PBMCs incubated with SARS‐CoV‐2 spike protein before and after IMM124E treatment was observed (Figure 2). Administration of IMM124E was associated with increased SFC, which was further increased by exposure to viral antigens. The number of SFC in PBMCs incubated with HBV virus surface antigen resulted in an 85%–100% increase in IFNγ SFC (Table 1).
Figure 2.
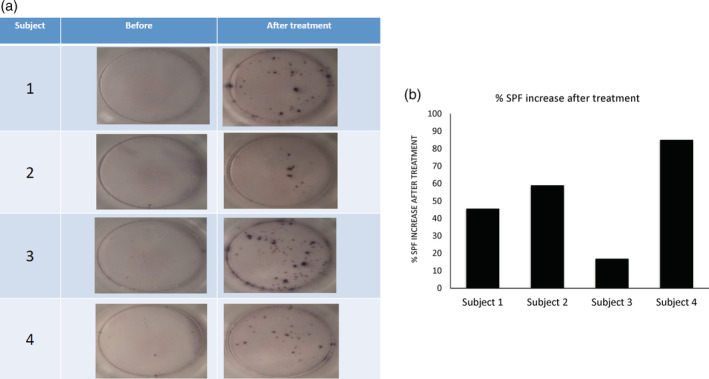
The effect of oral administration of IMM124E for 5 days on the number of T cell clones secreting IFNγ in response to the treatment of the spike antigen of SARS‐CoV‐2 (10 μg/ml). (a) Representative pictures of the clones in each of the four subjects prior to and after 5 days of IMM124E oral administration. (b) The percentage of increase in the number of IFNγ SFC clones on the last day of the study minus the number prior to drug administration in comparison to the number in negative controls. Percentages of increase in IFNγ SFC are presented after treatment following subtraction of the negative controls. IFNγ, interferonγ; SFC, spot forming colonies
Table 1.
The number of SFC in human PBMCs incubated with HBV surface antigen likewise resulted in an 85%–100% increase in IFNγ SFC before (A) and after (B) treatment with IMM124E
| A: Average # of spots before IMM124E | |||
|---|---|---|---|
| Negative | Covid‐19 | Hepatitis | |
| Patient 1 | 0 | 1.0 | 0.3 |
| Patient 2 | 0.5 | 1.3 | 0.3 |
| Patient 3 | 0.5 | 4.3 | 2.3 |
| Patient 5 | 0.5 | 2.7 | 2.0 |
| B: Average # of spots after IMM124E | |||
|---|---|---|---|
| Negative | Covid‐19 | Hepatitis | |
| Patient 1 | 9 | 14.3 | 13.7 |
| Patient 2 | 3.5 | 7.3 | 5.0 |
| Patient 3 | 22 | 34.7 | 65.3 |
| Patient 5 | 30 | 54.3 | 41.7 |
4. DISCUSSION
The results of the preclinical studies presented above show that IMM124E is a potent medication to enhance antiviral immunity across viral strains, by increasing the number of IFNγ‐secreting T cell clones in response to CMV and influenza viral antigens. The clinical data support a similar effect in humans in augmenting antiviral response against COVID‐19 and Hepatitis B.
Oral immunotherapy has been previously shown to be effective in augmenting antiviral immunity in preclinical models and humans (Gotsman et al., 2000; Gotsman et al., 2002; Ilan, 2002; Israeli et al., 2004; Safadi et al., 2003). It is a mean for altering the systemic immunity by targeting the gut immune system.
HBC contains sphingolipids that carry immune‐regulatory functions. Sphingolipids have been shown to play a major part in innate and adaptive cells, which drive the initial antiviral response and may later induce target organ damage including acute respiratory distress syndrome (ARDS).
Glycosphingolipids (GS), such as beta‐glucosylceramide (GC), are sphingolipids with the addition of a sugar moiety, and have also been proposed to promote an immune balance by changing different stages of the immune system in opposing environments (Ilan, 2019a). Evidence indicates that its beneficial effects may be mediated by (natural killer T) NKT cells, lipid rafts, and various signaling pathways including STAT (Bandyopadhyay et al., 2016; Ilan, 2019b; Lalazar et al., 2009; Shuvy et al., 2009).
GC increases the potency of antiviral vaccination by exerting a potent adjuvant effect (Mizrahi et al., 2008). Oral administration of GC could augment anti‐HBV immunity to viral proteins. Sphingolipids have also been shown to alter viral binding to cell membranes to prevent viral entry at the receptor level (Adar et al., 2020).
Exogenous sphingolipids are thought to modulate “sphingolipid rheostat,” a balance of pro/anti‐inflammatory and pro−/anti‐survival sphingolipids (Ilan, 2019a; Ishay, Nachman, et al., 2020). The addition of sphingolipid ceramide species drives cellular apoptosis and exerts anti‐inflammatory signaling (Gomez‐Munoz et al., 2010).
HBC induces T regulatory cells (Tregs) and affects NKT cell distribution and functions, which have been associated with alleviated inflammation in animal models of colitis, immune‐mediated hepatitis, and type 2 diabetes (Adar et al., 2012; Ben Ya'acov et al., 2015). The anti‐inflammatory effect of IMM124E in humans has been shown to be mediated by promoting Tregs while altering NKT cell distribution, and has been associated with significant improvement of inflammation indicated by reduced pro‐inflammatory cytokines and improved inflammation‐associated organ damage in the liver, pancreas, and adipose tissue (Adar et al., 2012; Mizrahi et al., 2012).
In addition, the antibody content in HBC may also contribute to its immunomodulatory effects. Antibodies directed at common gut bacteria may reduce chronic inflammation and gut permeability—two conditions associated with inflammation—and may improve responses toward acute insults such as viral infections.
Clinical trials in humans have shown that HBC has a high‐safety profile and can alleviate systemic inflammation and associated immune‐mediated organ damage via promoting Tregs and altering NKT cell distribution (Abdelmalek et al., (2018); Mizrahi et al., 2012).
In a controlled clinical trial in patients with chronic liver inflammation and type II diabetes, patients with biopsy‐proven nonalcoholic steatohepatitis (NASH) were orally administered with GC daily. No treatment‐related AEs were observed, and GC decreased the hepatic fat content as measured using MRI compared to that in the placebo. HbA1C and HDL levels also decreased in patients treated with GC. The beneficial effects were associated with the alteration of NKT distribution (Ilan, Maron, et al., 2010; Ilan, Zigmond, et al., 2010).
In a multicenter prospective randomized double‐blind placebo‐controlled multidose study, 133 patients with histologically proven NASH received placebo (n = 44), IMM124E 600 mg TID (n = 43), or IMM124E 1200 mg TID (n = 46) for 24 weeks. Both doses of HBC were well tolerated and safe comparable to that of placebo. A trend for a reduction in ALT by >30% was observed in both drug arms. In patients with baseline ALT levels higher than 50 IU/L, IMM124E (1200 mg) decreased ALT by >30% in 36.4% of the patients in comparison to 13.6% of placebo‐treated subjects. IMM124E (1200 mg) also improved aspartate aminotransferase (AST), CK18, interleukin (IL)‐1β, IFNγ, and IL‐17 (Manal, 2018).
Recent data have suggested that using digital systems‐based therapies might improve the response to antiviral agents and specifically against COVID‐19 (Gelman et al., 2020).
Taken together, the data of the preclinical and clinical studies suggest that IMM124E can be used to augment antiviral effectiveness against SARS‐CoV‐2 and other respiratory viruses, and reduce the incidence and/or severity of the associated immune‐mediated ARDS and organ damage, while potentially enabling the immune system to overcome viral resistance mechanisms.
The high‐safety profile of IMM124E enables its administration to patients at all disease stages to reduce disease severity. In addition, it may allow its application to prevent the transmission of SARS‐CoV‐2 and other respiratory pathogens in densely populated areas, among household contacts of infected patients, and among medical personnel and other workers who are at high risk of exposure to infected patients. The relatively low cost of IMM124E can potentially minimize care provider burdens and costs, and enable its global application. Future studies will assess the effect of IMM124E in patients infected with COVID‐19 to determine its ability to treat and prevent further infections.
CONFLICT OF INTEREST
No financial support was received for the study. Yaron Ilan is the founder of Oberon Sciences, and a consultant for Teva, ENZO, Protalix, Betalin Therapeutics, Immuron, SciM, Natural Shield, Tiziana Pharma, Plantylight, and Exalenz Bioscience.
Ishay, Y. , Potruch, A. , Weksler‐Zangen, S. , Shabat, Y. , & Ilan, Y. (2022). Augmented antiviral T cell immunity by oral administration of IMM‐124E in preclinical models and a phase I/IIa clinical trial: A method for the prevention and treatment of COVID‐19. Drug Development Research, 83, 615–621. 10.1002/ddr.21890
Yuval Ishay, Assaf Potruch, and Sarah Weksler‐Zangen contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abdelmalek, M. F. , Freilich, B. L. , Harrison, S. , Powell, E. E. , Rinella, M. E. , Tobis, N. , Peres, D. , Kanellos, J. , Lalazar, G. , & Sanyal, A. J. (2018). Imm‐124E improves metabolic endotoxemia and markers of liver injury in nonalcoholic steatohepatitis. Hepatology, 68(Suppl 1), 108. [Google Scholar]
- Adar, T. , Ben Ya'acov, A. , Lalazar, G. , Lichtenstein, Y. , Nahman, D. , Mizrahi, M. , Wong, V. , Muller, B. , Rawlin, G. , & Ilan, Y. (2012). Oral administration of immunoglobulin G‐enhanced colostrum alleviates insulin resistance and liver injury and is associated with alterations in natural killer T cells. Clinical and Experimental Immunology, 167(2), 252–260. 10.1111/j.1365-2249.2011.04511.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adar, T. , Shankar Lankalapalli, R. , Bittman, R. , & Ilan, Y. (2020). The assembly of glycosphingolipid determines their immunomodulatory effect: A novel method for structure‐based design of immunotherapy. Cell Immunology, 355, 104157. 10.1016/j.cellimm.2020.104157 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay, K. , Marrero, I. , & Kumar, V. (2016). NKT cell subsets as key participants in liver physiology and pathology. Cellular and Molecular Immunology, 13(3), 337–346. 10.1038/cmi.2015.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ya'acov, A. , Lichtenstein, Y. , Zolotarov, L. , & Ilan, Y. (2015). The gut microbiome as a target for regulatory T cell‐based immunotherapy: Induction of regulatory lymphocytes by oral administration of anti‐LPS enriched colostrum alleviates immune mediated colitis. BMC Gastroenterology, 15, 154. 10.1186/s12876-015-0388-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman, R. , Bayatra, A. , Kessler, A. , Schwartz, A. , & Ilan, Y. (2020). Targeting SARS‐CoV‐2 receptors as a means for reducing infectivity and improving antiviral and immune response: An algorithm‐based method for overcoming resistance to antiviral agents. Emerging Microbes Infections, 9(1), 1397–1406. 10.1080/22221751.2020.1776161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Munoz, A. , Gangoiti, P. , Granado, M. H. , Arana, L. , & Ouro, A. (2010). Ceramide‐1‐phosphate in cell survival and inflammatory signaling. Advances in Experimental Medicine and Biology, 688, 118–130. 10.1007/978-1-4419-6741-1_8 [DOI] [PubMed] [Google Scholar]
- Gotsman, I. , Alper, R. , Klein, A. , Rabbani, E. , Engelhardt, D. , & Ilan, Y. (2002). Inducing oral immune regulation of hepatitis B virus envelope proteins suppresses the growth of hepatocellular carcinoma in mice. Cancer, 94(2), 406–414. 10.1002/cncr.10237 [DOI] [PubMed] [Google Scholar]
- Gotsman, I. , Beinart, R. , Alper, R. , Rabbani, E. , Engelhardt, D. , & Ilan, Y. (2000). Induction of oral tolerance towards hepatitis B envelope antigens in a murine model. Antiviral Research, 48(1), 17–26. 10.1016/s0166-3542(00)00113-3 [DOI] [PubMed] [Google Scholar]
- Ilan, Y. (2002). Immune downregulation leads to upregulation of an antiviral response: A lesson from the hepatitis B virus. Microbes Infections, 4(13), 1317–1326. 10.1016/s1286-4579(02)00012-6 [DOI] [PubMed] [Google Scholar]
- Ilan, Y. (2019a). Beta‐Glycosphingolipids as mediators of both inflammation and immune tolerance: A manifestation of randomness in biological systems. Frontiers in Immunology, 10, 1143. 10.3389/fimmu.2019.01143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan, Y. (2019b). Immune rebalancing by oral immunotherapy: A novel method for getting the immune system back on track. Journal of Leukocyte Biology, 105(3), 463–472. 10.1002/JLB.5RU0718-276RR [DOI] [PubMed] [Google Scholar]
- Ilan, Y. , Ben Ya'acov, A. , Shabbat, Y. , Gingis‐Velitski, S. , Almon, E. , & Shaaltiel, Y. (2016). Oral administration of a non‐absorbable plant cell‐expressed recombinant anti‐TNF fusion protein induces immunomodulatory effects and alleviates nonalcoholic steatohepatitis. World Journal of Gastroenterology, 22(39), 8760–8769. 10.3748/wjg.v22.i39.8760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan, Y. , Maron, R. , Tukpah, A. M. , Maioli, T. U. , Murugaiyan, G. , Yang, K. , Wu, H. Y. , & Weiner, H. L. (2010). Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in Ob/Ob mice. Proceedings of the National Academy of Sciences of the United States of America, 107(21), 9765–9770. 10.1073/pnas.0908771107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan, Y. , Zigmond, E. , Lalazar, G. , Dembinsky, A. , Ben Ya'acov, A. , Hemed, N. , Kasis, I. , Axelrod, E. , Zolotarov, L. , Klein, A. , El Haj, M. , Gandhi, R. , Baecher‐Allan, C. , Wu, H. , Murugaiyan, G. , Kivisakk, P. , Farez, M. F. , Quintana, F. J. , Khoury, S. J. , & Weiner, H. L. (2010). Oral administration of OKT3 monoclonal antibody to human subjects induces a dose‐dependent immunologic effect in T cells and dendritic cells. Journal of Clinical Immunology, 30(1), 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishay, Y. , Kessler, A. , Schwarts, A. , & Ilan, Y. (2020). Antibody response to SARS‐co‐V‐2, diagnostic and therapeutic implications. Hepatology Communications, 4(12), 1731–1743. 10.1002/hep4.1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishay, Y. , Nachman, D. , Khoury, T. , & Ilan, Y. (2020). The role of the sphingolipid pathway in liver fibrosis: An emerging new potential target for novel therapies. American Journal of Physiology: Cell Physiology, 318(6), C1055–C1064. 10.1152/ajpcell.00003.2020 [DOI] [PubMed] [Google Scholar]
- Israeli, E. , Safadi, R. , Melhem, A. , Pappo, O. , Shibolet, O. , Klein, A. , Hemed, N. , Thalenfeld, B. , Engelhardt, D. , Rabbani, E. , & Ilan, Y. (2004). Induction of oral immune regulation towards liver‐extracted proteins for treatment of chronic HBV and HCV hepatitis: Results of a phase I clinical trial. Liver International, 24(4), 295–307. 10.1111/j.1478-3231.2004.0935.x [DOI] [PubMed] [Google Scholar]
- Lalazar, G. , Ben Ya'acov, A. , Livovsky, D. M. , El Haj, M. , Pappo, O. , Preston, S. , Zolotarov, L. , & Ilan, Y. (2009). Beta‐glycoglycosphingolipid‐induced alterations of the STAT signaling pathways are dependent on CD1d and the lipid raft protein flotillin‐2. American Journal of Pathology, 174(4), 1390–1399. 10.2353/ajpath.2009.080841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manal, F. , Abdelmalek, B. L. F. , Harrison, S. A. , Powel, E. E. , McCarthy Rinella, M. E. , Tobis, N. , Peres, D. , Kanellos, J. , Lalazar, G. , & Sanyal, A. J. (2018). Imm‐124E improves metabolic Endotoxemia and markers of liver injury in nonalcoholic Steatohepatitis. Hepatology, 68, 1. [Google Scholar]
- Mason, S. , Devincenzo, J. P. , Toovey, S. , Wu, J. Z. , & Whitley, R. J. (2018). Comparison of antiviral resistance across acute and chronic viral infections. Antiviral Research, 158, 103–112. 10.1016/j.antiviral.2018.07.020 [DOI] [PubMed] [Google Scholar]
- Mizrahi, M. , Lalazar, G. , Ben Ya'acov, A. , Livovsky, D. M. , Horowitz, Y. , Zolotarov, L. , Alder, R. , Shouval, D. , & Ilan, Y. (2008). Beta‐glycoglycosphingolipid‐induced augmentation of the anti‐HBV immune response is associated with altered CD8 and NKT lymphocyte distribution: A novel adjuvant for HBV vaccination. Vaccine, 26(21), 2589–2595. 10.1016/j.vaccine.2008.03.026 [DOI] [PubMed] [Google Scholar]
- Mizrahi, M. , Shabat, Y. , Ben Ya'acov, A. , Lalazar, G. , Adar, T. , Wong, V. , Muller, B. , Rawlin, G. , & Ilan, Y. (2012). Alleviation of insulin resistance and liver damage by oral administration of Imm124‐E is mediated by increased Tregs and associated with increased serum GLP‐1 and adiponectin: Results of a phase I/II clinical trial in NASH. Journal of Inflammation Research, 5, 141–150. 10.2147/JIR.S35227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosaddeghi, P. , Shahabinezhad, F. , Dehghani, Z. , Farahmandnejad, M. , Taghipour, M. J. , Moghadami, M. , Nezafat, N. , Masoompour, S. M. , & Negahdaripour, M. (2020). Therapeutic approaches for COVID‐19 based on the interferon‐mediated immune responses. Current Signal Transduction Therapy. 10.20944/preprints202003.0206.v1 [DOI] [Google Scholar]
- Omer, S. B. , Malani, P. , & Del Rio, C. (2020). The COVID‐19 pandemic in the US: A clinical update. JAMA, 323(18), 1767–1768. 10.1001/jama.2020.5788 [DOI] [PubMed] [Google Scholar]
- Otto, W. , Najnigier, B. , Stelmasiak, T. , & Robins‐Browne, R. M. (2011). Randomized control trials using a tablet formulation of hyperimmune bovine colostrum to prevent diarrhea caused by enterotoxigenic Escherichia coli in volunteers. Scandinavian Journal of Gastroenterology, 46(7–8), 862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safadi, R. , Israeli, E. , Papo, O. , Shibolet, O. , Melhem, A. , Bloch, A. , Rowe, M. , Alper, R. , Klein, A. , Hemed, N. , Segol, O. , Thalenfeld, B. , Engelhardt, D. , Rabbani, E. , & Ilan, Y. (2003). Treatment of chronic hepatitis B virus infection via oral immune regulation toward hepatitis B virus proteins. American Journal of Gastroenterology, 98(11), 2505–2515. 10.1111/j.1572-0241.2003.07700.x [DOI] [PubMed] [Google Scholar]
- Shuvy, M. , Ben Ya'acov, A. , Zolotarov, L. , Lotan, C. , & Ilan, Y. (2009). Beta glycosphingolipids suppress rank expression and inhibit natural killer T cell and CD8+ accumulation in alleviating aortic valve calcification. International Journal of Immunopathology and Pharmacology, 22(4), 911–918. 10.1177/039463200902200406 [DOI] [PubMed] [Google Scholar]
- Silva, E. G. D. S. O. , Rangel, A. H. D. N. , Mürmam, L. , Bezerra, M. F. , & Oliveira, J. P. F. D. (2019). Bovine colostrum: Benefits of its use in human food. Food Science and Technology, 39, 355–362. [Google Scholar]
- Sponseller, J. K. , Steele, J. A. , Schmidt, D. J. , Kim, H. B. , Beamer, G. , Sun, X. , & Tzipori, S. (2015). Hyperimmune bovine colostrum as a novel therapy to combat Clostridium difficile infection. The Journal of Infectious Diseases, 211(8), 1334–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. , Yu, Y. , Xu, J. , Shu, H. , Xia, J. , Liu, H. , Wu, Y. , Zhang, L. , Yu, Z. , Fang, M. , Yu, T. , Wang, Y. , Pan, S. , Zou, X. , Yuan, S. , & Shang, Y. (2020). Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: A single‐centered, retrospective, observational study. Lancet Respiratory Medicine, 8(5), 475–481. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa, T. , Hill, T. E. , Yoshikawa, N. , Popov, V. L. , Galindo, C. L. , Garner, H. R. , Peters, C. J. , & Tseng, C. T. (2010). Dynamic innate immune responses of human bronchial epithelial cells to severe acute respiratory syndrome‐associated coronavirus infection. PLoS One, 5(1), e8729. 10.1371/journal.pone.0008729 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


