Abstract
Background:
Maternal obesity and gestational diabetes mellitus (GDM) contribute to increased risk for type 2 diabetes mellitus (T2DM) among both mothers and their offspring. Randomized trials demonstrated T2DM risk reduction in adults following lifestyle behavior change and modest weight loss; the evidence base for at-risk children remains limited.
Purpose:
Evaluate the feasibility, acceptability, and preliminary efficacy of a T2DM prevention intervention for mother-child dyads delivered by Federally Qualified Health Center staff.
Methods:
A group randomized design tested the effects of a behavioral lifestyle intervention on T2DM risk factors in women with a history of GDM and their 8- to 12-year-old children. Mother-child dyads were recruited and randomized to intervention or wait-listed control conditions. Intervention participants completed the 13-week intervention; control participants received standard of care. Baseline and 13-week measures assessed program acceptability and feasibility, and explored effects on body weight, waist circumference, hemoglobin A1c, and lifestyle behaviors.
Results:
Forty-two dyads were randomized and 35 (83%) completed pre-/post-measurements. Participants and program leaders positively rated content and engagement. Nearly all strongly agreed that activities were enjoyable (97%), applicable (96%), useful (97%), and motivational (96%). Attendance averaged 65% across 2 cohorts; delivery costs were approximately $225/dyad. There were no significant differences in body weight, BMI (or BMI z-score), waist circumference, hemoglobin A1c, diet quality, physical activity, sleep, or home environment changes between intervention and control groups.
Conclusions:
A family T2DM prevention program was feasibly delivered by FQHC staff, and acceptable to mothers and children. Program efficacy will be evaluated in an adequately powered clinical trial.
Keywords: diabetes, gestational, mothers, diabetes mellitus, type 2, lifestyle, child, primordial prevention
Lay Summary: The feasibility and acceptability of a 13-week lifestyle behavior change program designed to reduce type 2 diabetes mellitus risk factors among low-income women and children was tested. Participants were patients at a Federally Qualified Health Center (FQHC) with a history of gestational diabetes or prediabetes and their 8- to 12-year-old children. Program acceptability and feasibility were assessed using surveys and interviews completed by participants and FQHC staff, and effects on weight, diabetes risk factors, and lifestyle behaviors were explored. Forty-two mother-child pairs were randomized to participate in intervention or control conditions. Thirty-five pairs (83%) completed measurements. Participants and program leaders positively rated content and engagement. A majority strongly agreed that weekly activities were enjoyable (97%), applicable (96%), useful (97%), and motivational (96%). Attendance averaged 65% across 2 intervention groups; delivery costs were estimated at $225 per pair. There were no significant differences in body weight, body mass index, waist circumference, blood glucose, diet quality, physical activity, sleep, or home environment changes between intervention and control mothers or children. Findings from this pilot study will inform a larger clinical trial to establish the efficacy of the intervention on diabetes risk factors in FQHC patients.
Background
Emerging evidence suggests that maternal obesity and gestational diabetes mellitus (GDM) contribute to increased risk for type 2 diabetes mellitus (T2DM) among offspring.1-5 Findings from the SEARCH for Diabetes in Youth Study suggested that exposure to GDM in utero predicted T2DM development in early adulthood. 6 Combined with genetic risk factors and increased prevalence of obesity among U.S. youth, 7 offspring of women with a history of gestational diabetes mellitus (GDM) are at increased risk for developing diabetes, particularly if excess adiposity is present. 8
Several large scale randomized trials have demonstrated that lifestyle modification interventions that promote modest weight loss and increased physical activity have reduced T2DM risk in adults.9-11 In contrast, the evidence base for diabetes prevention for at-risk youth is limited and represents a significant gap in the field of diabetes prevention.
Given the rising incidence of GDM in women12-14 and continued high levels of obesity among children and adolescents, 7 we addressed this evidence gap by developing and pilot testing a family-based behavioral-focused approach to T2DM prevention that prioritized mothers with history of GDM and their children. The intervention was embedded within a Federally Qualified Health Center (FQHC) and delivered by trained FQHC personnel to encourage changes to the home food and physical activity environment. The aims of this study were to evaluate the feasibility and acceptability of this intervention within the FQHC setting. Potential for adoption, integration, and scaling across the FQHC clinic network were also explored.
Methods
The study used a group randomized design to test the feasibility and acceptability of a behavioral lifestyle intervention on T2DM risk factors in women with a history of GDM and their 8- to 12-year-old children. The intervention was developed and delivered in collaboration with an FQHC in Southern Arizona serving more than 110 000 uninsured and underinsured patients. This work was university scholar-initiated and guided but involved active participation and support by FQHC colleagues through a formal subcontract and a research team consisting of university researchers and FQHC personnel. The research team met bimonthly for 2 years (2018-2020), and adaptation of intervention content was conducted in collaboration with these colleagues. 15 Two FQHC clinic sites were selected to participate in the study. Participants were recruited as mother-child dyads to either the intervention or a wait-listed control condition. Female FQHC patients with a history of GDM during any of their pregnancies and/or a prior diagnosis of prediabetes and who had children 8- to 12-years-old at time of study were identified through an electronic medical records search and contacted by phone and postal mail to participate. The participating child was required to be a biological child but did not have to be the direct product of a pregnancy with GDM. Respondents meeting eligibility criteria were invited to attend 1 of 6 information sessions held prior to the start of the intervention in September 2019, where those who wished to participate provided informed consent (mother) and assent (child), were assigned a study identification number, and completed baseline measurements. Participants completing baseline measurements were block randomized (block size of 4) to either the intervention or wait-listed control conditions by selecting a numbered envelope with a blinded treatment condition inside. Randomized participants were assigned to a clinic site based on their preferred language (English or Spanish). Participants began the 13-week face-to-face group-based intervention within 2 weeks of completing baseline measurements while wait-listed control families continued to receive FQHC standard of care for persons with pre-diabetes (referral to a Registered Dietitian, follow-ups with primary care provider every 6 months, redraw hemoglobin A1c every 6 months) over the same 13-week period. Intervention blinding was not possible with this design.
Intervention content and delivery was modeled after our previous T2DM prevention work with youth and families16-19; selection of program outcomes was guided by the 2017 U.S. Preventive Task Force (USPSTF) to meet national recommendations for nutrition, physical activity, and behavior change for youth. A description of the study protocol and how we adapted the curriculum from the Diabetes Prevention Program9,10 and our prior work are published elsewhere.15,20 Briefly, following a series of focus group discussions with FQHC patients who were mothers, we refined the program curriculum and structure to address potential barriers to participation, including access to childcare, transportation, and the desire to involve multiple family members to reinforce program objectives. 20 Intervention content focused on strategies for T2DM risk reduction (Table 1). Session topics focused on nutrition, physical activity, and behavioral health. Evidence-based behavior change techniques and behavioral targets21,22 were incorporated into each session along with activities such as cooking demonstrations and family physical activity. The intervention was delivered in a group-based format once weekly for 13 consecutive weeks in English or Spanish by bilingual group leaders. Group leaders, who were selected from FQHC staff based on their nutrition and health competencies (certified group fitness instructors and registered dietitian nutritionists), each completed 16 h of group-based experiential and didactic training led by the research team using standardized training materials that included a lifestyle coach manual to ensure program fidelity and consistent delivery across both languages. Dyads attended 1.5-h long sessions which were similarly structured, consisting of: family physical activity, a small group (2-3 families) discussion on goal setting, a food demonstration incorporating vegetables, legumes, and whole grains, and an activity designed to increase foundational knowledge and skills related to healthy food selection, family physical activity, and creating a home environment wherein healthy behaviors and choices become the default. Mothers and children were divided into 2 groups during the last half of each session. Mothers participated in parenting discussions which focused on strategies to engage the entire family in lifestyle modification, while children engaged in age-appropriate physical activities. Every third session, a behavioral health specialist led stress management and sleep exercises for all participants.
Table 1.
Intervention Curriculum Overview.
| Week | Session topics |
|---|---|
| 1 | Goals of the Program; Meet the Coaches; Understanding Diabetes Risk |
| 2 | Energy Density of Foods (Whoa, Slow, Go); Planning Family Meals; Goal Setting |
| 3 | Modifiable Diabetes Risk Factors; Importance of Physical Activity; Stress and Health |
| 4 | Enjoy Legumes, Whole Grains and Vegetables; Create a Low-Stress Eating Environment |
| 5 | Choosing Low-Kcal Tasty Beverages; Label Reading; Problem Solving |
| 6 | Managing Stress; Understanding Link Between Stress and Chronic Disease |
| 7 | Serve the Right Amount of Food; Whoa Foods; Reducing and Replacing Unhealthy Fats |
| 8 | Label Reading; Healthy Snacks |
| 9 | Food Marketing; Healthy Choices at Restaurants |
| 10 | Grocery Shopping and Meal Planning; Talking Back to Negative Thoughts |
| 11 | Practicing Stress Management; Getting Good Sleep |
| 12 | Understanding Hunger and Cravings; Food Traditions and Culture |
| 13 | Sustaining Healthy Behaviors; Family Physical Activity; Good Things About Me |
We explored the feasibility of collecting rigorous, T2DM-related outcomes at baseline and immediately following the intervention (13 weeks) in anticipation of a future definitive trial. The primary outcome was technician-measured pre-/post-intervention change in body weight and body mass index (BMI) in mothers and change in BMI z-scores for children. Standing height and weight measurements were completed using a Schorr measuring board (Schorr Products, Olney, MD) and a calibrated digital scale (model 880; SECA, Hamburg, Germany). The average of 2 measurements for both height and weight were used and BMI was calculated as weight (kg) divided by height squared (m2). 23 In children, BMI percentile was determined using age- and sex-specific growth charts 24 and BMI z-score change calculated. 25 Waist circumference was measured at the umbilicus, completed in duplicate with the average of the 2 measures used. Secondary physiological outcomes in both mothers and children included changes in blood pressure and hemoglobin A1c (HbA1c), collected using the Mindray Accutorr V Vital Signs Monitor and Siemens DCA Vantage Analyzer, respectively.
Behavioral outcomes associated with weight trajectory and T2DM risk in children and mothers were also assessed at baseline and 13 weeks (post-intervention). Child dietary intake was assessed with 2 nonconsecutive, interviewer-administered 24-h dietary recalls conducted telephonically by trained nutritionists and entered into the Nutrient Data System for Research (Minneapolis, MN, v. 2012). 26 Diet quality and its component scores were calculated using the Healthy Eating Index-2015, a valid and reliable measure of diet quality that assesses the degree to which individual intake conforms to dietary recommendations.27,28 Parent dietary intake was assessed using the Southwest Food Frequency Questionnaire, a Spanish bicultural and bilingual self-administered semi-quantitative 158-item questionnaire. 29 Child physical activity and sedentary behavior was assessed using the Youth Activity Profile, a self-administered 7-day recall questionnaire validated for use in children ages 8- to 12-years-old, 30 which estimated daily energy expenditure, hours per day spent in each activity, and number of activities reported for each category. Child sleep behavior was assessed using the Children’s Sleep Habits Questionnaire-Abbreviated, a 22-item parent report of key child sleep domains. 31 Parent physical activity was assessed using the validated Arizona Activity Frequency Questionnaire, a self-administered 59-item questionnaire in which participants reported whether they performed each activity during the past 28 days. 32 The Family Nutrition and Physical Activity Tool, a 21-item survey, was used to describe the family home environment and practices associated with children’s risk of becoming overweight.33,34 Participant demographic data were collected using the Protocol for Responding to and Assessing Patients’ Assets, Risks, and Experiences (PRAPARE) questionnaire developed for use in health care settings 35 and already routinely implemented by El Rio. All surveys were available in English and Spanish. Participants received $50 each upon completion of study measurements at each time point.
Program feasibility was assessed using participant recruitment and enrollment rates and by fidelity of intervention delivery by FQHC staff, assessed by trained researchers who observed 3 of 13 sessions for each group following an established rubric. Program acceptability was assessed by session attendance, weekly participant, and coach surveys (each consisting of 7 Likert-response questions evaluating weekly curriculum topics for relevance, ease of understanding/delivery, motivation, application to daily life, enjoyment, engagement, confidence) rated on a 0 to 3 scale; and, 3 open-ended questions soliciting best liked/least liked activities and suggestions), observations of engagement of participants during program activities by coaches and researchers, and participant retention rates. Program costs were documented throughout the intervention period using a bottom-up micro-costing approach that included direct medical and non-medical costs (eg, personnel gross hourly salaries, intervention material costs, and overhead costs related to use of facilities for prevention services). Potential for adoption, integration, and scale were explored through semi-structured interviews with FQHC staff and administrators. The interviews were conducted by a trained researcher who was not part of the original research team or involved in the intervention. Interviews were conducted after intervention and data collection were completed. The interviewer probed for successes, challenges, alignment with the FQHC’s needs and mission, leadership support, and opportunities for growth and sustainability.
All continuous variables were summarized using descriptive statistics and presented by treatment group. Categorical variables were summarized using frequency counts and percentages. Baseline sociodemographic and clinical data were compared between the intervention and wait-listed control groups. Dichotomous and ordinal variables were examined using either a chi-square test or Fishers exact test, and continuous measures were analyzed using a 2-sample t-test or 2 sample non-parametric Wilcoxon Rank Sum Test (if assumptions of normality were not met). Analyses compared the changes from baseline to follow-up between the intervention versus wait-listed control groups using 2-sample sample t-tests. Changes in body weight and BMI z-score from the investigators’ prior studies informed sample size and power calculations. For mothers, the calculation was based on mean change in body weight of −6.0% (standard deviation = 4.0%) after 6 months of intervention. 17 For child participants, the calculation was based on a mean change in BMI z-score of 0.054 (standard deviation = 0.02) after 3 months of intervention. 16 We assumed that there would be 3 groups with 10 dyads per study condition (n = 30 intervention vs n = 30 wait-listed control) over 2 intervention periods (total of n = 60 participants). The statistical power was >80% for the mothers and 95% for the children assuming an intra-group correlation or 0.15 or below (2-sided alpha level of .05). As this was a pilot study to provide estimates for a larger trial, there was no adjustment for multiple comparisons.
Results
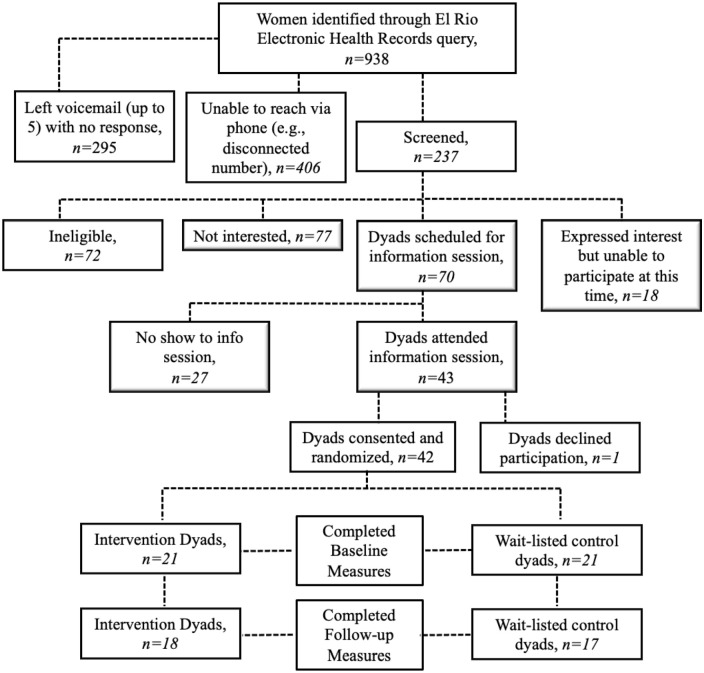
Nine hundred and thirty-eight women were identified through an electronic records query as potentially eligible per study criteria. Two hundred and thirty-seven were screened for eligibility. Of those, 70 were eligible and scheduled for an information session; 42 consented to participate and were randomized with their 8- to 12-year-old child. Thirty-five mother-child pairs (83%) completed post-intervention measurements at 13 weeks (Figure 1).
Figure 1.
Participant flow.
Participant characteristics are described in Table 2. Mothers were on average 39.9 ± 6.2 years old, 83% Hispanic or Latino, 54% White. Sixteen of 42 (38%) self-selected to receive Spanish-language study materials. Ninety-two percent had a BMI of ≥30 kg/m2 which indicated the presence of obesity. Median HbA1c was 5.8 (IQR = 5.6-6.0), indicative of prediabetes. 36 Fifty-seven percent of adults reported an annual household income of $25 000 or greater; 43% reported educational attainment beyond high school, and 60% were employed at the time of the study. Children had a median age of 10.2 years-old (IQR = 9.4-11.0), 89% Hispanic, 60% White, 46% female. Sixty-eight percent of children were overweight or obese with a mean HbA1c of 5.3 (normoglycemic). There were no statistically significant demographic differences between those who completed the program and those who did not.
Table 2.
Baseline Characteristics of Participants Completing 13-Week Measures, n = 35 Dyads.
| Mother | Intervention | Control | Total | P-value |
|---|---|---|---|---|
| N = 17 | N = 18 | N = 35 | ||
| Age (years) | 40.8 (6.1) | 39.1 (6.4) | 39.9 (6.2) | .44 a |
| Body weight (kg) | 85.3 (15.3) | 93.2 (17.7) | 89.4 (16.8) | .16 a |
| BMI (kg/m2) | 33.7 (5.3) | 36.0 (6.4) | 34.9 (5.9) | .25 a |
| Normal | 1 (6%) | 0 (0%) | 1 (3%) | .79 b |
| Overweight | 2 (12%) | 2 (11%) | 4 (11%) | |
| Obese | 14 (82%) | 16 (89%) | 30 (86%) | |
| Hemoglobin A1c (%) | 5.8 (5.4-6.0) | 5.8 (5.7-6.1) | 5.8 (5.6-6.0) | .65 c |
| Waist circumference (cm) | 102.8 (97.2-118.9) | 111.6 (100.7-121.8) | 109.3 (97.2-121.8) | .60 c |
| Systolic BP (mmHg) | 119.2 (15.0) | 121.6 (12.5) | 120.4 (13.6) | .61 a |
| Diastolic BP (mmHg) | 73.8 (8.8) | 70.6 (8.2) | 72.2 (8.5) | .28 a |
| Household size | 5.2 (1.7) | 4.9 (1.0) | 5.1 (1.3) | .62 a |
| Ethnicity | .03 b | |||
| Non-Hispanic | 5 (29%) | 0 (0%) | 5 (14%) | |
| Hispanic | 12 (71%) | 17 (94%) | 29 (83%) | |
| No response | 0 (0%) | 1 (6%) | 1 (3%) | |
| Race | .37 b | |||
| American Indian/Alaskan Native | 1 (6%) | 2 (11%) | 3 (9%) | |
| Black/African American | 3 (18%) | 0 (0%) | 3 (9%) | |
| White | 8 (47%) | 11 (61%) | 19 (54%) | |
| Other | 5 (29%) | 5 (28%) | 10 (29%) | |
| Income | .63 d | |||
| <$25 000 | 8 (47%) | 7 (39%) | 15 (43%) | |
| ≥$25 000 | 9 (53%) | 11 (61%) | 20 (57%) | |
| Benefits | .41 d | |||
| No benefit | 8 (47%) | 6 (33%) | 14 (40%) | |
| Any benefits | 9 (53%) | 12 (67%) | 21 (60%) | |
| Insurance | .40 b | |||
| Private insurance | 1 (6%) | 4 (22%) | 5 (14%) | |
| Public insurance | 14 (82%) | 12 (67%) | 26 (74%) | |
| No insurance | 2 (12%) | 2 (11%) | 4 (11%) | |
| Employment | .63 b | |||
| Employed | 11 (65%) | 10 (56%) | 21 (60%) | |
| Unemployed (seeking work) | 2 (12%) | 5 (28%) | 7 (20%) | |
| Unemployed (not seeking work) | 4 (24%) | 3 (17%) | 7 (20%) | |
| Education | .18 d | |||
| Less than a high school degree | 4 (24%) | 7 (39%) | 11 (31%) | |
| High school diploma or equivalent | 3 (18%) | 6 (33%) | 9 (26%) | |
| More than high school | 10 (59%) | 5 (28%) | 15 (43%) |
Data are presented as mean (SD) or median (IQR) for continuous measures, and n (%) for categorical measures.
Benefits include: Supplemental Nutrition Assistance Program; Social Security; Special Supplemental Nutrition Program for Women, Infants, and Children; Veteran’s Administration.
Public Insurance includes: Medicaid, Arizona Health Cost Containment System, KidsCare.
Two sample t-test.
Fishers’ Exact Test.
Wilcoxon Rank Sum Test.
Pearson’s Chi-squared Test.
| Child | Intervention | Control | Total | P-value |
|---|---|---|---|---|
| N = 17 | N = 18 | N = 35 | ||
| Age (years) | 10.2 (9.9-11.1) | 10.1 (9.1-10.7) | 10.2 (9.4-11.0) | .32 a |
| Weight (kg) | 46.5 (37.5-54.5) | 45.3 (35.0-49.3) | 46.5 (36.8-52.0) | .43 a |
| BMI (kg/m2) | 21.3 (18.6-22.7) | 21.4 (20.1-24.6) | 21.3 (18.7-24.6) | .84 a |
| <5th Percentile | 0 (0%) | 1 (6%) | 1 (3%) | .76 b |
| ≥5th <85th Percentile | 6 (35%) | 4 (22%) | 10 (29%) | |
| ≥85th <95th Percentile | 6 (35%) | 6 (33%) | 12 (34%) | |
| ≥95th Percentile | 5 (29%) | 7 (39%) | 12 (34%) | |
| Hemoglobin A1c (%) | 5.3 (0.3) | 5.3 (0.3) | 5.3 (0.3) | .85 c |
| Waist circumference | 79.7 (13.2) | 79.1 (12.1) | 79.4 (12.5) | .88 c |
| Systolic BP (mmHg) | 106.5 (10.9) | 104.2 (10.7) | 105.3 (10.7) | .54 c |
| Diastolic BP (mmHg) | 59.5 (8.2) | 58.8 (6.9) | 59.1 (7.5) | .81 c |
| Sex | .13 d | |||
| Female | 10 (59%) | 6 (33%) | 16 (46%) | |
| Male | 7 (41%) | 12 (67%) | 19 (54%) | |
| Ethnicity | .045 b | |||
| Non-Hispanic | 4 (24%) | 0 (0%) | 4 (11%) | |
| Hispanic | 13 (76%) | 18 (100%) | 31 (89%) | |
| Race | 1.00 b | |||
| American Indian/Alaskan Native | 1 (6%) | 2 (11%) | 3 (9%) | |
| Black/African American | 1 (6%) | 0 (0%) | 1 (3%) | |
| White | 10 (59%) | 11 (61%) | 21 (60%) | |
| Other | 5 (29%) | 5 (28%) | 10 (29%) |
Data are presented as mean (SD) or median (IQR) for continuous measures, and n (%) for categorical measures.
Wilcoxon Rank Sum Test.
Fishers’ Exact Test.
Two sample t-test.
Chi-squared Test.
Due to scheduling constraints of the participating FQHC clinics (which offered multiple programs in a limited space), the number of groups was consolidated from the originally planned 3 groups (up to 10 dyads per group) per intervention or wait-listed control condition to 2 groups (up to 15 dyads per group) per condition. The COVID19 pandemic disrupted the recruitment and conduct of a second cohort of participants planned for Spring 2020, reducing our sample size from N = 60 (anticipated) to N = 41 (actual). Program attendance averaged 65% across both language formats, with the Spanish language session attendance exceeding 75%. Session observations confirmed strong intervention adherence, and program delivery within the 1.5 h per week allotted time frame. Coaches used evidence-based information, encouraged participant comments and questions, and kept families engaged in intervention activities “all or almost all the time (>90%).” Participants and lifestyle coaches positively rated the program content and engagement strategies. Nearly all strongly agreed that weekly activities were enjoyable (97%), applicable (96%), useful (97%), and motivated them to make lifestyle changes (96%). Participants particularly enjoyed mindfulness/breathing activities, learning to read nutrition labels, understanding the role of sleep, and menu planning activities. Coaches positively rated ease of program implementation (average 4.8/5.0). A majority (88%) of coaches indicated the program topics motivated them to lead the sessions and that participants were actively engaged in session activities. Mothers and lifestyle coaches preferred that activities were delivered to the dyad unit versus separately to children and parents. Estimated intervention delivery costs were approximately $225 per mother-child pair.
Seven FQHC staff members participated in a one-on-one in-depth interview following the intervention. Program successes identified by interviewees included the development and successful delivery of a culturally sensitive, engaging, and empowering family-based T2DM prevention program. The integration of a behavioral health component was also considered a strength. Challenges included the limited number of participants served by the research study compared to the needs of the FQHC population, difficulties recruiting participants who met the study eligibility criteria, and the logistics of coordinating the intervention across multiple clinic locations. Despite these challenges, interviewees agreed that the intervention was responsive to the FQHC priorities by increasing evidence-based pediatric- and family-centric health promotion programming that addressed obesity and T2DM disparities among FQHC patients. Interviewees suggested that broader eligibility criteria, greater visibility of the program across the FQHC, and a reimbursement model that offset program costs would allow them to reach and serve more families, thereby enhancing the potential for adoption, integration, and sustainability of the program over the long-term.
While our primary goal was feasibility and acceptability, we assessed changes to anthropometric, physiological and behavioral outcomes among participants. There were no statistically significant differences in body weight, BMI (or BMI z-score), waist circumference, or HbA1c changes between intervention and control mothers or children. Table 3. Similarly, there were no statistically significant differences in any of the secondary outcomes, including diet quality, time spent in physical activity, child sleep, or the healthy home environment score (Data not shown).
Table 3.
Changes in Weight, BMI, Waist Circumference, and HbA1c in Participants by Intervention (Week 13-Baseline), n = 35 Dyads.
| Intervention | Control | Difference a | P-value b | |
|---|---|---|---|---|
| N = 17 | N = 18 | |||
| Mother | ||||
| Change in weight (kg) | −1.8 (2.3) | −0.5 (1.8) | −1.2 (2.1) | .07 |
| BMI (kg/m2) | −0.7 (0.8) | −0.2 (0.7) | −0.5 (0.8) | .06 |
| Waist circumference c | −1.9 (3.6) | −1.2 (5.0) | −0.7 (4.3) | .63 |
| HbA1c | 0.018 (0.527) | 0.006 (0.494) | −0.012 (0.503) | .94 |
| Child | ||||
| Change in weight (kg) | 1.5 (1.9) | 1.4 (1.0) | 0.01 (1.5) | .99 |
| BMI (z-score) | −0.055 (0.209) | −0.033 (0.166) | −.022 (0.186) | .74 |
| Waist circumference | −0.03 (3.697) | 1.06 (3.174) | −1.09 (3.43) | .36 |
| HbA1c | 0.038 (0.131) | 0.044 (0.146) | −.0.006 (0.137) | .89 |
Data are presented as mean (SD).
Mean (intervention)−mean (control).
t-Test.
One participant with an extreme change in waist circumference removed.
Discussion
Our intervention was developed in response to a call for pilot and feasibility studies 37 that sought to develop practical and sustainable strategies to improve processes of care and health outcomes for persons at risk of developing T2DM in primary care settings. Emphasis was placed on potential for improving routine diabetes and obesity practice and informing policy. Given that women with history of GDM and their offspring have elevated risk for developing T2DM, our approach engaged both mothers and their children. Populations who experience disparities in T2DM prevalence and care are disproportionately low-income and identify as persons of color, thus, we partnered with colleagues at FQHC in Southern Arizona, wherein a majority of patients are uninsured or underinsured, and identify as Hispanic or Latino.
Our intervention was among the first to address T2DM risk of both mother and child simultaneously using the concept of “primordial prevention,” which explicitly leverages the motivation of mothers to address potential social and environmental conditions that promote or amplify chronic disease risk factors such as T2DM, thereby slowing or halting risk of transmission to their children.18,19,38 Indeed, there is precedent for the application of primordial prevention to T2DM risk reduction. 39 Family-based pediatric obesity interventions in which parents or caregivers are actively involved and coached to use proactive food and physical activity parenting strategies have demonstrated higher rates of success compared with those that focus only on the child.40,41 Leveraging the FQHC infrastructure to deliver primordial prevention within low-income and otherwise vulnerable communities is an opportunity to definitively address T2DM familial risk. 42
We succeeded in demonstrating feasibility of implementing a diabetes prevention program that enrolled majority low income and Hispanic women, a population disproportionately burdened by diabetes. This program extended previous efforts by our team to introduce primordial prevention to the area of diabetes prevention, 19 and was highly accepted by participants and FQHC program staff. To our knowledge, ours was one of the first studies to integrate a family-based T2DM prevention program as part of a FQHC delivery system, and evaluate the feasibility and acceptability of such a program in this setting.
Due to the pilot nature of our study, we did not expect to observe statistically significant changes in weight and BMI—2 key risk factors for reducing diabetes risk—although there was evidence of changes in desired directions. Prior to proceeding with a larger, fully powered clinical trial, we will further refine the intervention to address additional drivers of T2DM risk identified in our study and by our FQHC partners (eg, social determinants of health such as food insecurity, and transportation challenges) and work closely with our FQHC colleagues to respond to these challenges. 42
Our study had several strengths. A behavioral health specialist was added to our intervention team to specifically address the links between stress and health. The inclusion of behavioral health components was novel to family-based diabetes prevention and was very highly rated by participants. The FQHC was able to bill for behavioral health services, thereby partially recovering program delivery costs. Our academic-community partnership was another strength of this study, allowing the team to understand and balance the needs of the FQHC and its patients with scientific rigor necessary to build an evidence base for family-based T2DM prevention in primary care. This collaboration could form the foundation of a scalable model in the FQHC network once program effectiveness is established.
Our study also had several limitations. Our focus on women with a history of GDM was narrow by design; however, this created challenges for FQHC staff using the electronic health record to identify patients with GDM, some of whom were diagnosed up to a decade prior. Our focus on GDM prevented us from testing the program on patients with prediabetes who comprise a much larger proportion of the FQHC patient population. We offered optional face-to-face visits with Registered Dietitian Nutritionists during the study, but current restrictions around Dietitian services in our State meant that patients were responsible for a co-pay or the entire consult depending on insurance status. Consequently, only 4 mother-child dyads took advantage of this opportunity. Finally, we were challenged by the COVID19 pandemic, which hindered recruitment of our second cohort of participants (limiting us to 42 participants instead of the 60 we had planned), slowed recruitment of additional families to the study in January and February of 2020, and halted all intervention plans in late February 2020. Although this lowered our statistical power to detect meaningful changes in weight and behavioral outcomes, we were able to gather enough data to estimate power for a future definitive clinical trial.
As we emerge from a pandemic, there are many opportunities for this program to expand and thrive. In the next iteration of this program, we will consider additional opportunities for cost recovery. Digital and hybrid (digital plus face-to-face) treatment options are more relevant now than ever and we anticipate that restrictions on Dietitian services and billing will continue to shift in this direction, providing an additional reimbursement avenues. Other critical next steps include the integration of the intervention with the existing referral system and electronic health record, greater visibility of provider “champions” who promote the program to patients and staff, dedicated recruitment staff, and a cost recovery model that can be replicated and scaled in other FQHC networks across the United States.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R34DK118486 and by the National Cancer Institute of the National Institutes of Health under award number P30 CA023074. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iD: Melanie Hingle  https://orcid.org/0000-0002-6696-5601
https://orcid.org/0000-0002-6696-5601
References
- 1. Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003;111(3):e221-e226. [DOI] [PubMed] [Google Scholar]
- 2. Lawlor DA, Fraser A, Lindsay RS, et al. Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia. 2010;53(1):89-97. [DOI] [PubMed] [Google Scholar]
- 3. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2010;54(1):87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dabelea D, Mayer-Davis EJ, Lamichhane AP, et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH case-control study. Diabetes Care. 2008;31(7):1422-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sartorius T, Staiger H, Ketterer C, et al. Association of common genetic variants in the MAP4K4 locus with prediabetic traits in humans. PLoS One. 2012;7(10):e47647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hamman RF, Bell RA, Dabelea D, et al. The SEARCH for diabetes in youth study: rationale, findings, and future directions. Diabetes Care. 2014;37(12):3336-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2017–2018. NCHS Health E-Stats, Centers for Disease Control and Prevention. January 29, 2021. Accessed July 6, 2021. https://www.cdc.gov/nchs/data/hestat/obesity-child-17-18/obesity-child.htm [Google Scholar]
- 8. Dabelea D, Crume T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes. 2011;60(7):1849-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lindström J, Ilanne-Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish diabetes prevention study. Lancet. 2006;368(9548):1673-1679. [DOI] [PubMed] [Google Scholar]
- 12. Lavery JA, Friedman AM, Keyes KM, Wright JD, Ananth CV. Gestational diabetes in the United States: temporal changes in prevalence rates between 1979 and 2010. BJOG. 2017;124(5):804-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou T, Sun D, Li X, et al. Prevalence and trends in gestational diabetes mellitus among women in the United States, 2006–2016. Diabetes. 2018;67:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999-2005. Diabetes Care. 2008;31(5):899-904. [DOI] [PubMed] [Google Scholar]
- 15. Hingle MD, Turner T, Going S, et al. Feasibility of a family-focused YMCA-based diabetes prevention program in youth: the E.P.I.C. Kids (encourage, practice, and inspire change) Study. Prev Med Rep. 2019;14:100840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY pilot study. Am J Prev Med. 2008;35(4):357-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hannon TS, Carroll AE, Palmer KN, Saha C, Childers WK, Marrero DG. Rationale and design of a comparative effectiveness trial to prevent type 2 diabetes in mothers and children: the ENCOURAGE healthy families study. Contemp Clin Trials. 2015;40:105-111. [DOI] [PubMed] [Google Scholar]
- 18. Hannon TS, Saha CK, Carroll AE, Palmer KN, O’Kelly Phillips E, Marrero DG. The ENCOURAGE healthy families study: a comparative effectiveness trial to reduce risk for type 2 diabetes in mothers and children. Pediatr Diabetes. Published online May 20, 2018. doi: 10.1111/pedi.12692 [DOI] [PubMed] [Google Scholar]
- 19. Marrero DG, Blew RM, Palmer KNB, James K, Roe DJ, Hingle MD. Rationale and design of a type 2 diabetes prevention intervention for at-risk mothers and children at a federally qualified healthcare center: EPIC El Rio families study protocol. BMC Public Health. 2021;21(1):346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rivers P, Hingle M, Ruiz-Braun G, Blew R, Mockbee J, Marrero D. Adapting a family-focused diabetes prevention program for a federally qualified health center: a qualitative report. Diabetes Educ. 2020;46(2):161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greaves CJ, Sheppard KE, Abraham C, et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health. 2011;11(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol. 2009;28(6):690-701. [DOI] [PubMed] [Google Scholar]
- 23. World Health Organization. Obesity and overweight. 2020. Accessed July 7, 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 24. Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72(5):1074-1081. [DOI] [PubMed] [Google Scholar]
- 25. Kakinami L, Henderson M, Chiolero A, Cole TJ, Paradis G. Identifying the best body mass index metric to assess adiposity change in children. Arch Dis Child. 2014;99(11):1020-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feskanich D, Sielaff BH, Chong K, Buzzard IM. Computerized collection and analysis of dietary intake information. Comput Methods Programs Biomed. 1989;30(1):47-57. [DOI] [PubMed] [Google Scholar]
- 27. Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reedy J, Lerman JL, Krebs-Smith SM, et al. Evaluation of the healthy eating index-2015. J Acad Nutr Diet. 2018;118(9):1622-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taren D, de Tobar M, Ritenbaugh C, Graver E, Whitacre R, Aickin M. Evaluation of the Southwest Food Frequency Questionnaire. Ecol Food Nutr. 2000;38(6):515-547. [Google Scholar]
- 30. Saint-Maurice PF, Welk GJ. Validity and calibration of the youth activity profile. PLoS One. 2015;10(12):e0143949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Owens JA, Spirito A, McGuinn M. The children’s sleep habits questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043-1051. [PubMed] [Google Scholar]
- 32. Staten LK, Taren DL, Howell WH, et al. Validation of the Arizona activity frequency questionnaire using doubly labeled water. Med Sci Sports Exerc. 2001;33(11):1959-1967. [DOI] [PubMed] [Google Scholar]
- 33. Ihmels MA, Welk GJ, Eisenmann JC, Nusser SM. Development and preliminary validation of a Family Nutrition and Physical Activity (FNPA) screening tool. Int J Behav Nutr Phys Act. 2009;6(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ihmels MA, Welk GJ, Eisenmann JC, Nusser SM, Myers EF. Prediction of BMI change in young children with the family nutrition and physical activity (FNPA) screening tool. Ann Behav Med. 2009;38(1):60-68. [DOI] [PubMed] [Google Scholar]
- 35. National Association of Community Health Centers. PRAPARE protocol for responding to and assessing patients’ assets, risks and experiences. 2019. Accessed July 7, 2021. https://www.nachc.org/research-and-data/prapare/about-the-prapare-assessment-tool/
- 36. Mann DM, Carson AP, Shimbo D, Fonseca V, Fox CS, Muntner P. Impact of A1C screening criterion on the diagnosis of pre-diabetes among U.S. adults. Diabetes Care. 2010;33(10):2190-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. National Institutes of Health. Planning grants for pragmatic research in healthcare settings to improve diabetes and obesity prevention and Care (R34 clinical trial required) – PAR-18-924. Accessed July 7, 2021. https://grants.nih.gov/grants/guide/pa-files/par-18-924.html
- 38. Strasser T. Reflections on cardiovascular diseases. Interdiscip Sci Rev. 1978;3(3):225-230. [Google Scholar]
- 39. Wessel J, Marrero D. Genetic testing for type 2 diabetes in high-risk children: the case for primordial prevention. Res Ideas Outcomes. 2017;3:e20695. [Google Scholar]
- 40. Chai LK, Collins C, May C, Brain K, Wong See D, Burrows T. Effectiveness of family-based weight management interventions for children with overweight and obesity: an umbrella review. JBI Database System Rev Implement Rep. 2019;17(7):1341-1427. [DOI] [PubMed] [Google Scholar]
- 41. Rajjo T, Mohammed K, Alsawas M, et al. Treatment of pediatric obesity: an umbrella systematic review. J Clin Endocrinol Metab. 2017;102(3):763-775. [DOI] [PubMed] [Google Scholar]
- 42. Soltero EG, Ramos C, Williams AN, et al. ¡Viva Maryvale!: a multilevel, multisector model to community-based diabetes prevention. Am J Prev Med. 2019;56(1):58-65. [DOI] [PMC free article] [PubMed] [Google Scholar]