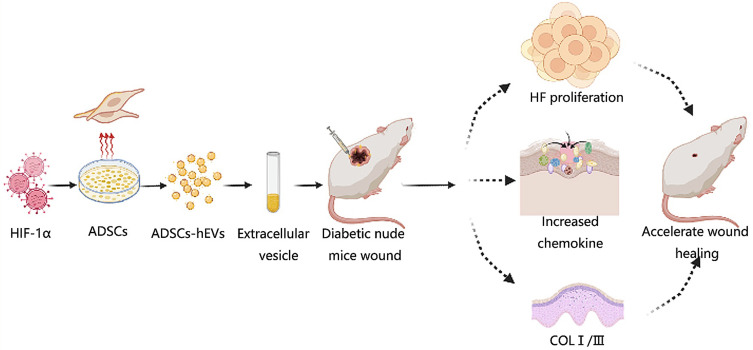
Abstract
Purpose
Inhibition of cellular adaptation to hypoxia can cause persistent inflammation, thereby increasing tissue damage and complicating wound healing in diabetes patients. Regulating cellular adaptation to hypoxic environments can help in effective wound repair. Hypoxia-inducible factor (HIF)-1α is a key regulator of cell hypoxia. Extracellular vesicles (EVs) regulate wound repair. This study investigated the mechanism of HIF-1α overexpression in adipose-derived stem cell extracellular vesicles (ADSCs-hEVs) in the repair of diabetic wounds.
Materials and Methods
HIF-1α expression in diabetes patients and healthy participants was studied. High-throughput sequencing, GO, and KEGG analysis revealed that ADSCs small extracellular vesicle hypoxia environments may increase HIF-1α expression by affecting cell metabolism, differentiation, and TGF-β secretion, or by altering the PI3K/AKT pathway. Effect of addition of ADSCs-hEVs on cell proliferation and migration was investigated using Western blotting, EdU assay, transwell assay, and migration. In vivo, after 7, 14, and 21 days, important factors for diabetic wound healing were evaluated by immunohistochemistry, qRT-PCR, Masson staining, and H&E staining.
Results
HIF-1α expression decreased in the skin of diabetes patients; interleukin (IL)-6 expression increased, and growth factor-related indexes decreased. ADSCs-hEVs significantly increased the expression and secretion of growth factors, compared with ADSCs-EVs. In vivo, ADSC-hEV treatment accelerated the healing rate and improved the healing quality of diabetic wounds compared with ADSCs-EVs.
Conclusion
Speed and quality of wound healing increased significantly in the ADSCs-hEVs group, which could inhibit early inflammation while promoting the secretion and expression of growth factors and extracellular matrix-related indexes.
Keywords: diabetic wound, HIF-1α, ADSCs, extracellular vesicles, fibroblast
Graphical Abstract
Schematic illustration of ADSCs-hEVs therefore accelerates the healing rate of diabetic wounds and improves healing quality by inhibiting inflammation and regulating extracellular matrix secretion. ADSCs-hEVs can promote the proliferation and migration of fibroblasts through the PI3K/AKT signaling pathway and increase the secretion of related vascular factors, growth factors, and the extracellular matrix.
Introduction
Diabetes is a common disease; oxidative stress, inflammation, or glycolipid toxic pancreatic β cell dysfunction play a key role in diabetes.1–3 Wound healing in diabetes patients is typically extremely slow; some wounds do not even heal completely, with infection further complicating treatment; however, there is no effective treatment.4 New methods for treating wounds in diabetes patients have been reported, such as the application of carbon monoxide and phototherapy; however, these methods are not widely used and the treatment is not sufficiently thorough. Thus, further research and understanding of wound healing in diabetes patients are needed.5,6 Here, through a study of hypoxia in diabetic wounds, we found that hypoxia-inducible factor (HIF)-1α may be effective in promoting refractory wound healing. HIF-1α decomposes rapidly in hypoxic environments and rapidly mediates cellular adaptation to hypoxia under hypoxic conditions. HIF-1α is an important factor for cell metabolism, inflammation, fibrosis, vascular remodeling, and angiogenesis.7,8 More than 200 target proteins were reported to be directly regulated by HIF-1α.9 HIF-1α also regulates several processes required for wound repair. Hypoxia is a prognostic determinant of wound repair, and HIF-1α mediates cellular adaptation to hypoxia and contributes to wound healing. Chronic hypoxia in the skin can lead to delayed healing, as well as a gradual decrease in HIF-1α levels.10–12
New evidence suggests that cells can communicate by secreting extracellular vesicles that are typically stored in the cavity, thereby affecting cell activity and growth.13 Adipose-derived stem cell (ADSCs) paracrine mechanisms, particularly extracellular vesicle secretion, can prevent apoptosis in rat ischemic myocardium and play a critical role in.14 Extracellular vesicles can directly fuse with receptor cells and transport proteins, nucleic acids, and other molecules in these cells to regulate their activity.15 Compared with ADSCs adjuvant therapy, the “cell-free therapy” using extracellular vesicles effectively mimics the ability of primordial cells to avoid limited cell survival, immune-mediated rejection, and the possibility of malignant transformation, thus enhancing their potential to play an unprecedented mediating role in cell-cell communication.16
Generally, delayed healing or non-healing wounds are caused by the low oxygen utilization rate of the damaged area. Combined transplantation of ADSCs and extracellular vesicles (ADSCs-EVs) in a hypoxic environment can promote wound healing in diabetes patients; however, the specific mechanism of action remains to be elucidated.17 HIF-1α plays an important role in improving the cell hypoxia state. Therefore, we can enhance HIF-1α expression in extracellular vesicles to improve blood perfusion, reduce inflammatory cell numbers, improve the quality and speed of wound healing, and achieve the goal of complete treatment of refractory diabetic wounds.
In this study, we speculated that HIF-1α plays a key regulatory role in the healing process of diabetic wounds. To verify this hypothesis, we first analyzed differences in the expression of skin tissue-related factors between the tissues of diabetes patients and normal skin tissues. Then, we evaluated the effect of ADSCs-EVs on cells using high-throughput sequencing. Furthermore, we evaluated the effects of ADSCs-hEVs on fibroblast proliferation, migration, chemokine fraction, and extracellular matrix secretion in vitro. We also investigated the in vivo effects of ADSCs-hEVs on inflammation, growth factors, and extracellular matrix secretion in diabetic wound healing.
Materials and Methods
Cell Culture
Adipose tissue and skin tissue samples was harvested from the Second Affiliated Hospital of Harbin Medical University. All patients signed the informed consent form. All study was approved by the ethics committee of the hospital. First, we conducted primary culture of human adipose stem cells (hADSCs) and finally cultured in a humidified 5% CO2 atmosphere at 37°C. Similarly, we conducted primary cultures of human fibroblasts (HF). The skin tissue of diabetic patients and normal people was immediately −80°C preserved for qRT-PCR detection.
Cell Extraction and Identification
Third-generation mesenchymal hADSCs were extracted. hADSCs were immunolabeled with antibodies against CD44, CD34, CD14, CD29, and CD105 (Abcam, Cambridge, UK) and analyzed using a flow cytometer (BD Biosciences, San Jose, CA, USA). HADSCs were seeded in 6-well plates and incubated in an adipogenic medium for 7 days. Adipogenic differentiation was determined by Oil red O (Sigma‐Aldrich) staining. Homoplastically, hADSCs were seeded in 6-well plates and incubated in an osteogenic medium for 21 days. Osteogenesis differentiation was determined by Alizarin Red (Sigma-Aldrich) staining.
Cell Transfection
HIF-1α-overexpressing vector (pLVX-HIF-1α-GFP-3FLAG-ZsGreen–puromycin) and controls (pLVX-GFP-3FLAG-ZsGreen-puromycin) were provided by Hanheng gene (HANBIO, Shanghai, China). When hADSCs reached 50% confluency (passage 3), lentiviral vectors were transfected into them and incubated for 8 h (multiplicity of infection = 120). Non-transfected cells were eliminated with puromycin.
QRT- PCR
RNA was extracted from the exosome-treated cells and wound tissue. Next, cDNA was reverse transcribed from the extracted RNA. Real-time PCR was performed using a 10-fold dilution of the cDNA template (ABI, CA, USA). Primer sequences are listed in Table 1.
Table 1.
Primer Sequences of the Study
| hADSCs Genes | Forward Primer 5ʹ→3’ | Reverse Primer 5ʹ→3’ |
|---|---|---|
| Actin-Mouse | CACTGTCGAGTCGCGTCC | TCATCCATGGCGAACTGGTG |
| Actin-Human | TGGCACCCAGCACAATGAA | CTAAGTCATAGTCCGCCTAGAA |
| HIF1-α-Human | ATTACAGCAGCCAGACGATCA | ATTGATTGCCCCAGCAGTCT |
| MMP1-Human | AGAGCAGATGTGGACCATGC | TTGTCCCGATGATCTCCCCT |
| PDGF-Human | AGGATTCTTTGGACACCAGC | AGATCAGGAAGTTGGCGGAC |
| SDF-1-Human | GCCCTTCAGATTGTAGCCCG | GTAAGGGTTCCTCAGGCGTC |
| bFGF-Human | AAGAGCGACCCTCACATCAA | ACGGTTAGCACACACTCCTT |
| EGF-Human | TCCAAGTGCATCAACACCGA | GGGTGGAGTAGAGTCAAGACA |
| TGF-β-Human | GTGAGCTAGATCGGTTGCTT | CTTGCTAGATGGGAACTGAGAC |
| VEGF-Human | GGCGAAGAGAAGAGACACATT | TTCAATGGTGTGAGGACATAGG |
| IL-6-Human | CCTAGAGTACCTCCAGAACAGA | CAGGAACTGGATCAGGACTTT |
| Col I-Human | GAGGGCAACAGCAGGTTCACTTA | TCAGCACCACCGATGTCCA |
| TGF-β-Mouse | CGAAGCGGACTACTATGCTAAA | TCCCGAATGTCTGACGTATTG |
| bFGF-Mouse | CTTACCGGTCACGGAAATACTC | AGCTCTTAGCAGACATTGGAAG |
| Col I-Mouse | GAGGGCAACAGCAGGTTCACTTA | TCAGCACCACCGATGTCCA |
| VEGF-Mouse | AGGCTGCTGTAACGATGAAG | TCTCCTATGTGCTGGCTTTG |
| PDGF-Mouse | TAACACCAGCAGCGTCAAGT | TTCCCTACGCCTTCCTGTCT |
Identification and Treatment with ADSCs-hEVs
HADSCs (HIF-1α overexpression, HIF-1α overexpression control, and control groups) at 90% confluence were cultured in a medium containing 10% serum without extracellular vesicles (SBI, California, USA, EXO-FBS-50A-1) for an additional 24 h. The cell supernatant was then collected and centrifuged at 300 g for 10 min, and then at 2000 g for 10 min. Then, the cell supernatant was filtered with a 0.22-μm sterile filter (sterile micropores, Burlington). We followed the manufacturer’s instructions to add the ExoQuick‐TC reagent (SBI, EXOTC10A-1) overnight at 4°C. After centrifugation at 1500 g for 30 min, the cell supernatant was discarded and the extracellular vesicle pellet was resuspended in PBS, and the extracellular vesicle protein concentrations were measured using a BCA Protein Assay Kit (Beyotime, P0010-1). Extracellular vesicles were either stored at −80°C or used immediately for the downstream experiments.
Identification of Extracellular Vesicles
We analyzed the size distribution of the purified extracellular vesicles by nanoparticle tracking analysis (Malvern’s NanoSight NS300), and the Western blot assay was performed to assess the levels of the proteins CD63, TSG101, and CD9 to identify the purified extracellular vesicles (Abcam, UK). In addition, we followed the manufacturer’s instructions to label ADSCs-EVs using PKH26 Red Fluorescent Cell Linker Kits (Sigma, USA), and examined the ultrastructure of vesicles by transmission electron microscopy (Libra 120 instrument, Zeiss). Next, ADSCs-EVs, ADSCs-hEVs, negative control of adipose stem cell extracellular vesicles (ADSCs-cEVs) (100 μg/mL), or PBS were added to 80–90% confluent human fibroblasts and incubated in an incubator for 24 h with the PI3K/AKT inhibitor LY294002 (50 nM, Monmouth Junction, NJ, USA) or for 48 h for the Edu assays (cell proliferation ELISA, Roche Diagnostics).
miRNA Sequence Analysis of the Extracellular Vesicles
Extracellular vesicles were extracted from cells and the miRNAs from extracellular vesicles were isolated. Verification and sequence analysis were performed to ensure that the miRNA samples met the requirements. Thermal graph quality analysis was performed for the genes in extracellular vesicles and GO and KEGG analyses were performed for the related differentially expressed miRNAs.
Protein Extraction and Western Blotting
The lysed cells were analyzed by Western blotting with the following rabbit primary antibodies: anti-GAPDH (ab181602, Abcam), anti-HIF-1α (WL01607, WanleiBio), anti-type I collagen (COL I) (ab34710, Abcam), anti-type III collagen (COL III) (ab184993, Abcam), anti-P-AKT (13038, Cell Signaling Technology), anti-AKT (9272, Cell Signaling Technology), and anti-VEGF (ab52917, Abcam). Horseradish peroxidase-conjugated goat anti-rabbit IgG (1:8000, Abcam) was used as the secondary antibody. A chemical discharge imaging system was used for the visualization of the blots (MageQuant LAS 4000 mini machine, GE).
Scratch Assay
After 90% cell growth in the 6-well plate, a 100-μL pipette head was used to draw a smooth line vertically in the center of the plate. Extracellular vesicles were added to serum-free medium, followed by culturing. The cell migration area was calculated by examining the plate under a microscope.
Transwell Assay
We inoculated 5000 cells in the upper compartment of Transwells and cultured them in serum-free medium, while extracellular vesicles were added to the lower compartment. After 24 h of culture, the upper chamber was resuspended with clean water, and the cells at the bottom were wiped off with a cotton ball. After staining with crystal violet for 30 min, photographs were captured for analysis.
Surgical Procedure and Treatment
Nude BALB/c (4–5-week-old) mice were fed a high-fat diet for 3–4 weeks till they weighed 20 g. To establish a diabetes mouse model, the mice were injected with streptozotocin 35 mg/kg in 0.1 M citrate-buffered saline. All animals were obtained from the Second Affiliated Hospital of Harbin Medical University (Harbin, Heilongjiang, China). All animal experimental protocols were approved by the IACUC (approval number: 2014116).
After a week, the changes in blood glucose were evaluated to verify the establishment of the model (blood glucose levels ≥ 16.7 mM for up to a week). Next, we performed a surgical operation and excised the same amount of skin from the back as in the wound model (0.8 × 0.8 cm). Nude mice were administered EVs (2 mg in 100 μL PBS) or only 100 μL PBS subcutaneously at four points of the wound edge using a 1-mL syringe. After feeding on days 0, 3, 7, and 14, the wound tissues were photographed and analyzed of wound healing. The following groups were assessed: control, no wound in mice with diabetes; DM + PBS, nude mice with diabetes treated with PBS; DM + EVs, nude mice with diabetes treated with ADSCs-EVs; DM + hEVs, nude mice with diabetes treated with ADSCs-hEVs; DM + cEVs, nude mice with diabetes treated with ADSCs-cEVs.
Histopathological and Immunohistochemical Analyses
The skin tissue samples from the diabetes nude mouse model were fixed in 4% polyformaldehyde and processed to obtain tissue sections. Then, they were incubated overnight at 4°C with primary antibodies against β-actin, IL-6, α-SMA, COLIII, and transforming growth factor (TGF)-β. On the next day, they were incubated with secondary antibodies against COLI and IL-10. We performed immunofluorescence staining, H&E, and Mason trichrome staining using using standard protocols. Images were obtained using a Leica microscope, and five different fields were randomly assessed. The skin tissue excised from the mice with diabetes were stored at −80°C for qRT-PCR analysis to determine the expression levels of COLI, TGF-β, PDGF, bFGF, VEGF (Table 1).
Statistical Analyses
All values are expressed as the mean ± SD. The data were analyzed using GraphPad Prism 9.0 software, and the images were analyzed using ImageJ. Quantitative data across all groups were analyzed using one-way ANOVA and t-test. A P value less than 0.05 indicated statistical significance.
Results
Characterization of hADSCs and ADSCs-EVs
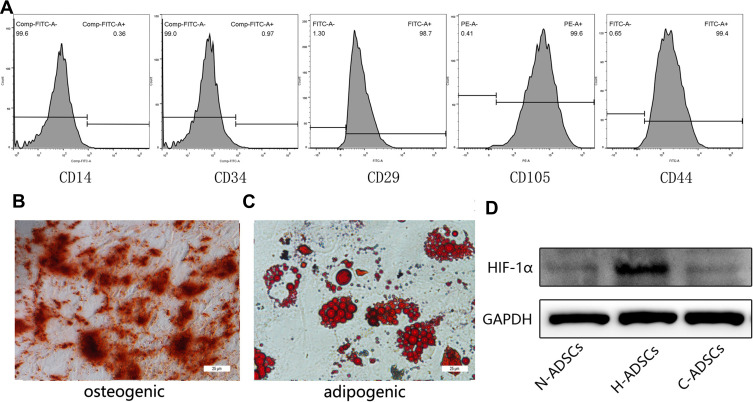
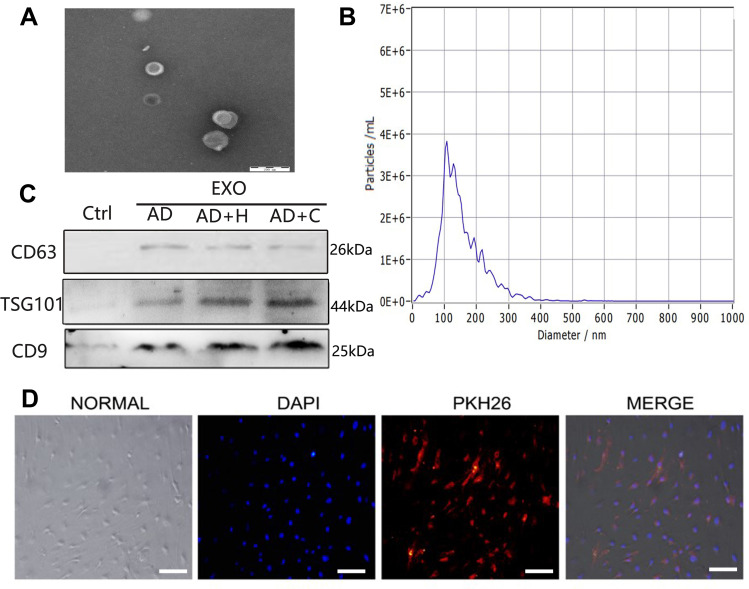
We identified hADSCs that were CD44, CD29, and CD105 positive and CD14 and CD34 negative (Figure 1A). Alizarin red staining revealed that hADSCs showed calcified nodules after 21 days of differentiation, indicating osteogenic differentiation (Figure 1B). Oil red O-stained hADSCs showed lipid droplets after 7 days of adipogenic differentiation (Figure 1C). HIF-1α expression was upregulated in ADSCs (H-ADSCs) after lentivirus transfection. The protein levels of HIF-1α were significantly higher in H-ADSCs than in N-ADSCs and C-ADSCs (Figure 1D). As shown in Figure 2, the EVs microstructure demonstrates a mean size 110 nm. High expression levels of CD63, TSG101, and CD9 were found in fibroblasts using Western blotting. After labeling EVs with the PKH26 red dye, we discovered that ADSCs-EVs were internalized by fibroblasts.
Figure 1.
Identification of hADSCs. (A) Flow cytometry revealed immunophenotypic characteristics of hADSCs. (B) Calcium nodules were detected by alizarin red staining (100 ×). (C) Lipid clusters were detected by oil red O staining. (D) Protein levels of HIF-1α in different groups of hADSCs as analyzed by Western blot. Bars, 25 μm.
Figure 2.
Characterization of ADSCs-EVs. (A) Morphology observed under transmission electron microscope. Bars, 200 μm. (B) Particle size distribution. (C) Western blot was used to detect EVs surface markers. (D) Fluorescent microscopy analysis of PKH26-labeled ADSCs-EVs internalization . Bars, 100 μm.
HIF-1α Expression in Tissues of Diabetes Patients and High-Throughput Sequencing and KEGG and GO Analyses of the Effect of Extracellular Vesicles on Cells
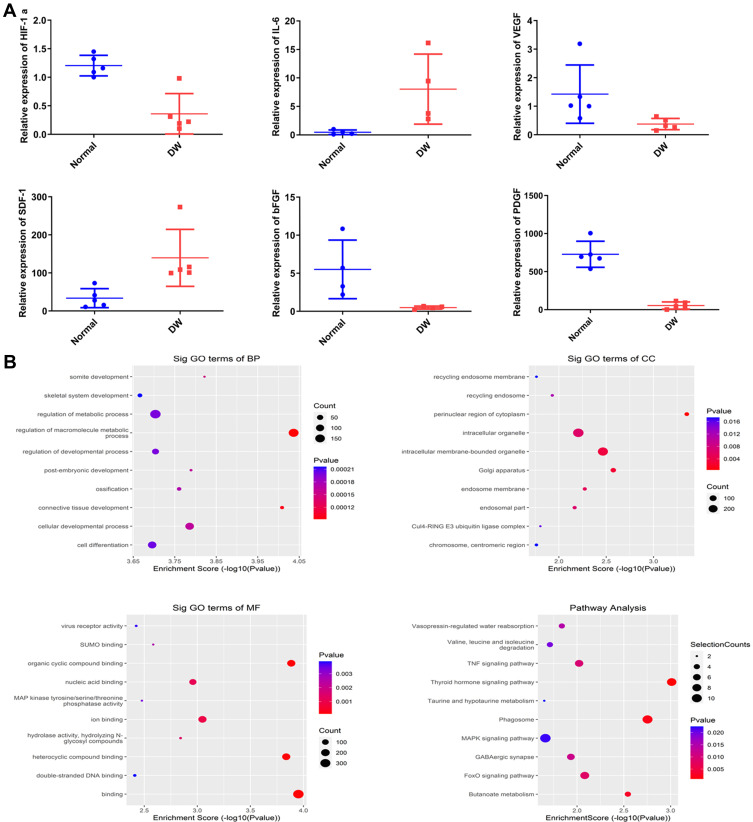
HIF-1α is a key factor that regulates cellular adaptation to hypoxia and promotes wound healing.18 To determine whether the expression of inflammatory factors and HIF-1α differed between the skin tissue of diabetes patients and healthy individuals, we analyzed the skin tissue of diabetes patients and healthy individuals (N = 6) using qRT-PCR (Figure 3; P < 0.05). The mRNA levels of bFGF, SDF-1, IL-6, VEGF, PDGF, and HIF-1α were lower in diabetes patients than in healthy individuals (Figure 3A). Studies have demonstrated reduced anti-inflammatory response in the skin tissue and cellular adaptation to hypoxia in diabetes patients. Thus, the healing of wounds in diabetes patients is delayed.
Figure 3.
The differential levels of mRNA levels between normal human skin samples and Diabetic skin samples. (A) QRT-PCR analysis of mRNA levels, including HIF-1α, bFGF, IL-6, PDGF, VEGF, SDF-1. (B) GO and KEGG enrichment of differentially expressed miRNAs. Significant terms in KEGG pathways. n=5. Data are represented as mean ± SD.
We also performed high-throughput sequencing of ADSCs-EVs and hypoxic ADSCs-EVs. The results showed that 584 miRNAs were differentially expressed. KEGG and GO analyses showed that extracellular vesicles in a hypoxic environment may enhance the ability to produce cells via the PI3K/AKT pathway and significantly promote cell metabolism and differentiation, thus suggesting that cell function can be regulated through HIF-1α-overexpressed extracellular vesicles (Figure 3B).
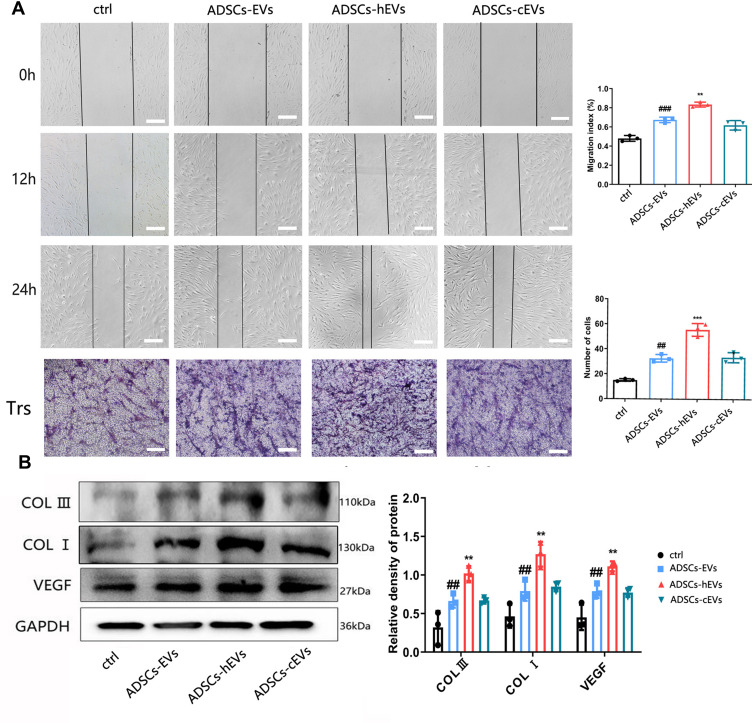
Cell Proliferation, Migration, and Extracellular Matrix Formation
We first explored the effects of ADSCs-hEVs on fibroblasts by evaluating their effects on fibroblast proliferation. The scratch and transwell assays showed significantly increased proliferation and migration of cells after treatment of fibroblasts with ADSCs-hEVs (Figure 4A). In the ADSCs-hEVs group, COL I, COL III, and VEGF protein expression levels were significantly increased (Figure 4B).
Figure 4.
ADSCs-hEVs significantly promote the proliferation and migration of HF. (A) The migration ability of HF treated with ADSCs-hEVs, measured by scratch and Transwell test assays. (B) Analysis of protein expression levels in treated HF. Data are represented as mean ± SD. *, vs ADSCs-EVs; #, vs ctrl; n = 3. **P < 0.001, ##P < 0.001, ***P < 0.0001, ###P < 0.0001. Bars, 100μm.
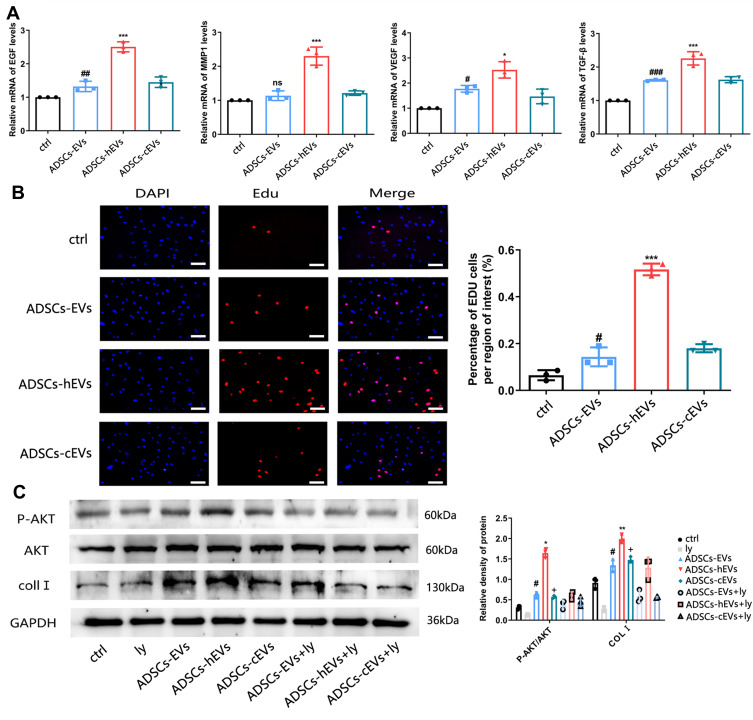
Fibroblasts can secrete a variety of chemokines and facilitate extracellular matrix formation to accelerate wound repair and improve wound healing quality.19 We also observed the effects of ADSCs-hEVs-related factors on fibroblasts. qRT-PCR showed that the expression levels of the VEGF, TGF-β, EGF, and MMP1 genes in fibroblasts were significantly increased (Figure 5A). Moreover, we found that ADSCs-hEVs significantly enhanced fibroblast proliferation (Figure 5B). Thus, ADSCs-hEVs can regulate the production of extracellular proteins and chemokines in fibroblasts and exert potential effects to promote angiogenesis.
Figure 5.
ADSCs-hEVs promote the expression and secretion of extracellular matrix and growth factors in HF. (A) QRT-PCR analysis of mRNA levels, including bFGF, MMP1, VEGF, TGF-β. (B) The proliferation of cells by Edu assays. *, vs ADSCs-EVs; #, vs Ctrl; (C) Western-blot analysis of protein levels of p-akt induced by different concentrations of control, ADSCs-EVs, ADSCs-hEVs and ADSCs-cEVs. LY294002 inhibit the activation of PI3K/ AKT induced by control, ADSCs-EVs, ADSCs-hEVs and ADSCs-cEVs. Data are represented as mean ± SD. *, vs ADSCs-hEVs+Ly; #, vs ADSCs-EVs+Ly; +, vs ADSCs-cEVs+Ly; n = 3. *P < 0.05, **P < 0.01, ***P < 0.0001, #P < 0.05,##P < 0.001, ###P < 0.0001, +P < 0.05.
ADSCs-hEVs Regulated Fibroblast Proliferation and Extracellular Matrix Formation by Activating the PI3K/AKT Pathway
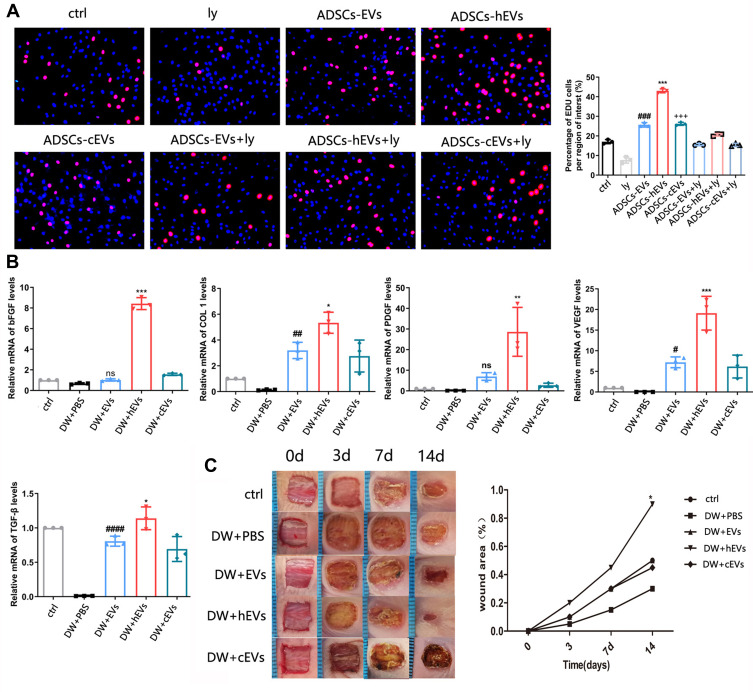
The PI3K/AKT pathway plays an important role in increasing cell proliferation and migration and promoting wound healing. Functionally, after inhibiting PI3K/AKT signaling, western spot detection showed that ADSCs-hEVs significantly improved p-AKT protein levels in HF in a dose-dependent manner (Figure 5C). This finding suggests that the regulation of the externally dependent proliferation and migration of ADSCs-hEVs may occur through the PI3K/AKT signaling pathway. To further investigate the regulatory effects of this signaling pathway on fibroblasts, we pretreated fibroblasts with the signal inhibitor LY294002. Subsequently, Edu analysis showed that the effect of ADSCs-hEVs on fibroblast proliferation was significantly attenuated (Figure 6A). Simultaneously, the signal inhibitor significantly inhibited the increase in AKT phosphorylation and the important factor COL I induced by ADSCs-hEVs expression (Figure 5C). This suggests that the regulation of the externally dependent proliferation and migration of ADSCs-hEVs occurs through the PI3K/AKT signaling pathway.
Figure 6.
ADSCs-hEVs promote the proliferation and migration of cells via the PI3K/AKT. (A) Edu assay showed that ADSCs-hEVs-treated HF proliferation was suppressed by inhibitors LY294002, compared with ADSCs-EVs. *, vs ADSCs-hEVs+Ly; #, vs ADSCs-EVs+Ly; +, vs ADSCs-cEVs+Ly; (B) QRT-PCR analysis of wound tissue mRNA level 14 days after operation, including bFGF, COLI, PDGF, VEGF, TGF-β. (C) After ADSCs-hEVs-treated 0, 3, 7 and 14 days were taken. Data are represented as mean ± SD. *, vs DW+EVs; #, vs DW+PBS; ns, vs DW+PBS; n = 3. *P < 0.05, **P < 0.01, ***P < 0.0001, #P < 0.05, ##P < 0.01, ####P < 0.00001, +++P < 0.0001. Bars, 100μm.
ADSCs-Hexo Significantly Increased the Healing Effect of Diabetic Wounds
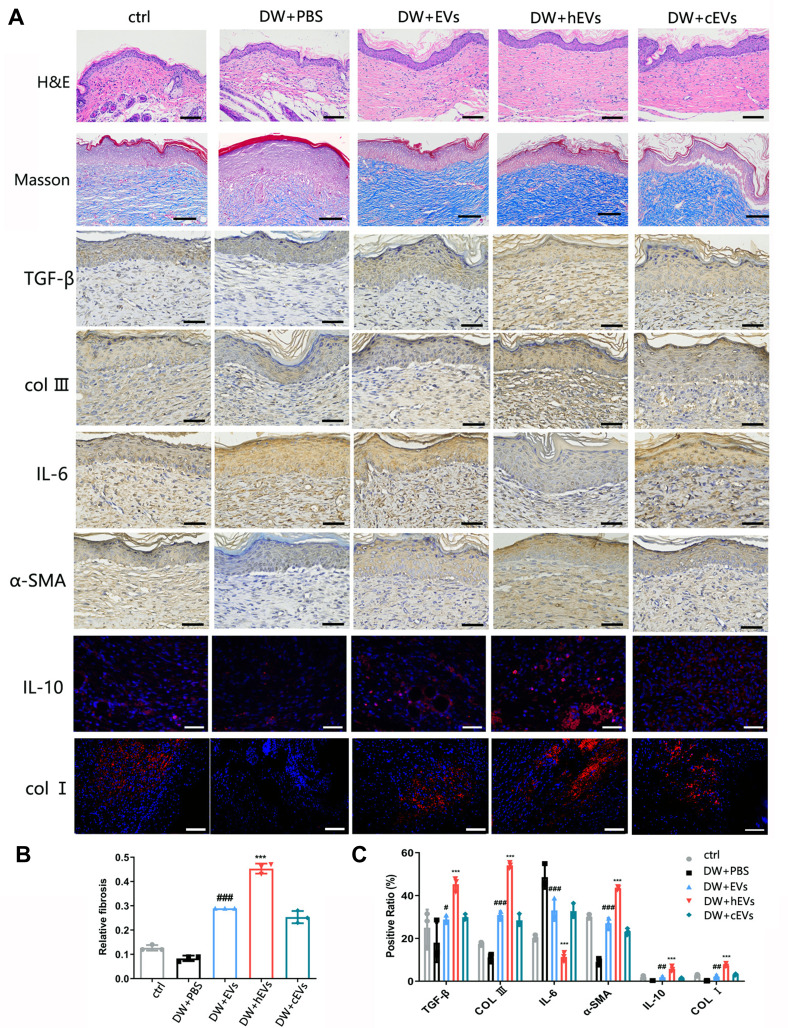
Extracellular vesicles were injected into the subcutaneous wounds of diabetic nude mice to observe and study wound healing. From the images taken on days 0, 3, 7, and 14, the size of the ADSCs-hEVs-treated wounds was smaller than that of the ADSCs-EVs and control-treated wounds (Figure 6C). Next, we also found that the ADSC-hEVs wounds closed on day 14. An analysis of the wound closure rates confirmed that ADSCs-hexo-treated wounds contracted much faster than ADSCs-EVs-treated wounds on days 7 and 14. From the general observations of H&E staining (Figure 7A) and Masson-stained sections, ADSCs-hEVs-treated skin wounds exhibited complete re-epithelialization and cuticle covering of the epidermis and continuous tissue arrangement. After Masson’s trichrome staining, the skin fibers appeared blue (Figure 7A and B). In contrast, skin wounds treated with ADSCs-EVs exhibited low re-epithelialization.
Figure 7.
Observation of wound healing quality and velocity after operation. (A) HE and Masson staining of tissue wounds 14 days after operation and expression of IL-6 and IL-10 in wound tissue was observed 7 days after operation. Expression of α-SMA, TGF-β, COLI, COLIII in wound tissue was observed 21 days after operation. n=3. (B and C) Masson, immunofluorescence and immunohistochemical statistical analysis. Data are represented as mean ± SD. *, vs DW+EVs; #, vs DW+PBS; n = 6. **P < 0.001, ***P < 0.0001, #P < 0.05, ##P < 0.001, ###P < 0.0001. Bars, 100μm.
ADSCs-hEVs Regulate Inflammation in the Early Stages of Diabetic Wound Healing and Promote Fibroblast Proliferation and Extracellular Matrix Synthesis
To evaluate the effects of ADSCs-hEVs on wound healing in bare diabetic nude mice, we determined the levels of wound-related inflammatory factors and chemokines as well as collagen proteogenesis. The increase in TGF-β, bFGF, PDGF, COL I, and VEGF gene expression in wound tissue indicated that ADSCs-hEVs promoted collagen and growth factor expression in skin tissue cells to accelerate wound healing (Figure 6B). We also investigated the effects of ADSCs-hEVs on TGF-β, α-SMA, COL I, and COLIII and the inflammatory factors IL-6 and IL-10 in diabetic wounds. TGF-β, IL-10, α-SMA, COL I, and COLIII expression was upregulated in the ADSCs-hEVs treatment group at week 1 and 2, whereas IL-6 expression was downregulated (Figure 7A and C), compared to the expression levels in the control group. These findings indicate that ADSCs-hEVs can significantly inhibit the inflammatory production of diabetic wounds, promote angiogenesis, and promote high-quality wound healing in diabetic nude mice.
Discussion
Refractory diabetic wounds are a common health concern worldwide and require effective treatment. Healing of wounds, burns, or diabetic ulcers present on the skin and their softening after recurrence are extremely slow and difficult to manage.20 The metabolic abnormalities in diabetic patients can further complicate the wound healing process due to hyperglycemia, which may also cause chronic wound stagnation. Hypoxia is an extremely important prognostic determinant for wound healing. Inhibition of cell adaptation to hypoxia makes it difficult for cells to multiply and migrate in hypoxic environments. Cellular adaptation to hypoxia is mediated by HIF-1α, which can increase the quality and speed of wound healing. Accumulating evidence suggests that extracellular vesicles play a regulatory role in wound healing mechanisms.3,21 Although this study presents some important results related to wound healing, an effective treatment remains elusive at present. In this study, we found that ADSCs-hEVs can accelerate high-quality healing of diabetic wounds by activating the PI3K/AKT pathway to promote fibroblast proliferation and migration.
Adaptation of trauma cells to a hypoxic environment, the inflammatory response, and the expression of collagen proteins affect the process of late wound healing.22,23 It has been shown that insufficient tissue or cellular oxygen supply is a common feature of multiple diseases, such as cancer, obesity, diabetes, and ischemic diseases. In diabetic diseases, elevated blood glucose can degrade the stability of HIF-1α through related proteins, which affects genes dependent on HIF-1 α regulation. Meanwhile, the decreased binding of HIF-1α to coactivator P300 due to high glucose response can also lead to slow or even non-healing of diabetic wounds.24 New reports also suggest reduced expression levels of chemokines, such as VEGF, due to defects in the traumatic tissues of HIF-1α transactivation.10 Therefore, we initially evaluated the differences in epidermal gene expression between patients with diabetes and healthy individuals. We found that the expression of anti-inflammatory factors PDGF, bFGF, SDF-1, VEGF, and HIF-1α decreased compared to that in normal skin, but the expression of IL-6 was upregulated in diabetic skin tissue. PDGF released by platelets binds to fibroblast surface receptors to promotes wound healing through fibroblast proliferation,25 and SDF-1 is typically secreted by fibroblasts during the late stage of wound healing.26 Several studies have shown that HIF-1α is an important adaptive gene that regulates the hypoxic environment of cells.18 Through high-throughput sequencing, GO, and KEGG analyses, we found that hADSCs extracellular vesicles under hypoxia may increase HIF-1α expression by affecting cell metabolism, differentiation, and TGF-β secretion or through the PI3K/AKT signaling pathway. These findings may provide insights for future research.
In this study, ADSCs-hEVs promoted fibroblast proliferation and migration through the PI3K/AKT pathway and increased the production of extracellular matrix components and multiple growth factors. Fibroblasts are important cells for wound healing because they release a variety of growth factors and extracellular matrix for scar formation. Type I and III collagen are two important components of the extracellular matrix that are secreted by HF and are contribute to the structural and mechanical properties of newly formed tissues.27 Thus, the proliferation and migration of HF determine the speed of wound healing. Studies have shown that the growth factor TGF-β can promote fibroblast proliferation and can coordinate with bFGF to stimulate fibroblast proliferation in normal rats.28 Moreover, MMPs participate in extracellular matrix remodeling, and EGF is a chemokine that promotes fibroblast proliferation and migration.29 EVs are important transmitters between cells and have tremendous potential for the healing of chronic diabetic wounds,30,31 as they have been reported to promote cell proliferation and migration and wound healing.32,33 Notably, because HIF-1α can target VEGF expression, it is an important factor for promoting angiogenesis.34 The VEGF protein level increased after EVs treatment, promoting the wound healing process and increasing angiogenesis.35 EVs can activate the phosphorylation of survival pathways, especially the PI3K/AKT signaling pathway.36,37 As a result, ADSCs-hEVs induce HF proliferation and migration, thereby promoting wound healing. This is an important finding in the study of diabetic wound treatment.
Studies in diabetic nude mouse wounds revealed that ADSCs-hEVs can accelerate the healing rate of diabetic wounds by increasing the secretion of COLI/III, IL-10, TGF-β, α-SMA, and VEGF and reducing the expression of IL-6 inflammatory factors. Previous studies analyzing factors related to extracellular matrix secretion during wound healing have shown that the secretion of COLI and COL III is an important factor that facilitates rapid wound healing.38,39 The anti-inflammatory factors IL-6 and IL-10 are involved in wound healing and angiogenesis in the surrounding tissues.40,41 HIF-1a is an important regulator of inflammatory cells that is capable of regulating macrophage motility,42 and its high expression promotes blood flow, angiogenesis, and tissue healing by regulating VEGF and SDF-1 expression in hypoxic wound tissue.43 Based on the above findings, we deduced that ADSCs-hEVs treatment can accelerate diabetic wound healing and contribute to high-quality wound healing. The present study thus provides strong evidence supporting the utility of ADSCs-hEVs in clinical treatment.
The following limitations of study should be noted. We did not study the specific effects of ADSCs-hEVs on the proliferation and migration of fibroblasts. Future studies should investigate these effects as well as the involvement of other pathways in ADSC-hEVs-mediated wound healing. Similarly, future studies exploring the treatment applications of ADSCs-hEVs should determine accurate injection doses to achieve the best therapeutic effect.
Conclusions
In summary, this study shows that ADSCs-hEVs can increase HF proliferation and migration through the PI3K/AKT signaling pathway and increase the secretion of related vascular factors, growth factors, inflammatory factors, and the extracellular matrix. ADSCs-hEVs can therefore accelerate the healing rate of diabetic wounds and improve healing quality by inhibiting inflammation and regulating extracellular matrix secretion. These studies provide more possibilities for ADSCs-hEVs in diabetic wound repair.
Acknowledgments
The authors thank the scientific research center at the Second Affiliated Hospital of Harbin Medical University for technical support. They also extend a special thanks to the Experimental Animal Center of the Second Affiliated Hospital of Harbin Medical University for helping with our animal experiments.
Funding Statement
This work was supported by the National Natural Science of China (Grant 81471796) and Heilongjiang Province Science Foundation for Excellent Youth Scholars (Grant JC2017019).
Abbreviations
ADSCs-EVs, Adipose stem cells extracellular vesicles; ADSCs-hEVs, Overexpression HIF-1α of adipose stem cell extracellular vesicles; ADSCs-cEVs¸ Negative control of adipose stem cell extracellular vesicles; N-ADSCs, Normal; C-ADSCs, Negative control; H-ADSCs, HIF-1α overexpression; bFGF, Basic fibroblast growth factor; COL I, Collagen I; COL III, Collagen III; GO, Gene Ontology; HIF-1α, Hypoxia inducible factor-1α; HF, Human fibroblast; H&E, Hematoxylin and eosin; IRB, Institutional Review Board; IL-6, Interleukin-6; IL-10, Interleukin-10; KEGG, Kyoto Encyclopedia of Genes and Genomes; SDF-1, Stromal cell-derived factor-1; TGF-β, Transforming growth factor-β; TEM, Transmission electron microscopy; Tras, Transwell; MMP1, Matrix metalloproteinase 1; MSCs, Mesenchymal stem cells; P-AKT, Phosphate-AKT; PMSF, Phenylmethanesulfonyl fluoride; PDGF, Platelet derived growth factor; qRT-PCR, Quantitative reverse transcription-polymerase chain reaction; SD, Standard deviation; VEGF, Vascular endothelial growth factor; α-SMA, Alpha-smooth muscle actin.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval
This study was approved by the Institutional Review Board of the Second Hospital of Harbin Medical University and conducted in accordance with the 2000 Helsinki Declaration. All patients signed the informed consent form. All experiments involving animals were approved by the Animal Ethics Committee of the Second Hospital of Harbin Medical University (approval number: 2014116). Animal care and handling procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Harbin Medical University and conducted by following the Guiding Opinions on the Ethical Treatment of Laboratory Animals issued by the Science and Technology Ministry of People’s Republic of China, and the National Standard Guidelines for Laboratory Animal Welfare and Ethical Review of the People’s Republic of China.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Francia P, Anichini R, Seghieri G, De Bellis A, Gulisano M. History, prevalence and assessment of limited joint mobility, from stiff hand syndrome to diabetic foot ulcer prevention: a narrative review of the literature. Curr Diabetes Rev. 2018;14(5):411–426. doi: 10.2174/1573399813666170816142731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamed S, Ullmann Y, Masoud M, Hellou E, Khamaysi Z, Teot L. Topical erythropoietin promotes wound repair in diabetic rats. J Invest Dermatol. 2010;130(1):287–294. doi: 10.1038/jid.2009.219 [DOI] [PubMed] [Google Scholar]
- 3.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–746. doi: 10.1056/NEJM199909023411006 [DOI] [PubMed] [Google Scholar]
- 4.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–1743. doi: 10.1016/S0140-6736(05)67700-8 [DOI] [PubMed] [Google Scholar]
- 5.Malone-Povolny MJ, Maloney SE, Schoenfisch MH. Nitric oxide therapy for diabetic wound healing. Adv Healthc Mater. 2019;8(12):e1801210. doi: 10.1002/adhm.201801210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houreld NN. Shedding light on a new treatment for diabetic wound healing: a review on phototherapy. ScientificWorldJournal. 2014;2014:398412. doi: 10.1155/2014/398412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamama K, Kawasaki H, Kerpedjieva SS, Guan J, Ganju RK, Sen CK. Differential roles of hypoxia inducible factor subunits in multipotential stromal cells under hypoxic condition. J Cell Biochem. 2011;112(3):804–817. doi: 10.1002/jcb.22961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M, Cui Y, He W, et al. Effects of triple-mutated hypoxia-inducible factor-1α on angiogenesis and cardiac function improvement in rats with myocardial infarction. Cell Physiol Biochem. 2018;50(6):2329–2340. doi: 10.1159/000495094 [DOI] [PubMed] [Google Scholar]
- 9.Tekin D, Dursun AD, Xi L. Hypoxia inducible factor 1 (HIF-1) and cardioprotection. Acta Pharmacol Sin. 2010;31(9):1085–1094. doi: 10.1038/aps.2010.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thangarajah H, Yao D, Chang EI, et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci USA. 2009;106(32):13505–13510. doi: 10.1073/pnas.0906670106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thangarajah H, Vial IN, Grogan RH, et al. HIF-1alpha dysfunction in diabetes. Cell Cycle. 2010;9(1):75–79. doi: 10.4161/cc.9.1.10371 [DOI] [PubMed] [Google Scholar]
- 12.Chang EI, Loh SA, Ceradini DJ, et al. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation. 2007;116(24):2818–2829. doi: 10.1161/CIRCULATIONAHA.107.715847 [DOI] [PubMed] [Google Scholar]
- 13.An Y, Lin S, Tan X, et al. extracellular vesicles from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021;54(3):e12993. doi: 10.1111/cpr.12993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y, El Andaloussi S, Wood MJ. extracellular vesicles and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21(R1):R125–R134. doi: 10.1093/hmg/dds317 [DOI] [PubMed] [Google Scholar]
- 15.Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bang C, Thum T. extracellular vesicles: new players in cell-cell communication. Int J Biochem Cell Biol. 2012;44(11):2060–2064. doi: 10.1016/j.biocel.2012.08.007 [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Wu H, Peng Y, et al. Hypoxia adipose stem cell-derived extracellular vesicles promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J Nanobiotechnology. 2021;19(1):202. doi: 10.1186/s12951-021-00942-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botusan IR, Sunkari VG, Savu O, et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci USA. 2008;105(49):19426–19431. doi: 10.1073/pnas.0805230105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Opalenik SR, Davidson JM. Fibroblast differentiation of bone marrow-derived cells during wound repair. FASEB J. 2005;19(11):1561–1563. doi: 10.1096/fj.04-2978fje [DOI] [PubMed] [Google Scholar]
- 20.Ding J, Wang X, Chen B, Zhang J, Xu J. extracellular vesicles derived from human bone marrow mesenchymal stem cells stimulated by deferoxamine accelerate cutaneous wound healing by promoting angiogenesis. Biomed Res Int. 2019;2019:9742765. doi: 10.1155/2019/9742765 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Li X, Liu L, Yang J, et al. Exosome derived from human umbilical cord mesenchymal stem Cell mediates MiR-181c attenuating burn-induced excessive inflammation. EBioMedicine. 2016;8:72–82. doi: 10.1016/j.ebiom.2016.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braun L, Kim PJ, Margolis D, Peters EJ, Lavery LA; Wound Healing Society. What’s new in the literature: an update of new research since the original WHS diabetic foot ulcer guidelines in 2006. Wound Repair Regen. 2014;22(5):594–604. doi: 10.1111/wrr.12220 [DOI] [PubMed] [Google Scholar]
- 23.Markuson M, Hanson D, Anderson J, et al. The relationship between hemoglobin a1c values and healing time for lower extremity ulcers in individuals with diabetes. Adv Skin Wound Care. 2009;22(8):365–372. doi: 10.1097/01.ASW.0000358639.45784.cd [DOI] [PubMed] [Google Scholar]
- 24.Gunton JE. Hypoxia-inducible factors and diabetes. J Clin Invest. 2020;130(10):5063–5073. doi: 10.1172/JCI137556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piazuelo E, Lanas A, Jimenez P, García-Gonzalez A, Esteva F. In vitro wound repair by human gastric fibroblasts: implications for ulcer healing. Dig Dis Sci. 1998;43(6):1230–1240. doi: 10.1023/a:1018803707179 [DOI] [PubMed] [Google Scholar]
- 26.Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor–HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem. 2012;19(1):90–97. doi: 10.2174/092986712803413944 [DOI] [PubMed] [Google Scholar]
- 27.Wen Q, Zhou C, Luo W, Zhou M, Ma L. Pro-osteogenic effects of fibrin glue in treatment of avascular necrosis of the femoral head in vivo by hepatocyte growth factor-transgenic mesenchymal stem cells. J Transl Med. 2014;12(1):114. doi: 10.1186/1479-5876-12-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark RA, McCoy GA, Folkvord JM, McPherson JM. TGF-beta 1 stimulates cultured human fibroblasts to proliferate and produce tissue-like fibroplasia: a fibronectin matrix-dependent event. J Cell Physiol. 1997;170(1):69–80. doi: [DOI] [PubMed] [Google Scholar]
- 29.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–870. doi: 10.1152/physrev.2003.83.3.835 [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Yang Y, Li Y, et al. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9(13):4430–4438. doi: 10.1039/c7nr00352h [DOI] [PubMed] [Google Scholar]
- 31.Watson DC, Bayik D, Srivatsan A, et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195–205. doi: 10.1016/j.biomaterials.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu L, Wang J, Zhou X, et al. Extracellular vesicles derived from human adipose mesenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts [published correction appears in Sci Rep. 2020 Apr 16;10(1):6693]. Sci Rep. 2016;6(1):32993. doi: 10.1038/srep32993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Guan J, Niu X, et al. Extracellular vesicles released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13(1):49. doi: 10.1186/s12967-015-0417-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koyasu S, Kobayashi M, Goto Y, Hiraoka M, Harada H. Regulatory mechanisms of hypoxia-inducible factor 1 activity: two decades of knowledge. Cancer Sci. 2018;109(3):560–571. doi: 10.1111/cas.13483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narayanan R, Huang CC, Ravindran S. Hijacking the cellular mail: exosome mediated differentiation of mesenchymal stem cells. Stem Cells Int. 2016;2016:3808674. doi: 10.1155/2016/3808674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vrijsen KR, Maring JA, Chamuleau SA, et al. Extracellular vesicles from cardiomyocyte progenitor cells and mesenchymal stem cells stimulate angiogenesis via EMMPRIN. Adv Healthc Mater. 2016;5(19):2555–2565. doi: 10.1002/adhm.201600308 [DOI] [PubMed] [Google Scholar]
- 37.Kim S, Lee SK, Kim H, Kim TM. extracellular vesicles secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int J Mol Sci. 2018;19(10):3119. doi: 10.3390/ijms19103119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu K, Sun Y, Kh Al-Ani M, et al. Synergistic promoting effects of bone morphogenetic protein 12/connective tissue growth factor on functional differentiation of tendon derived stem cells and patellar tendon window defect regeneration. J Biomech. 2018;66:95–102. doi: 10.1016/j.jbiomech.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 39.Tsai WC, Pang JH, Hsu CC, Chu NK, Lin MS, Hu CF. Ultrasound stimulation of types I and III collagen expression of tendon cell and upregulation of transforming growth factor beta. J Orthop Res. 2006;24(6):1310–1316. doi: 10.1002/jor.20130 [DOI] [PubMed] [Google Scholar]
- 40.Gallucci RM, Simeonova PP, Matheson JM, et al. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000;14(15):2525–2531. doi: 10.1096/fj.00-0073com [DOI] [PubMed] [Google Scholar]
- 41.Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol. 2003;73(6):713–721. doi: 10.1189/jlb.0802397 [DOI] [PubMed] [Google Scholar]
- 42.Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation [published correction appears in Cell. Cell. 2003;112(5):645–657. doi: 10.1016/s0092-8674(03)00154-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol. 2001;280(6):C1358–C1366. doi: 10.1152/ajpcell.2001.280.6.C1358 [DOI] [PubMed] [Google Scholar]