Dear Editor,
The recently extended use of mRNA vaccines BNT162b2 (Pfizer–BioNTech) and mRNA‐1273 (Moderna) due to the COVID‐19 pandemic has allowed the description of multiple cutaneous adverse effects linked to this new type of immunization. Although erythema multiforme (EM) is a recognized rare adverse effect of many other vaccines, reports linking this reaction to mRNA ones are very scarce so far. We describe a case of atypical EM occurring shortly after the second dose of BNT162b2 with no other evident cause.
Erythema multiforme is an inflammatory skin condition classically linked to infections (herpes simplex virus and mycoplasma are the most common causes), but a wide array of triggers, including many other infectious agents, drugs, immunizations, and even internal diseases, have also been described. Hallmark lesions consist of predominantly acral, targetoid papules, made up of three concentric distinct zones. 1 However, clinical manifestations of EM are diverse, and it can present as atypical palpable lesions with an erythematous dusky body surrounded by a paler halo.
A 91‐year‐old woman presented with diffuse, erythematous, and pruriginous papules with a tendency to confluence into plaques, which started over her left deltoid area 6 days after receiving the second dose of BNT162b2 (Pfizer–BioNTech COVID‐19 vaccine) injected in that location. The rash progressed over the following 10 days, involving her back, “V” of the neck, and extremities.
At the time of assessment, she presented large plaques in the injection site and in the central dorsolumbar area and buttocks, and also multiple papules on her extremities and trunk (Fig. 1). A discreet light pinkish erythematous border could be noted around some of the lesions. There were no signs of epidermal detachment. Mucosae were unaltered. She was afebrile and denied systemic symptoms except for mild asthenia. She also denied prodromal semiology or recent medication changes.
Figure 1.
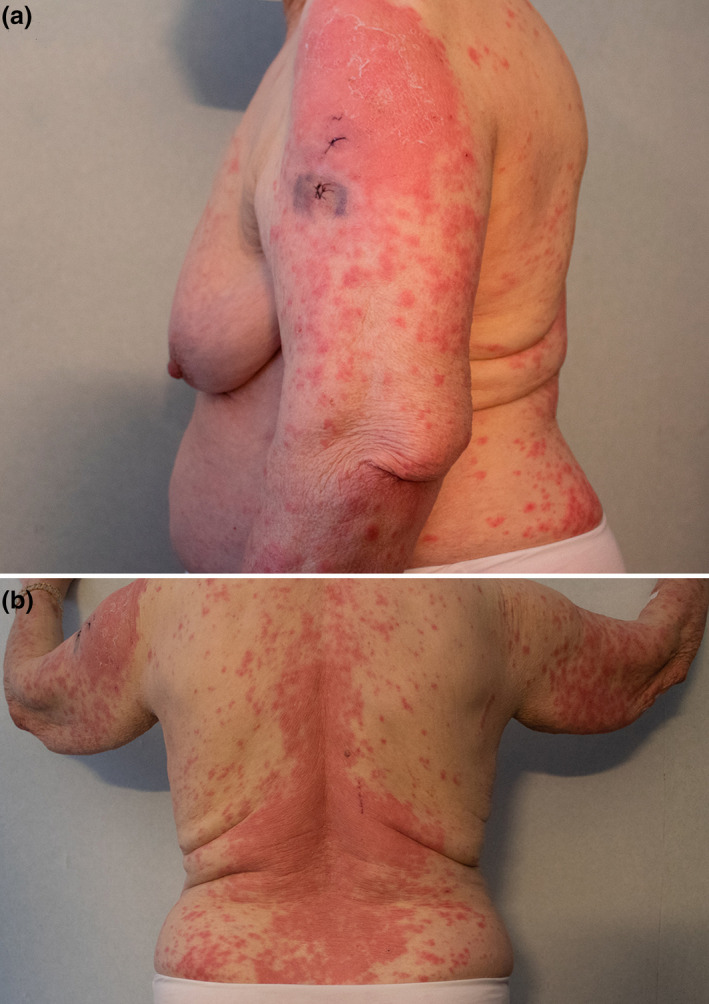
A large erythematous plaque on the site of the injection of the second dose of BNT162b2 COVID‐19 vaccine (left deltoid). The main plaque displays peripheral scale, representing an early stage of the resolution process of initial lesions. Peripheral erythematous papules, many of them surrounded by a discreet paler halo, can be seen. Sutures over both biopsy wounds can be noted (a). After the progression of the initial plaque on the left deltoid, similar lesions can be seen profusely involving the back and upper extremities of the patient (b)
Two biopsies from the left deltoid plaque and a peripheral papule showed superficial dermal lymphocytic infiltrate obscuring the dermo‐epidermal junction associated with hydropic changes and dyskeratosis of isolated or grouped keratinocytes not confined to the basal layer. Intraepidermal lymphocytes were also noted (Fig. 2).
Figure 2.
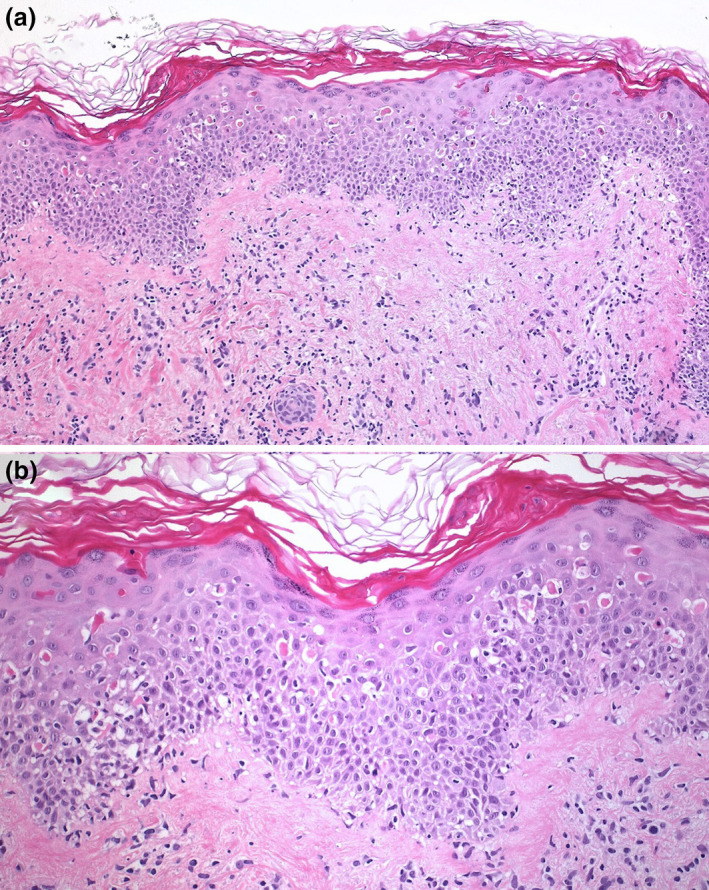
(a) Skin biopsy demonstrated moderate perivascular, dermal lymphoid infiltration with focal interface dermatitis (Hematoxylin‐eosin, 100×). (b) Higher magnification revealed multiple apoptotic keratinocytes at all epidermal layers, sometimes with satellite lymphocytes (Hematoxylin‐eosin, 200×)
These findings, along with the clinical appearance of individual lesions, allowed the diagnosis of atypical EM to be made. Due to the extent of the lesions, she was admitted for close surveillance and initial treatment with high potency topical corticosteroids (clobetasol propionate ointment). She did not develop complications, and the lesions gradually subsided, leaving residual hyperpigmentation. On the eighth day of admission, she was discharged owing to favorable evolution.
EM has recently been described as a rare cutaneous adverse effect of the COVID‐19 vaccine. A registry‐based study involving 414 health workers who suffered skin reactions to COVID‐19 vaccines included three cases of EM related to the first doses of mRNA‐127 (Moderna COVID‐19 vaccine), but additional clinical data of these patients are not provided. 2 Additionally, there are only two case reports of EM secondary to the COVID‐19 vaccine. The first one is a 58‐year‐old woman with a history of EM reactive to herpes labialis who developed typical targetoid lesions limited to her palms and soles hours after each of the BNT162b2 vaccine doses. 3 The second one described a 74‐year‐old woman who was admitted to the hospital because of disseminated atypical EM lesions that presented a day after the BNT162b2 first dose. 4 This clinical picture has been labeled as Rowell syndrome by authors due to the patient showing antinuclear antibody (ANA) titers of 1/640.
It is necessary to underline that vaccine‐induced EM has been known for a long time, with 984 cases reported to the Vaccine Adverse Event Reporting System. 1 Moreover, EM‐like reactions have already been linked to COVID‐19 infection, both as typical acral lesions in younger individuals and more widespread, atypical lesions in adults. 5 SARS‐CoV‐2 spike protein, the structure codified in mRNA COVID‐19 vaccines, has been demonstrated immunohistochemically in endothelial cells and eccrine ducts epithelium in those cases. 5 Consequently, EM secondary to COVID‐19 vaccination would be an expected complication in some cases.
It is also notable that this is the first COVID‐19 vaccine‐related EM having its onset after the second dose.
Conflict of interest: None.
Funding source: None.
References
- 1. Su JR, Haber P, Ng CS, et al. Erythema multiforme, Stevens Johnson syndrome, and toxic epidermal necrolysis reported after vaccination, 1999–2017. Vaccine 2020; 38: 1746–1752. 10.1016/j.vaccine.2019.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol 2021; S0190–9622: 00658–667. 10.1016/j.jaad.2021.03.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lavery MJ, Nawimana S, Parslew R, et al. A flare of pre‐existing erythema multiforme following BNT162b2 (Pfizer‐BioNTech) COVID‐19 vaccine. Clin Exp Dermatol 2021; 10.1111/ced.14714. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gambichler T, Scholl L, Dickel H, et al. Prompt onset of Rowell's syndrome following the first BNT162b2 SARS‐CoV‐2 vaccination. J Eur Acad Dermatol Venereol 2021; 35: 10.1111/jdv.17225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rongioletti F, Ferreli C, Sena P, et al. Clinicopathologic correlations of COVID‐19‐related cutaneous manifestations with special emphasis on histopathologic patterns. Clin Dermatol 2021; 39: 149–162. 10.1016/j.clindermatol.2020.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]


