Abstract
Knowledge on the immunogenicity of vector-based and mRNA-vaccines in solid organ transplant recipients is limited. Therefore, SARS-CoV-2–specific T cells and antibodies were analyzed in 40 transplant recipients and 70 controls after homologous or heterologous vaccine-regimens. Plasmablasts and SARS-CoV-2–specific CD4 and CD8 T cells were quantified using flow cytometry. Specific antibodies were analyzed by ELISA and neutralization assay. The two vaccine types differed after the first vaccination, as IgG and neutralizing activity were more pronounced after mRNA priming (p = .0001 each), whereas CD4 and CD8 T cell levels were higher after vector priming (p = .009; p = .0001). All regimens were well tolerated, and SARS-CoV-2–specific antibodies and/or T cells after second vaccination were induced in 100% of controls and 70.6% of transplant recipients. Although antibody and T cell levels were lower in patients, heterologous vaccination led to the most pronounced induction of antibodies and CD4 T cells. Plasmablast numbers were significantly higher in controls and correlated with SARS-CoV-2–specific IgG- and T cell levels. While antibodies were only detected in 35.3% of patients, cellular immunity was more frequently found (64.7%) indicating that assessment of antibodies is insufficient to identify COVID-19-vaccine responders. In conclusion, heterologous vaccination seems promising in transplant recipients, and combined analysis of humoral and cellular immunity improves the identification of responders among immunocompromised individuals.
KEYWORDS: clinical research/practice, flow cytometry, infection and infectious agents - viral, infectious disease, T cell biology, vaccine
Abbreviations: BAU, binding antibody units; COVID-19, coronavirus disease 2019; DL, detection limit; ELISA, enzyme-linked immunosorbent assay; IFN, interferon; IH, percentage of inhibition; IL, interleukin; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2; SEB, Staphylococcus aureus enterotoxin B; TNF, tumor necrosis factor
1. INTRODUCTION
Currently authorized vaccines toward the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) include mRNA vaccines and adenovirus-based replication-incompetent vector vaccines. Both vaccine types including the heterologous vector-mRNA combination have proved to be strongly immunogenic1, 2, 3, 4 and highly efficient in preventing severe coronavirus disease (COVID-19) in immunocompetent individuals.1, 2, 3 Immunocompromised individuals such as patients after solid organ transplantation are at higher risk to suffer from more severe disease.5 Therefore, COVID-19 vaccination is generally recommended for transplant recipients with no preference towards the use of either mRNA or vector-based vaccines.6 , 7 It has become evident that humoral immunity in transplant recipients immunized with mRNA-based COVID-19 vaccines was only induced in approximately 6%–17% after the first dose,8, 9, 10 and up to 59% after the second dose,11, 12, 13, 14, 15, 16 respectively. Risk factors for poor response included older age, more intense immunosuppressive drug regimens including depleting antibodies and anti-metabolites, and earlier time after transplantation.8 , 11 While the ability to induce antibodies has primarily been reported so far, knowledge on vaccine-induced cellular immunity in transplant recipients is limited and restricted to mRNA vaccines,17 , 18 whereas immunogenicity in transplant recipients after administration of vector-based vaccines is currently unknown, and general knowledge on potential differences of immunogenicity between the two vaccine types is scarce. Although both the mRNA vaccines and the currently licensed vector vaccine ChAdOx1 nCoV-19 are administered twice, the recommended time interval between the first and the second dose varies from 3–6 weeks for mRNA vaccines to 9–12 weeks for the ChAdOx1 nCoV-19 vaccine.6
Here we report the results of a prospective study assessing the vaccine-induced humoral and cellular immune response in solid organ transplant recipients in comparison with a healthy age-matched control group. The vector vaccine ChAdOx1 nCoV-19 and the mRNA vaccines BNT162b2 or mRNA-1273 including homologous and heterologous regimens were assigned as per national policies.6 , 7 To allow direct comparison of immunogenicity of the two vaccine types independent of the recommended time interval between the first and the second dose, we analyzed primary induction of humoral and cellular immunity after the first vaccine dose. In addition, immunogenicity and reactogenicity after a second homologous and heterologous dose was characterized to estimate the overall response after a complete vaccine regimen.
2. METHODS
2.1. Study design and patient population
Solid organ transplant recipients and age-matched immunocompetent controls with no known history of SARS-CoV-2 infection were included in the study. Individuals either received homologous or heterologous regimens consisting of the adenovirus-vector vaccine ChAdOx1 nCoV-19 or mRNA-vaccines (BNT162b2 or mRNA-1273) as per national recommendation.7 Lymphocyte subpopulations as well as vaccine-induced SARS-CoV-2–specific humoral and cellular immune responses were analyzed from heparinized whole blood 13–30 days after the first and the second vaccination (except for one healthy control tested 7 days after the second vaccination). Results after secondary vaccination of 32 controls were included as part of a previous study on 216 immunocompetent controls.4 Analyses of lymphocyte subpopulations and antigen-specific T cells were carried out within 24 h. Antibody testing was performed from frozen plasma samples. Baseline levels of SARS-CoV-2-reactive antibodies were determined to control for pre-existing immunity. Antibodies toward the nucleocapsid protein were analyzed to estimate infection after vaccination. Local and systemic adverse events within 7 days after vaccinations were recorded using a questionnaire. The study was approved by the ethics committee of the Ärztekammer des Saarlandes (reference 76/20), and all individuals gave written informed consent.
2.2. Quantification of lymphocyte populations and plasmablasts
Quantification of T cells, B cells and plasmablasts was performed on 100 µl heparinized whole blood exactly as described before4 , 19 using monoclonal antibodies towards CD3 (clone SK7), CD19 (clone HIB19), CD27 (clone L128), CD38 (clone HB7), and IgD (clone IA6-2). Cells were analyzed using flow cytometry (FACS-Canto-II or FACSLyric) and FACS-Diva-V6.1.3 or FlowJo software (BD Biosciences). Among total lymphocytes, T and B cells were identified by expression of CD3 and CD19, respectively. Plasmablasts were gated as CD38 positive cells among IgD-CD27+ CD19 positive switched-memory B cells as described before.4 Differential blood counts were used to calculate absolute lymphocyte numbers.
2.3. Quantification of vaccine-induced SARS-CoV-2–specific T cells
SARS-CoV-2–specific T cells were determined as described previously.4 , 19 In brief, heparinized whole blood samples were stimulated with overlapping peptides spanning the SARS-CoV-2 spike protein (N-terminal receptor-binding domain and C-terminal portion including the transmembrane domain, 2 µg/ml/peptide; JPT, Berlin, Germany). Stimulations with the peptide diluent (0.64% DMSO) and with 2.5 μg/ml of the polyclonal stimulus Staphylococcus aureus enterotoxin-B (SEB; Sigma) served as negative and positive controls, respectively. All stimulations were carried out in presence of co-stimulatory antibodies against CD28 and CD49d (1 μg/ml each). Cells were stimulated for 6 h, processed as described before4 , 19 and immunostained using anti-CD4 (clone SK3), anti-CD8 (clone SK1), anti-CD69 (clone L78), anti-IFNγ (clone 4S.B3), anti-IL-2 (clone MQ1-17H12), and anti-TNFα (clone MAb11). SARS-CoV-2-reactive CD4 or CD8 T cells were identified as activated CD69 positive T cells producing IFNγ using flow-cytometry based on a gating strategy as described before.4 In addition, co-expression of IL-2 and TNFα was analyzed after Boolean gating to characterize functionality in more detail. Reactive CD4 and CD8 T cell levels after control stimulations were subtracted from levels obtained after SARS-CoV-2–specific stimulation, and 0.03% of reactive T cells was set as detection limit as described before4 based on the distribution of T cell frequencies after control stimulations (97.5% of control stimulations below this threshold).
2.4. Determination of SARS-CoV-2–specific antibodies and neutralization capacity
SARS-CoV-2–specific IgG antibodies towards the receptor binding domain of SARS-CoV-2 spike protein were quantified using an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (SARS-CoV-2-QuantiVac, Euroimmun, Lübeck, Germany). Antibody-binding units (BAU/ml) <25.6 were scored negative, ≥25.6-35.2 BAU/ml were scored intermediate, and ≥35.2 BAU/ml were scored positive. A neutralization assay based on antibody-mediated inhibition of soluble ACE2 binding to the plate-bound S1 receptor binding domain was used according to the manufacturer’s instructions (SARS-CoV-2-NeutraLISA, Euroimmun). Neutralizing antibodies were determined in all individuals with SARS-CoV-2 positive IgG titers, and scored negative in IgG-negatives. Neutralizing capacity is expressed as percentage of inhibition (IH) calculated by 1 minus the ratio of the extinction of the patient sample and the blank value. IH <20% were scored negative, IH ≥20–35 were scored intermediate, and IH ≥35% were scored positive. A semiquantitative IgG ELISA towards the SARS-CoV-2 nucleocapsid protein was performed according to the manufacturer´s instructions (anti-SARS-CoV-2-NCP-ELISA, Euroimmun).
2.5. Statistical analysis
Mann–Whitney and Kruskall–Wallis tests were performed to compare unpaired non-parametric data between groups. Paired analyses of T cell and antibody levels were performed using the Wilcoxon matched pairs test. Data with normal distribution were analyzed using unpaired t test. Categorial analyses were performed using Fisher’s exact and Chi-square test as indicated. Correlations were analyzed according to Spearman. Statistical analysis was carried out using GraphPad Prism 9.0 software (GraphPad, San Diego, CA) using two-tailed tests. Multivariate analysis on vaccine response was performed using IBM-SPSS Statistics-26. A p-value < .05 was considered statistically significant.
3. RESULTS
3.1. Study population
Forty solid organ transplant recipients and 70 immunocompetent controls were recruited and tested at a median of 15 (IQR 6) days after the first vaccination with either vector or mRNA-vaccine, which was applied in 75% and 25% of individuals, respectively. Among vector-primed individuals, approximately two-thirds received heterologous boosting with mRNA. One patient refused the second dose, and one patient was lost to follow-up. Immunogenicity after secondary vaccination was analyzed at a median of 14 (IQR 1) days. All individuals had no known history of SARS-CoV-2 infection. The two groups were matched regarding age, and vaccine combinations, but there were more males among patients ( Table 1). Patients were transplanted for 6.5 (IQR 9.9) years, and the majority of patients (28/40, 70%) were on an immunosuppressive triple-drug regimen including a calcineurin inhibitor, glucocorticoids and an antimetabolite (Table 1). Three patients were within one year after transplantation (8.5–11.8 months), and no patient had received T or B cell depleting therapy for induction or rejection within two years before vaccination and thereafter. One patient had received rejection treatment with glucocorticoids within the first two months after transplantation, but more than eleven months before vaccination. Patients had a leukocytosis mainly affecting neutrophils and had significantly less lymphocytes. Among lymphocytes, significantly lower numbers of CD3 T cells and CD19 positive B cells including plasmablasts were observed in patients (Table 1).
TABLE 1.
Demographic and clinical characteristics of the study population
| Controls |
Transplant recipients |
p-value | |
|---|---|---|---|
| n = 70 | n = 40# | ||
| Years of age (mean ± SD) | 50.6 ± 11.9 | 54.5 ± 12.7 | .11 |
| Female gender, n (%) | 49 (70.0) | 18 (45.0) | .01 |
| Vaccine regimen (vector§ vs. mRNA) | |||
| vector/vector, n (%)x | 17 (24.3) | 9 (23.7) | |
| vector/mRNA, n (%)† | 34 (48.6) | 20 (52.6) | |
| mRNA/mRNA, n (%)‡ | 19 (27.1) | 9 (23.7) | |
| Analysis time (days after vaccination), median (IQR) | |||
| 1st vaccination | 15 (5) | 16 (6) | .292 |
| 2nd vaccination | 14 (1) | 14 (3) | .270 |
| Organ transplant | |||
| Kidney, n (%) | n.a. | 35 (87.5) | n.a. |
| Heart, n (%) | n.a. | 1 (2.5) | n.a. |
| Lung, n (%) | n.a. | 1 (2.5) | n.a. |
| Liver, n (%) | n.a. | 1 (2.5) | n.a. |
| Liver and kidney, n (%) | n.a. | 2 (5.0) | n.a. |
| Years since transplantation, median (IQR) | n.a. | 6.5 (9.9) | n.a. |
| Immunosuppressive regimen | |||
| CNI, antimetabolite, GC (%) | n.a. | 28 (70.0) | n.a. |
| CNI, antimetabolite (%) | n.a. | 5 (12.5) | n.a. |
| CNI, GC (%) | n.a. | 2 (5.0) | n.a. |
| CNI (%) | n.a. | 2 (5.0) | n.a. |
| mTOR-I, antimetabolite, GC (%) | n.a. | 2 (5.0) | n.a. |
| mTOR-I, GC (%) | n.a. | 1 (2.5) | n.a. |
| Differential blood cell counts | n = 68 | n = 38 | |
| Leukocytes (cells/µl), median (IQR) | 6450 (1975) | 7250 (2550) | .029 |
| Granulocytes (cells/µl), median (IQR) | 3716 (1548) | 4710 (1868) | .002 |
| Monocytes (cells/µl), median (IQR) | 557 (212) | 627 (419) | .099 |
| Lymphocytes (cells/µl), median (IQR) | 2126 (831) | 1434 (1055) | .001 |
| CD3 T cells (cells/µl), median (IQR)* | 1471 (632) | 1083 (995) | .026 |
| CD19 B cells (cells/µl), median (IQR)* | 202 (139) | 70 (116) | < .0001 |
| Plasmablast (% of B cells), median (IQR) | 0.315 (0.364) | 0.112 (0.426) | .002 |
Note: All data refer to time point after first vaccination, unless indicated differently.
Abbreviations: antimetabolite, azathioprine or mycophenolate mofetil/mycophenolic acid; CNI, calcineurin inhibitor; GC, glucocorticoids; mTOR-I, m-TOR inhibitor.
One transplant recipient refused the second vaccination, and one was lost to follow-up; both had received the vector vaccine as first vaccination.
B and T cell counts were calculated on 56 controls and 37 transplant recipients and plasmablasts on 58 and 38, respectively.
Vector refers to the ChAdOx1 nCoV-19 vaccine, whereas mRNA refers to BNT162b2 unless indicated differently.
Numbers include one control with mRNA-1273.
Numbers include five controls and one transplant recipient with mRNA-1273.
3.2. Lower SARS-CoV-2–specific antibody and T cell levels in transplant recipients
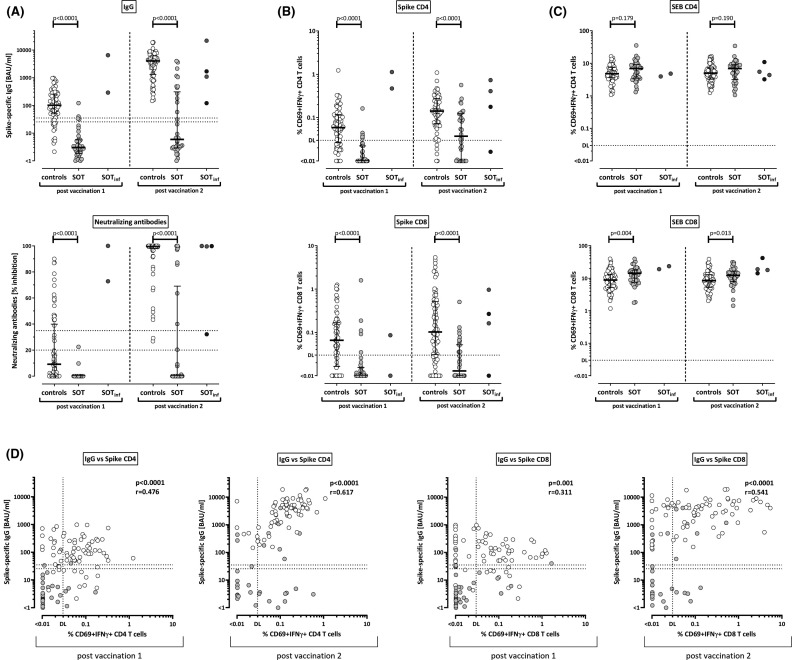
Spike-specific IgG levels were significantly higher among immunocompetent controls than in transplant recipients both after the first and the second vaccination ( Figure 1A, p < .0001). Two kidney transplant recipients with the highest antibody titers after first vaccination (6511 and 292 BAU/ml, respectively) already had detectable SARS-CoV-2 antibodies at baseline (384 and 306 BAU/ml, respectively), which identified a history of an asymptomatic SARS-CoV-2 infection. Two additional patients underwent asymptomatic infection after the first vaccination as determined by emergence of antibodies toward the SARS-CoV-2 nucleocapsid. The results of these patients are displayed separately from the remaining transplant recipients (Figure 1A, SOTinf). All other individuals were anti-nucleocapsid antibody negative (data not shown). Likewise, neutralizing antibody activity was higher among healthy controls both after the first and second vaccination (Figure 1A). Among transplant recipients, neutralizing antibodies after primary vaccination were only found in the two patients with prior infection, who had strong neutralizing activity (100% and 72.9% inhibition, respectively). In all other patients, neutralizing activity ≥35% was only found after the second vaccination.
FIGURE 1.
T cell and antibody responses in controls and transplant recipients after SARS-CoV-2–specific vaccination. (A) Levels of spike-specific IgG and neutralizing antibodies were determined by ELISA and neutralization assay, respectively, and compared between healthy controls (open symbols) and solid organ transplant recipients (SOT, filled symbols). Levels of SARS-CoV-2–specific (B) and SEB-reactive (C) CD4 and CD8 T cells were flow cytometrically determined after antigen-specific stimulation in vitro followed by intracellular cytokine staining, and compared between groups. In panels A–C, results of four vaccinated transplant recipients with a previously unknown history of asymptomatic SARS-CoV-2 infection (two before [gray symbols] and two after the first vaccination [black symbols]) are displayed separate from the remaining baseline-negative transplant recipients and excluded from statistical analyses. Bars represent medians with interquartile ranges. Differences between the groups were calculated using Mann–Whitney test. (D) Correlations between IgG titers and SARS-CoV-2–specific CD4 and CD8 T cells are shown (calculated according to Spearman) for controls (white symbols) and transplant recipients (gray symbols). Correlation parameters for controls and transplant recipients as individual groups are summarized in Table S1. Dotted lines indicate limits for IgG and neutralizing antibodies, including negative, intermediate, and positive results, respectively, as per the manufacturer’s instructions, or detection limits (DL) for SARS-CoV-2–specific and SEB-reactive CD4 and CD8 T cells, respectively. All analyses were performed after the first and after the second vaccination. IFN, interferon
Spike-specific cellular immune responses were characterized after stimulation with overlapping peptide pools of the SARS-CoV-2 spike protein followed by intracellular staining of IFNγ. Polyclonal T cell reactivity in SEB-stimulated samples served as positive controls. SARS-CoV-2- and SEB-reactive CD4 and CD8 T cells were identified by co-expression of the activation marker CD69 and IFNγ. Representative dot plots of a patient and a control are shown in supplementary Figure S1. In line with antibody levels, both the first and the second vaccination induced significantly higher SARS-CoV-2–specific CD4 and CD8 T cell levels in healthy controls than in transplant recipients (Figure 1B, each p < .0001). This was not due to a general non-responsiveness, as CD4 and CD8 T cell reactivity after polyclonal stimulation was not impaired in patients (Figure 1C). Among the four transplant recipients with evidence of infection, three showed a pronounced SARS-CoV-2–specific cellular immunity, which was similar or even higher in magnitude as compared to vaccine responses in immunocompetent infection-naïve individuals (Figure 1B). Overall, SARS-CoV-2–specific IgG levels showed a significant correlation with specific CD4 and CD8 T cells after both the first and the second vaccination, with differences among patients and controls (Figure 1D, white and gray symbols, and Table S1).
The percentages of individuals with cellular and humoral immunity above the respective detection limits are summarized in Table 2. While 56/70 controls (80.0%) and only 2/38 (5.3%) of transplant recipients had IgG levels above the threshold after the first vaccination, this increased to 100% and 35.3% after booster vaccination (Table 2). In contrast, a larger fraction of patients showed a vaccine-induced SARS-CoV-2–specific cellular immune response after priming (9/38 [23.7%] transplant recipients and 59/70 [84.3%] controls), which increased to 64.7% in patients and 95.7% in controls after boosting. Finally, while 67/70 (95.7%) controls and 10/38 (26.3%) of transplant recipients mounted any type of vaccine-induced immune response (antibodies and/or T cells) after priming, this increased to 100% of controls and 70.6% of patients after boosting.
TABLE 2.
Vaccine-induced immune responses and reported adverse events in the study population
| Post vaccination 1 |
Post vaccination 2 |
|||||
|---|---|---|---|---|---|---|
| Controls |
Transplant recipients |
Controls |
Transplant recipients |
|||
| n = 70 | n = 38* | p-value | n = 70 | n = 34* | p-value | |
| Individuals with SARS-CoV-2 spike-specific immunity | ||||||
| IgG (≥35.2 BAU/ml), n (%) | 56 (80.0) | 2 (5.3) | < .0001 | 70 (100.0) | 12 (35.3) | < .0001 |
| Neutralizing antibodies (IH ≥35%), n (%) | 18 (25.7) | 0 (0) | .0002 | 68 (97.1) | 10 (29.4) | < .0001 |
| CD4 T cells, n (%) | 52 (74.3) | 5 (13.2) | < .0001 | 64 (91.4) | 21 (61.8) | .0007 |
| CD8 T cells, n (%) | 49 (70.0) | 5 (13.2) | < .0001 | 53 (75.7) | 14 (41.2) | .0009 |
| Combined cellular and/or humoral analysis | ||||||
| CD4 and/or CD8 T cells, n (%) | 59 (84.3) | 9 (23.7) | < .0001 | 67 (95.7) | 22 (64.7) | < .0001 |
| T cells and/or IgG; n (%) | 67 (95.7) | 10 (26.3) | < .0001 | 70 (100.0) | 24 (70.6) | < .0001 |
| Adverse events | n = 70 | n = 38* | .018# | n = 70 | n = 34* | .140# |
| None, n (%) | 8 (11.4) | 12 (31.6) | 13 (18.6) | 11 (32.4) | ||
| Local, n (%) | 11 (15.7) | 4 (10.5) | 16 (22.9) | 9 (26.5) | ||
| Systemic, n (%) | 17 (24.3) | 11 (28.9) | 8 (11.4) | 2 (5.9) | ||
| Local and systemic, n (%) | 34 (48.6) | 11 (28.9) | 33 (47.1) | 12 (35.3) | ||
Abbreviations: Ab, antibody; BAU, binding antibody units; IH, percentage of inhibition.
The patients with evidence of prior infection before vaccination 1 (n = 2) and vaccination 2 (n = 2) are not included in the respective evaluation; one patient refused the second vaccination and one patient was lost to follow-up.
Comparison of none versus any adverse event (Fisher´s exact test), for differences in adverse events between the vaccines see Figure 4.
Multivariate analysis revealed increasing age and shorter time since transplantation as significant factors associated with non-responsiveness after the second vaccination (p = .035 and p = .039, respectively, Table S2).
3.3. Differential induction of SARS-CoV-2–specific T cells and antibodies after priming with vector-based vaccine and mRNA vaccines
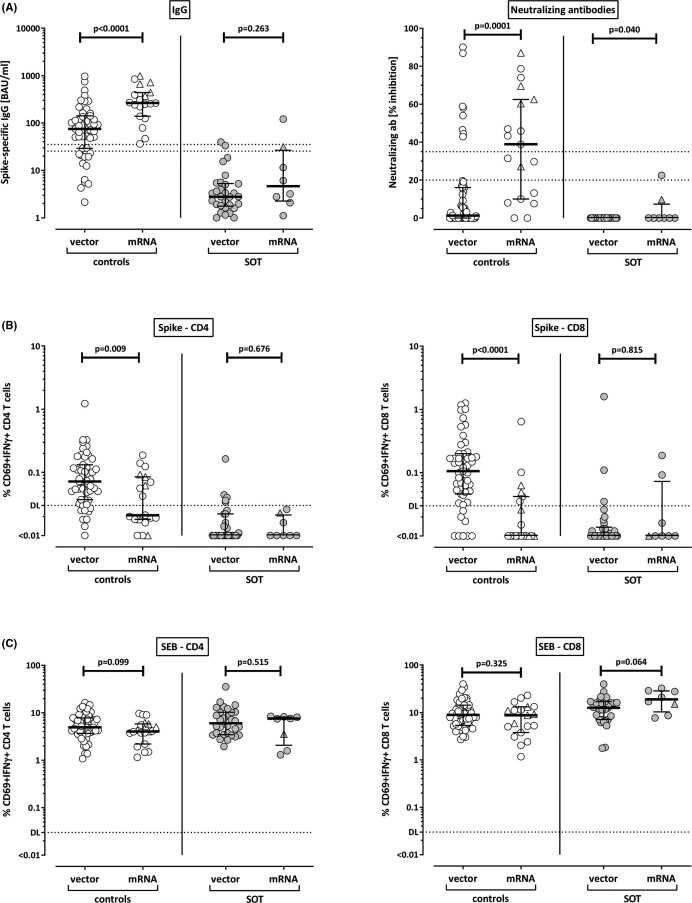
As two types of vaccines were administered in our study, we separately analyzed antibody and T cell responses in controls and patients after priming with either vector or mRNA vaccines. As shown in Figure 2A, induction of SARS-CoV-2–specific IgG and neutralizing antibodies in healthy controls was far more pronounced after immunization with the mRNA vaccines (median IgG 264.1 [IQR 302.2] BAU/ml) as compared to titers after vector-based vaccination (median IgG 75.3 [IQR 112.7] BAU/ml, p < .0001). This contrasts with cellular immunity, where both SARS-CoV-2–specific CD4 and CD8 T cell levels were significantly higher after vector priming (0.072% of CD4 T cells and 0.106% of CD8 T cells) as compared to mRNA priming (0.021% of CD4 T cells, p = .009, and 0.007% of CD8 T cells, p < .0001, Figure 2B). In transplant recipients, this distinction is less discernable, as neutralizing titers of very low inhibitory activity were found exclusively in two patients after mRNA priming, and SARS-CoV-2–specific CD4 T cells only occurred in five patients after vector priming. Apart from this, there were no striking differences between the vaccines in transplant recipients after priming, which may result from a lower general immune reactivity in patients. Likewise, no differences were observed for SEB-reactive T cells (Figure 2C).
FIGURE 2.
SARS-CoV-2–specific antibodies and T cells after priming stratified according to vector and mRNA vaccines. (A) Levels of SARS-CoV-2–specific IgG and neutralizing antibodies, as well as levels of SARS-CoV-2–specific (B) and SEB-reactive (C) CD4 and CD8 T cells after priming with vector or mRNA vaccines were compared among controls (open symbols) or among solid organ transplant recipients (SOT, filled symbols). Individuals who received the mRNA-1273 vaccine are indicated by triangles (5 controls, 1 SOT). Bars represent medians with interquartile ranges. Differences between the groups were calculated using Mann–Whitney test. Dotted lines in (A) indicate limits for IgG and neutralizing antibodies, including negative, intermediate and positive results, respectively as per manufacturer’s instructions. In (B) and (C) dotted lines indicate detection limits (DL) for SARS-CoV-2–specific and SEB-reactive CD4 and CD8 T cells. IFN, interferon
3.4. Heterologous vaccination leads to the strongest induction of antibodies and CD4 T cells in transplant recipients
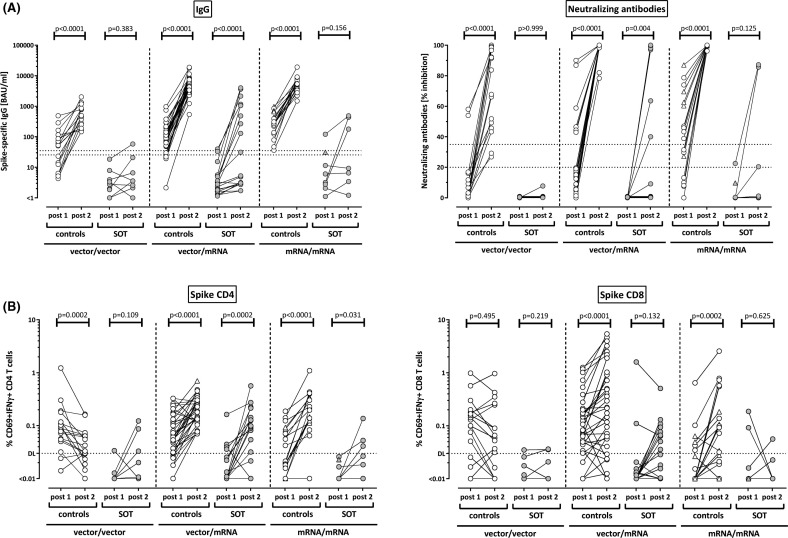
In immunocompetent controls, the heterologous and both homologous regimens led to a significant increase in IgG levels and neutralizing activities ( Figure 3A, p < .0001 for all regimens). Likewise, both CD4 and CD8 T cell levels significantly increased after heterologous and homologous mRNA boosting (Figure 3B). In contrast, CD4 T cell levels after homologous vector boosting were lower than after priming (p = .0002), and CD8 T cell levels remained unaltered (p = .495, Figure 3B). In transplant recipients, the heterologous regimen led to a significant induction in IgG levels (p < .0001) and neutralizing activity (p = .004), which was not significant for the homologous regimens. Likewise, CD4 T cell levels significantly increased after heterologous vaccination (p = .0002), and to a lesser extent after homologous mRNA boosting (p = .03, Figure 3B). Although CD8 T cell levels also showed a numerical increase after heterologous vaccination, this difference did not reach statistical significance (p = .132).
FIGURE 3.
Induction of humoral and cellular immunity after homologous and heterologous vaccine regimens in controls and transplant recipients. (A) Levels of SARS-CoV-2–specific IgG and neutralizing antibodies, as well as (B) levels of SARS-CoV-2–specific CD4 and CD8 T cells after homologous vector vaccination (vector/vector), after heterologous vaccination (vector/mRNA) or after homologous mRNA vaccination (mRNA/mRNA) were compared among controls or among solid organ transplant recipients (SOT). Individuals who received the mRNA-1273 vaccine are indicated by triangles (one control in the heterologous vector/mRNA group, five controls and the transplant recipient in the homologous mRNA/mRNA group, who had evidence of infection after the first vaccination). Differences between priming and boosting were calculated by the Wilcoxon matched pairs test. Dotted lines in (A) indicate limits for IgG and neutralizing antibodies, including negative, intermediate, and positive results, respectively, as per the manufacturer’s instructions. In (B) dotted lines indicate detection limits (DL) for SARS-CoV-2–specific CD4 and CD8 T cells. IFN, interferon
We also analyzed qualitative differences in specific T cell responses between patients and controls based on cytokine profiling of IFNγ, IL-2, and TNFα in individuals with detectable specific immunity. This analysis was restricted to individuals who had at least 30 cytokine-positive CD4 T cells which included 46 controls and 5 patients after first, and 59 controls and 15 patients after the second vaccination. As shown in Figure S2, most cells after vaccination in controls were multifunctional simultaneously producing all three cytokines, followed by cells producing IL-2 and TNFα. In contrast, the percentage of triple-positive CD4 T cells was significantly lower in patients, with a concomitantly higher percentage of cells producing IL-2 or TNFα. CD8 T cells in both controls and patients were dominated by IFNγ and TNFα production and showed only marginal IL-2 production (data not shown).
3.5. The number of plasmablasts correlates with SARS-CoV-2–specific antibody and T cell levels
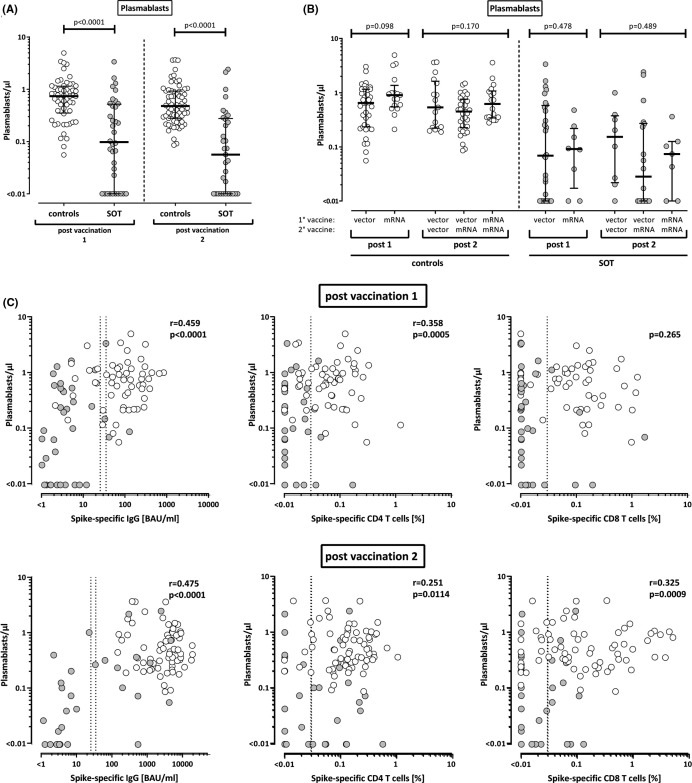
We have previously shown that induction of SARS-CoV-2–specific immunity after natural infection was associated with an expansion of plasmablasts.19 To elucidate whether this is also observed after vaccination, vaccine-induced plasmablasts were quantified from whole blood as CD38 positive cells among IgD-CD27+ CD19 positive switched-memory B cells. As shown from 15 healthy controls analyzed before and after the first vaccination, there was a significant increase from 0.88/µl to 1.29/µl (p = .022, data not shown). In the whole group, controls had significantly higher numbers of vaccine-induced plasmablasts compared to transplant recipients after both the first and the second vaccination (p < .0001, Figure 4A). In line with higher IgG titers in mRNA-vaccinated controls, median numbers of plasmablasts after mRNA priming were slightly higher than after vector priming, although the difference did not reach statistical significance (Figure 4B). After secondary vaccination, the three prime-boost regimens did not show any difference in neither patients nor controls (Figure 4B). However, plasmablast numbers showed a correlation with vaccine-induced IgG titers as well as with SARS-CoV-2–specific CD4 T cells after both the first and the second vaccination (Figure 4C). In contrast, a correlation with specific CD8 T cells was only found after the second vaccination (Figure 4C).
FIGURE 4.
Levels of plasmablasts in healthy controls and transplant recipients after SARS-CoV-2 vaccination and correlation with antibody and T cell responses. Numbers of plasmablasts were compared (A) between healthy controls (n = 56 after first and n = 68 after second vaccination) and solid organ transplant recipients (SOT, n = 36 and n = 33, respectively) and (B) between individuals after immunization with vector-based and/or mRNA vaccines. (C) Plasmablasts were correlated with levels of SARS-CoV-2 spike-specific IgG and CD4 and CD8 T cells. Correlation parameters for controls (white symbols) and transplant recipients (gray symbols) as individual groups are summarized in Table S1. Individuals who received the mRNA-1273 vaccine are indicated by triangles in (B) (1 control in the heterologous vector/mRNA group, 4 controls and 1 transplant recipient in the homologous mRNA/mRNA group). Bars in (A) and (B) represent medians with interquartile ranges. Differences between the groups were calculated using Mann-Whitney test or Kruskal-Wallis test for comparison of two or three groups, respectively. Correlations in (C) were analyzed according to Spearman. Dotted lines indicate detection limits for IgG, indicating negative, intermediate, and positive levels, respectively, as per the manufacturer’s instructions (left panel) or detection limit for SARS-CoV-2–specific CD4 and CD8 T cells (middle and right panels, respectively)
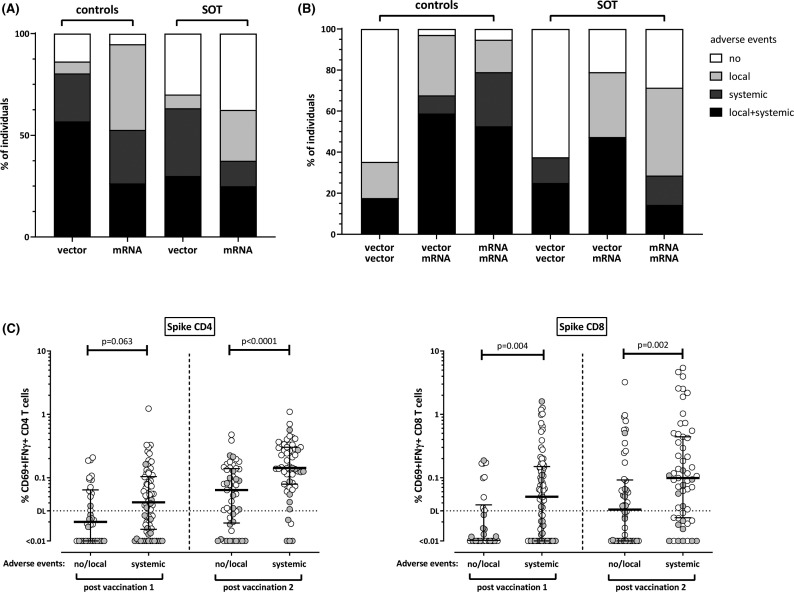
3.6. Transplant recipients reported less adverse events after vaccination than healthy controls
Adverse events in the first week after each vaccination were recorded using a questionnaire. The vaccine was better tolerated among transplant recipients (Table 2); the percentage of transplant recipients with neither local nor systemic symptoms was similar after the first and second vaccination (approximately 32%), whereas this was lower among controls (11% after first and 17% after second vaccination). After first vaccination, the vector vaccine caused more systemic adverse events than the mRNA vaccine, which only reached statistical significance in controls (p = .033, Figure 5A). After secondary vaccination, mRNA-boosted controls had more systemic adverse events as compared to patients, whereas the majority of controls and patients after homologous vector boosting did not report any adverse events (Figure 5B). It is interesting to note, that systemic adverse events were linked to cellular immunity, as individuals with systemic symptoms had higher levels of vaccine-induced SARS-CoV-2–specific CD4 and CD8 T cells as compared to individuals who only had local or no symptoms (Figure 5C).
FIGURE 5.
Increased levels of SARS-CoV-2–specific T cells in individuals with systemic adverse events after vaccination. Controls and solid organ transplant recipients (SOT) were subdivided according to occurrence of adverse events (no, local, systemic, local, and systemic) after immunization with vector-based or mRNA vaccines after (A) first and (B) second vaccination. Local adverse events included pain, swelling, or redness at the injection site. Systemic adverse events included tiredness, fatigue, fever, headache, chills, myalgia, arthralgia, nausea, vomiting, diarrhea, dizziness, and or allergic reactions. Controls after primary vector vaccination reported more systemic adverse events than controls after mRNA priming (p = .033, Fisher´s test). (C) SARS-CoV-2–specific CD4 and CD8 T cell levels were compared between individuals with no or local adverse events and individuals with systemic symptoms. Controls and transplant recipients are denoted with white and grey symbols, respectively. Bars represent medians with interquartile ranges. Differences between the groups were calculated using Mann–Whitney test. Dotted lines indicate detection limits for SARS-CoV-2–specific T cells
4. DISCUSSION
Based on the lack of efficacy trials, knowledge on the adaptive immune response after COVID-19 vaccination in transplant recipients will have the potential to provide guidance for clinical practice. Up to now, data in this patient population are limited to immunity after mRNA vaccination,8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 and no comparative data exist on vaccine-induced immunity in patients after mRNA or vector-based homologous or heterologous vaccination. In this study, we show that SARS-CoV-2–specific antibodies and/or T cells are detectable in 95.7% of immunocompetent controls and 26.3% of transplant recipients after a single dose of mRNA- or vector-based vaccine. Although the majority of controls had specific immunity well above the detection limit already after the first vaccination, antibody titers were higher after mRNA vaccination, whereas the vector vaccine elicited higher levels of T cells. Response rate increased to 100% in controls and 70.6% in transplant recipients after the second vaccination. Although both antibody and T cell levels were significantly lower in transplant recipients, heterologous boosting with mRNA after vector priming was the regimen that led to the most pronounced induction of antibodies and CD4 T cells. While all immunocompetent individuals had detectable antibodies after the second vaccination, combined analysis with cellular immunity was superior in identifying vaccine responders among immunocompromised individuals. Finally, we show that a single vaccine dose in transplant recipients with a history of asymptomatic SARS-CoV-2 infection led to a strong booster effect on T cells and antibodies with neutralizing function, which was similar or even higher in magnitude as primary induction of vaccine immunity in healthy controls.
Initial reports on humoral immunity after the second dose of an mRNA vaccine have shown that antibody titers were still lower as in controls, but were observed in 38%–59% of transplant recipients.11, 12, 13, 14 , 16 While this clearly exceeds response rates after the first dose, it is lower than in studies evaluating cellular and humoral immunity. In line with a 70% response in our study, recent studies on patients after mRNA-1273 vaccine reported combined response rates of 65% in kidney transplant recipients18 and of 87% and 93% in liver and heart transplant recipients,17 respectively. One study even reported T cell response rates of more than >90% after BNT162b2 vaccination.20 Underestimation based on humoral immunity may be particularly pronounced in individuals after vector priming, where antibody titers in healthy controls were lower than after priming with mRNA. Conversely, as vector priming inducted higher levels of T cells, cellular immunity may be superior in identifying responders. This is further illustrated by the fact that all transplant recipients with detectable CD4 T cells after the first vaccination had been immunized with the ChAdOx1 nCoV-19 vaccine. Interestingly, vector priming was also associated with more systemic adverse events, which was more frequent in individuals with strong T cell responses. Thus, a vector-induced inflammatory response may favor an efficient priming and expansion of T cells. On the B cell/antibody axis, we observed differences in the number of circulating plasmablasts between patients and controls, which directly correlated with spike-specific IgG and T cells. Among controls, plasmablasts after first vaccination were numerically higher after mRNA vaccination which may directly be linked to higher titers of IgG and neutralizing antibodies.
The observed differences in humoral and cellular immunity after priming may affect vaccine-induced immunity after boosting with homologous and heterologous vaccines. In line with our recent observations,4 this is illustrated by the fact that the strongest induction of antibodies and T cells in immunocompetent controls was observed for the heterologous vector-mRNA- and the homologous mRNA regimens, whereas the magnitude in immune responses was significantly lower in individuals after homologous vector vaccination. In our study, the majority of transplant recipients received the heterologous regimen. Although we did not observe any significant differences in the overall percentage of responders between homologous and heterologous regimens among both controls and transplant recipients, the heterologous regimen led to the strongest increase in both antibodies and CD4 T cells. Given the promising results in immunocompetent controls,4 future studies with larger sample size are necessary to confirm whether heterologous vaccine regimens may lead to a more comprehensive booster response exceeding that of a series of identical vaccines. As humoral immunity in transplant recipients have been shown to benefit from a third booster dose,21, 22, 23 heterologous combinations may also hold promise as additional options to boost immunity after non-response from homologous regimens.
Safety and immunogenicity trials of the licensed vaccines have reported more frequent systemic adverse events in individuals immunized with the vector vaccine compared to mRNA vaccines, where adverse events predominated after the second vaccination.24 , 25 Similar observations were made in transplant recipients, although adverse events were generally less frequent than in controls. In line with recent observations in transplant recipients after the first and second dose of the mRNA vaccine,26 , 27 patients after mRNA vaccination only had mild to moderate symptoms that were largely restricted to local pain at the injection site. Knowledge on adverse events was now extended to transplant recipients after homologous vector and heterologous vector-mRNA regimens, who reported more systemic symptoms after vector priming as compared to mRNA priming. Systemic adverse events were less frequent after secondary vaccination irrespective of vector or mRNA-boost. In contrast, local adverse events were more frequently reported after heterologous boosting with mRNA. Together this shows that all three regimens are well tolerated in transplant recipients.
Our study is novel in that patients on both vector- and mRNA regimens were prospectively included which allowed direct comparison of primary immunogenicity in vaccine naïve individuals independent from variabilities resulting from vaccine-specific differences in the time interval between the first and the second dose. As a further strength of our study, we comprehensively assessed both the humoral immune response with IgG and neutralization capacity, and the cellular immune response that included both CD4 and CD8 T cells and their cytokine profiles. The inclusion of an age-matched control group is considered as an additional asset to provide comparative data on immunogenicity of immunocompetent individuals in a real-world setting. Our definition of responders may be limited by the fact that our threshold for defining a positive T cell response was based on natural variability of T cell immunity after control stimulation as previously established.4 In the absence of effectiveness data it remains to be determined for both antibodies and T cells, whether the chosen thresholds represent clinically relevant indicators for protection. Our study is further limited by convenience sampling, where assignment of the vaccine regimen was determined by national guidelines, that changed for vector-primed individuals after initiation of our study due to an increased general incidence of cerebral venous thrombosis28 , 29; hence, the study participants were divided into smaller subgroups than initially planned. While we acknowledge the small sample size as a limitation, this offered the possibility to provide first data on the immunogenicity of the heterologous vector-mRNA regimen in transplant recipients.
In conclusion, we show that serology considerably underestimates the immunogenicity of COVID-19 vaccines in both immunocompetent controls and transplant recipients, with an improved ability to identify vaccine responders after combined analysis of humoral and cellular immunity. Despite this advantage, the proportion of responders and the level of immunity among transplant recipients is significantly lower than among controls, which may result in a higher risk of post-vaccination infection.30 Nevertheless, despite the occurrence of breakthrough infections, there is the first evidence of vaccine-mediated protection from severe disease.31, 32, 33 This is exemplified by registry data among transplant recipients that indicate a reduction in infection-related mortality from 12% in unvaccinated to 7.7% in recipients of two vaccinations.32 Until further data on effectiveness and immunological correlates of protection are defined, patients should be advocated for continued adherence to hand hygiene, physical distancing, and use of facemasks.34
ACKNOWLEDGMENTS
The authors thank Candida Guckelmus and Rebecca Urschel for excellent technical assistance, and Susanne Brehmer, Inna Vallar, Fabio Lizzi and the team of the Saarland University Transplant center for their valuable support. The authors also thank all participants to this study who contributed to the gain in knowledge from this project. Financial support was given by the State Chancellery of the Saarland.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. H.W. has received fees for lectures and/or consultations from Actelion, Boehringer, Bayer, Biotest, GlaxeSmithKline, Janssen, MSD, Pfizer, and Roche. M.S. has received grant support from Astellas and Biotest to the organization Saarland University outside the submitted work, and honoraria for lectures from Biotest and Novartis. The other authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
D.S., T.S., U.S., S.S., and M.S. designed the study. D.S., T.S., U.S., and M.S. designed the experiments. V.K. and T.S. performed experiments. S.S., H.W., M.C.R., J.M., and U.S. contributed to study design, patient recruitment, and clinical data acquisition. D.S., V.K., T.S., U.S., J.M., and M.S. supervised all parts of the study, performed analyses, and wrote the manuscript. All authors approved the final version of the manuscript.
DATA AVAILABILITY STATEMENT
All figures have associated raw data. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding information State Chancellery of the Saarland, Grant/Award Number: Saarland Vaccine Project
Footnotes
Verena Klemis and David Schub contributed equally.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
Supplementary Material
REFERENCES
- 1.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt T, Klemis V, Schub D, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021; 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed]
- 5.Danziger-Isakov L, Blumberg EA, Manuel O, Sester M. Impact of COVID-19 in solid organ transplant recipients. Am J Transplant. 2021;21(3):925–937. doi: 10.1111/ajt.16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vygen-Bonnet S, Koch J, Bogdan C, et al. Beschluss der STIKO zur 3. Aktualisierung der COVID-19-Impfempfehlung und die dazugehörige wissenschaftliche Begründung. Epid Bull. 2021;12:13–25. [Google Scholar]
- 7.Vygen-Bonnet S, Koch J, Bogdan C, et al. Beschluss der STIKO zur 5. Aktualisierung der COVID-19-Impfempfehlung und die dazugehörige wissenschaftliche Begründung. Epid Bull. 2021;19:24–36. [Google Scholar]
- 8.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benotmane I, Gautier -Vargas G, Cognard N, et al. Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021;99(6):1487–1489. doi: 10.1016/j.kint.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi S, Knight RJ, Graviss EA, et al. Kidney transplant recipients rarely show an early antibody response following the first COVID-19 vaccine administration. Transplantation. 2021;105(7):e72–e73. doi: 10.1097/TP.0000000000003764. [DOI] [PubMed] [Google Scholar]
- 11.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-Cov-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21(8):2719–2726. doi: 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marinaki S, Adamopoulos S, Degiannis D, et al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21(8):2913–2915. doi: 10.1111/ajt.16607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peled Y, Ram E, Lavee J, et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40(8):759–762. doi: 10.1016/j.healun.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korth J, Jahn M, Dorsch O, et al. Impaired humoral response in renal transplant recipients to SARS-CoV-2 vaccination with BNT162b2 (Pfizer-BioNTech) Viruses. 2021;13(5) doi: 10.3390/v13050756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrera S, Colmenero J, Pascal M, et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am J Transplant. 2021; 10.1111/ajt.16768. [DOI] [PMC free article] [PubMed]
- 18.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21(8):2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schub D, Klemis V, Schneitler S, et al. High levels of SARS-CoV-2-specific T cells with restricted functionality in severe courses of COVID-19. JCI Insight. 2020;5(20) doi: 10.1172/jci.insight.142167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131(14) doi: 10.1172/JCI150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021; 10.1001/jama.2021.12339. [DOI] [PMC free article] [PubMed]
- 23.Del Bello A, Abravanel F, Marion O, et al. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am J Transplant. 2021; 10.1111/ajt.16775. [DOI] [PMC free article] [PubMed]
- 24.Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyarsky BJ, Ou MT, Greenberg RS, et al. Safety of the first dose of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021;105(5):e56–e57. doi: 10.1097/TP.0000000000003654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ou MT, Boyarsky BJ, Motter JD, et al. Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021;105(9):2119–2123. doi: 10.1097/TP.0000000000003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schultz NH, Sorvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wadei HM, Gonwa TA, Leoni JC, Shah SZ, Aslam N, Speicher LL. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am J Transplant. 2021; 10.1111/ajt.16618. [DOI] [PMC free article] [PubMed]
- 31.Qin CX, Moore LW, Anjan S, et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation. 2021; 10.1097/TP.0000000000003907. [DOI] [PMC free article] [PubMed]
- 32.Ravanan R, Mumford L, Ushiro-Lumb I, et al. Two doses of SARS-CoV-2 vaccines reduce risk of death due to COVID-19 in solid organ transplant recipients: preliminary outcomes from a UK registry linkage analysis. Transplantation. 2021; 10.1097/TP.0000000000003908. [DOI] [PMC free article] [PubMed]
- 33.Tsapepas D, Husain SA, King KL, Burgos Y, Cohen DJ, Mohan S. Perspectives on COVID-19 vaccination among kidney and pancreas transplant recipients living in New York City. Am J Health Syst Pharm. 2021; 10.1093/ajhp/zxab272. [DOI] [PMC free article] [PubMed]
- 34.Blumberg EA, Manuel O, Sester M, Ison MG. The future of SARS-CoV-2 vaccines in transplant recipients: to be determined. Am J Transplant. 2021;21(8):2629–2630. doi: 10.1111/ajt.16598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
All figures have associated raw data. The data that support the findings of this study are available from the corresponding author upon reasonable request.