Abstract
Introduction
In early 2020, many services modified their delivery of opioid treatment in response to the COVID‐19 pandemic, to limit viral spread and maintain treatment continuity. We describe the changes to treatment and preliminary analysis of the association with patients' substance use and well‐being.
Methods
A pre‐post comparison of treatment conditions and patient self‐reported outcomes using data extracted from electronic medical records in the 5 months before (December 2019–April 2020) and after (May 2020–September 2020) changes were implemented in three public treatment services in South Eastern Sydney Local Health District.
Results
Data are available for 429/460 (93%) patients. Few (21, 5%) dropped out of treatment. In the ‘post’ period there was significantly more use of depot buprenorphine (12–24%), access to any take‐away doses (TAD; 24–69%), access to ≥6 TAD per week (7–31%), pharmacy dosing (24–52%) and telehealth services. There were significant reductions in average opioid and benzodiazepine use, increases in cannabis use, with limited group changes in social conditions, or quality of life, psychological and physical health. At an individual level, 22% of patients reported increases in their use of either alcohol, opioids, benzodiazepines or stimulants of ≥4 days in the past 4 weeks. Regression analysis indicates increases in substance use were associated with higher levels of supervised dosing.
Discussion and Conclusions
These preliminary findings suggest that the modified model of care continued to provide safe and effective treatment, during the pandemic. Notably, there was no association between more TAD and significant increases in substance use. Limitations are discussed and further evaluation is needed.
Keywords: opiate substitution treatment, outcome assessment, health care, organisational innovation, clinical protocol
Introduction
Opioid agonist treatment (OAT) for opioid dependence combines medication, medical and psychosocial interventions, with demonstrated reductions in mortality, illicit opioid use, blood‐borne viral infections and criminal behaviour [1, 2]. There were over 50 000 individuals in OAT treatment across Australia in 2019, with the largest number (21 400) in New South Wales (NSW), treated with either methadone (66%) or buprenorphine (34%), prescribed by public (state government) programs (34%), private prescribers (57%) and correctional facilities (9%); and dosed at community pharmacies (59%), specialist clinics (31%) or correctional facilities (9%) [3].
Safety concerns over non‐medical use of OAT medications (overdose, injecting, diversion) has resulted in a historical model of care for OAT across Australia predicated on daily supervised dosing of medications, with the availability of non‐supervised or take‐away doses (TAD) based on a risk assessment framework [4]. In NSW, patients considered to be at low risk of misuse are able to access up to four methadone TADs per week; with more generous TAD allowances for sublingual buprenorphine‐naloxone—with up to 1 month TADs allowed, although historically, few patients received monthly TADs and most attend several times a week for dosing [5]. Two subcutaneous depot buprenorphine formulations—obviating the role of supervised dosing or TADs—were introduced [6]: Buvidal® (weekly [7] or monthly [8]) in October 2019 and Sublocade® (monthly [9]) in May 2020.
The emergence of COVID‐19 in early 2020 generated a number of concerns for OAT patients and service providers [10, 11, 12], including:
The need for effective social distancing and social isolation to protect patients, health workers and their social contacts.
The increased vulnerability of an ageing patient population to COVID‐related morbidity and mortality.
Potential for increased demand for opioid treatment arising through reductions in access to other alcohol and other drugs treatment services during the pandemic (e.g. hospital admissions, residential rehabilitation programs, self‐help groups), increased release of patients into the community from prison and disruption of illicit drug distribution networks.
A number of features of the traditional Australian OAT model of care, such as daily supervised dosing, were considered incompatible with the principles of social distancing and isolation required during the COVID‐19 pandemic. In response, national guidance [10] was developed by peak professional and consumer groups, recommending the following adaptations:
Less supervised dosing and increased provision of TADs.
Increased use of depot buprenorphine.
Increased provision of take‐home naloxone (THN) to patients and carers to mitigate overdose risks associated with increased TADs.
Reduced ‘face to face’ patient contact—with greater use of telehealth services (telephone or audio‐visual modalities) and reduced patient monitoring activities (e.g. urine drug screens).
Reduced congregation of patients—with transfer of dosing from large clinics to community pharmacy settings.
Strategies to ensure continuity of treatment for patients in quarantine (e.g. home delivery of medication, engagement of carers in supervising TADs).
Several state health departments issued statements enabling local service providers to incorporate these changes [10], although changes were not mandated, and service providers retained autonomy over their clinical practice. It remains unclear as to the extent to which these changes were actually implemented in OAT services, and importantly whether changes were associated with positive or (inadvertent) negative consequences, such as prevention of COVID‐19 spread amongst patients and providers; changes in patient outcomes (e.g. substance use, health and social issues such as violence); adverse outcomes from non‐medical use of TADs (e.g. diversion to others, injecting TADs, overdose); or changes in treatment retention.
Given that the changes to OAT may contribute to increased mortality, morbidity and social harms, it is incumbent upon services to evaluate the changes, which in turn should inform future OAT models of care. The aims of this paper are to:
Describe changes to how OAT services were delivered in response to COVID‐19 across three public OAT services in south east Sydney, NSW—including changes in medications, dosing sites, provision of TADs and THN, and use of telehealth.
Examine changes in patient outcomes (including substance use, general health and quality of life, housing, experience of violence, treatment retention) in the 6 months following the implementation of OAT changes in response to COVID‐19.
Examine whether particular changes in treatment (e.g. TADs, community dosing) were associated with adverse patient outcomes.
The relationship between treatment changes and client outcomes cannot be interpreted as causal—this was a service response to a public health crisis therefore there was no control group and there were co‐occurring socio‐economic changes described further below—however, given the ongoing COVID‐19 pandemic and associated service changes it is important to analyse and report on the available data with an understanding of the potential confounding factors.
A brief timeline of COVID‐19 related events in NSW is necessary to understand the context of this evaluation. The first cases of COVID‐19 infection were recorded in NSW on 25 January 2020, and on 20 March the first large cluster of cases in Sydney emerged. New case numbers increased (between 100 and 200 cases daily) during late March and early April, with major reductions by May, although low numbers (e.g. 2–10 daily cases) of community‐acquired infections persisted throughout 2020.
Social restrictions were incrementally introduced during March 2020. Bans on major gatherings (>500 people) and non‐essential indoor gatherings (>100 people) were introduced on 18 March. Non‐essential activities and businesses were closed during the last week of March and stay at home orders were introduced. In late May social restrictions started to ease, with schools reopening and limited household gatherings allowed, and non‐essential businesses opening (with some restrictions) in early July [13].
Methods
The study employed a pre‐post cohort design of patients in South Eastern Sydney Local Health District (SESLHD), examining treatment conditions and patient outcomes in the 5 months before and after the introduction of COVID‐19 related OAT changes (April 2020). Routinely collected clinical information was extracted from the electronic medical record system used in NSW Health facilities.
Services
SESLHD Drug and Alcohol (D&A) Services operates state‐funded specialist opioid treatment services across three metropolitan sites for approximately 500–550 patients at any time. Services are provided by multidisciplinary teams, and informed by state regulations and guidelines [5], with no expenses for patients—with the exception of dispensing fees for patients dosed at community pharmacies (approximately $40 per week). Rapid changes to SESLHD services in response to COVID‐19 were developed and endorsed through local governance processes in March 2020, with implementation in early April. The key changes to service models, including a framework for determining TAD conditions, were broadly consistent with national recommendations [10] (see Tables S1 and S2, Supporting Information). The framework was rapidly implemented in services through written and verbal communication to inform staff and patients about the changes, clinical and business meetings to support OAT staff to carry out the changes to treatment, liaison with community pharmacies, peer worker provision of advice and support, and clinical leadership.
Study team and participants
The study team included clinicians, consumer workers and researchers from SESLHD D&A Services and the University of Sydney, who collectively identified the key evaluation questions, data sources and undertook analysis and write‐up. The study design was pragmatic—in that COVID‐related service changes occurred very rapidly, without time to introduce bespoke study procedures or data collection sources. The use of routinely collected data enabled the capture of changes in treatment conditions and patient outcomes without delays. Participants were patients enrolled in OAT at SESLHD services. Use of non‐identifiable clinical data for this quality improvement project was approved by the SESLHD Human Research Ethics Committee without the need for additional patient consent.
Evaluation period, eligibility, data sources and measures
The pre‐COVID period captured data from 1 December 2019 to 9 April 2020; with follow‐up from 1 May to 11 September 2020. Participants included all patients who were enrolled in OAT in SESLHD in the pre‐COVID study period, were still a patient on 1 May 2020 and had a pre‐COVID Australian Treatment Outcome Profile (ATOP) [14, 15] in the study period.
Specific data sources accessed within the electronic medical record system [16] are detailed in Table S3 (Supporting Information). Data included patient demographics (age, sex, Indigenous status), reason for treatment cessation (for those discharged during the follow‐up period), the number and modality (face‐to‐face, telehealth) of occasions of service for each patient per month over the study period. Although time in treatment with SESLHD D&A services was available, it should be noted that total time in OAT can be much longer as many clients entering SESLHD D&A transfer in from other programs (e.g. prison release); therefore it was not included in statistical analyses as it is not an accurate representation of total time in OAT.
The ATOP is a 21‐item instrument validated for face‐to‐face [17] and telephone administration [18], assessing patient‐reported substance use (days used), social conditions (housing, violence, vocation) and psychological health, physical health and quality of life (each measured on a 0–10 scale, 0 = poor, 10 = good) in the preceding 4‐week period. SESLHD OAT services aim to routinely complete ATOPs every 2 to 3 months. One pre‐COVID and one follow‐up ATOP was extracted for each patient. Where more than one ATOP was available, the ATOP completed closest to 9 April 2020 was selected.
The Opioid Substitution Treatment (OST) module summarises patients' OAT conditions: medication (methadone, sublingual buprenorphine, depot buprenorphine), dosing location (public clinic, community pharmacy) and level of TAD access (No TAD, 1–5 per week; 6+ per week). OST modules were selected that were completed on the same or nearest date to the ATOP for each patient.
Statistical analysis
Extracted data were analysed in SPSS 24.0 [19]. Descriptive analyses of participant demographics, OAT conditions, occasions of service and THN provision were described using frequencies and proportions. Changes in categorical ATOP variables (e.g. homelessness, any alcohol use) were assessed using McNemar's tests, and mean changes in ATOP health ratings were assessed using paired t‐tests. Median change in days of substance use variables was assessed using paired Wilcoxon signed‐rank tests. As group changes in mean substance use or health status over time do not always reflect changes in individual patients, we developed a threshold for statistically reliable change for ATOP scores based on the Jacobson and Truax's Reliable Change Index method [20] using standard deviations of the study sample pre‐COVID‐19 and the test–retest reliability statistics previously reported [15], enabling us to identify the proportion of patients who made statistically reliable increases in their substance use—calculated to be ≥4 days out of 28 at a 95% significance level, and the proportion of patients deteriorating on psychological, physical health and quality of life—calculated as a decrease of ≥2 points at the 80% significance level.
A composite variable, the ‘clinically relevant increase in substance use’ was calculated as an increase in the use by ≥4 days (in the past 28) of any of the four substance classes—alcohol, benzodiazepines, opioids and stimulants, considered particularly important in patient safety and determining treatment conditions (e.g. access to TADs) [5]. Inferential analysis was used to identify factors associated with an increase. Single‐observation outcomes were tested using logistic regression. A multinomial logistic regression model was developed using demographic variables and treatment conditions (medication type, dosing site, TAD conditions) to assess which characteristics of OAT patients were significantly associated with clinical relevant increases in substance use. As depot buprenorphine has no TADs and was only administered at clinics, depot buprenorphine participants were excluded from some analyses.
Results
Participants and participant flow
There were 707 SESLHD OAT patients at some point between 1 December 2019 and 9 April 2020 (see Figure 1). Patients who were discharged before the follow‐up period (n = 247) were excluded from further analysis. Of the 460 patients in treatment in April 2020, data were available for 429 (93.2%), with 31 patients excluded (no ATOP available from pre‐COVID period). Most (n = 366, 85.3%) had complete follow‐up data available (ATOP and OST module), 49 (11.4%) patients had an OST module but no follow‐up ATOP completed and 14 (3.2%) had no OST module or ATOP completed in the follow‐up period. Seventy‐four (17.2%) patients were discharged from the services during the follow‐up period, albeit 60 of these had OST module and/or ATOP data available and are included in analyses.
Figure 1.
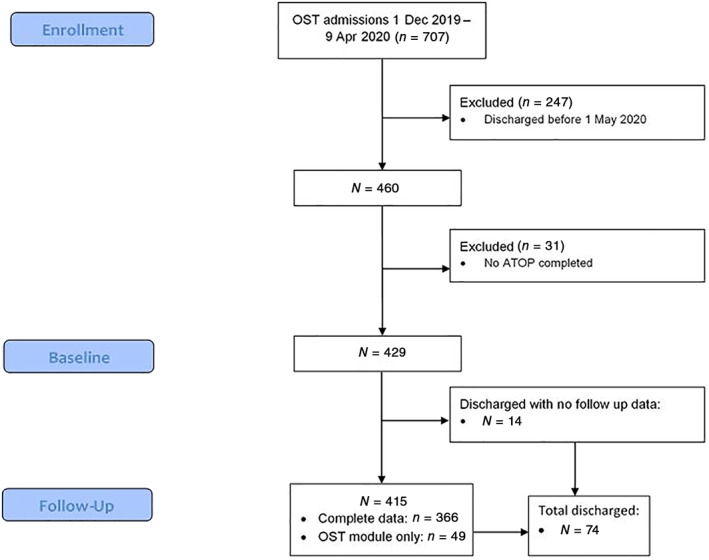
CONSORT patient flow diagram for the evaluation of the impact of COVID‐19 on patients attending opioid agonist treatment services in South‐Eastern Sydney. ATOP, Australian Treatment Outcome Profile; OST, opioid substitution treatment.
Demographics and treatment characteristics for the 429 participants, including those retained (n = 355) and those discharged (n = 74), are shown in Table 1. The proportion of patients with a shorter than 6 months duration of enrolment in the SESLHD D&A service prior to 9 April 2020 was 28% (121/429). However, given n = 46 were referred from Justice Health OAT, at least 38% of the 121 were not treatment‐naïve. Discharged patients were significantly younger (mean 39 vs. 44 years, one‐way analysis of variance F (1,427) = 17.02, P < 0.001) and more likely to be treated with sublingual buprenorphine than methadone or depot buprenorphine (Fisher's z (2) = 11.74, P = 0.003). Of the 74 patients discharged from the treatment services in the follow‐up period, 18 (24%) were imprisoned, 16 (22%) transferred to another OAT service, 17 (23%) withdrew from opioid treatment with staff consent as part of their treatment plan and 21 (28%) discharged ‘against medical advice’ or left without notice. Two patients (3%) died, with review at the local morbidity and mortality committee finding neither death was related to their OAT or substance use.
Table 1.
Pre‐COVID patient demographics, opioid substitution treatment (OST) medication type and take‐away dose conditions for patients in the evaluation of treatment characteristics and patient outcomes before and after the introduction of COVID‐19 related opioid agonist treatment changes in South‐Eastern Sydney
| All (n = 429) | Remained in treatment (n = 355) | Discharged during follow up (n = 74) | |
|---|---|---|---|
| Age, mean (SD) in years | 43 (10) | 44 (10) | 39 (10) |
| Sex, female | 33% | 35% | 24% |
| Indigenous | 18% | 19% | 14% |
| OST type pre‐COVID | |||
| Methadone | 245 (57%) | 210 (59%) | 35 (47%) |
| Sublingual buprenorphine | 133 (31%) | 98 (28%) | 35 (47%) |
| Depot buprenorphine | 51 (12%) | 47 (13%) | 4 (5%) |
| Takeaway dose category pre‐COVID (excluding depot buprenorphine patients) | |||
| None | 292 (77%) | 231 (75%) | 61 (87%) |
| 1–5 per week | 60 (16%) | 52 (17%) | 8 (11%) |
| 6+ per week | 26 (7%) | 25 (8%) | 1 (1%) |
| Dosing location pre‐COVID | |||
| Public clinic | 285 (66%) | 223 (63%) | 62 (84%) |
| Community pharmacy | 93 (22%) | 85 (24%) | 8 (11%) |
| Depot buprenorphine administered at public clinic | 51 (12%) | 47 (13%) | 4 (5%) |
To our knowledge, no staff and only one patient tested positive for COVID‐19 during the follow‐up period with no loss of continuity of their treatment.
Changes in OAT services
Table 2 and Table S4 (Supporting Information) describe the changes in medication and dosing conditions between pre‐COVID and at follow up. Key changes observed were:
An increase in patients treated with depot buprenorphine [from 12% (51/429) to 24% (101/415)], a reduction in sublingual buprenorphine use [from 31% (133/429) to 21% (89/415)] and similar proportion treated with methadone [57% (245/429) to 54% (225/415); P < 0.001, χ 2(2) = 25.79].
An increase in patients dosed at community pharmacies—from 22% (93/429) to 37% (154/415) for all patients (P < 0.001; χ 2(1) = 24.26) and from 25% (93/378) to 49% (154/314; P < 0.001, χ 2(1) = 44.64) when excluding depot buprenorphine.
An increase in patients accessing any TADs: from 20% (86/429) to 51% (210/415; P < 0.001, χ 2(1) = 86.49) for all patients and from 23% (86/378) to 67% (210/314; P < 0.001, χ 2(1) = 136.45) when excluding depot buprenorphine.
An increase in patients receiving 6+ TADs: from 6% (26/429) to 23% (95/415; P < 0.001, χ 2(1) = 48.66) for all patients and from 7% (26/378) to 30% (95/314; P < 0.001, χ 2(1) = 64.96) when excluding depot buprenorphine.
The mean number of direct staff‐patient contacts per month in the 3 months before COVID‐19 (December–January–February 2020) was 2.42, of which 0.33 (14%) were conducted by telehealth, 2.48 direct contacts in the first 3 months (April–May–June 2020) following the changes, of which 0.44 were by telehealth (18%) and 1.83 direct contacts per month in the subsequent 3‐month period (July–August–September 2020), of which 0.44 (24%) were by telehealth. The overwhelming majority of telehealth services were provided by telephone (directly with the client) rather than audio‐visual platforms, reflecting the logistic delays in establishing audio‐visual services for all staff and limited access for most clients to audio‐visual platforms. In most cases, telephone‐based consultations were adequate for communicating with clients (e.g. regarding service changes) and conducting routine clinical reviews, although telephone communication was unsatisfactory for some vulnerable clients (e.g. domestic violence concerns).
Table 2.
Opioid substitution treatment (OST) medication, take‐away dose (TAD) and dosing site locations for 429 patients pre‐COVID, and at follow up for the n = 415 patients with data available
| Pre‐COVID (429) | Follow up (n = 415) | |||
|---|---|---|---|---|
| Public clinic dosing | Community dosing | Public clinic dosing | Community dosing | |
| Methadone | ||||
| No TADs | 188 (44%) | 7 (2%) | 79 (19%) | 8 (2%) |
| 1–5 TADs | 1 (0%) | 49 (11%) | 30 (7%) | 69 (17%) |
| 6+ TADs | — | — | 13 (3%) | 26 (6%) |
| Sublingual buprenorphine | ||||
| No TADs | 94 (22%) | 3 (1%) | 16 (4%) | 1 (<1%) |
| 1–5 TADs | 2 (0%) | 8 (2%) | 8 (2%) | 8 (2%) |
| 6+ TADs | — | 26 (6%) | 14 (3%) | 42 (10%) |
| Depot buprenorphine | 51 (12%) | — | 101 (24%) | — |
| Total | 336 (78%) | 93 (22%) | 261 (63%) | 154 (37%) |
Percentages are calculated using total pre‐COVID or follow up sample size as the denominator.
THN intervention occurred in 178 patients (41%) between December 2019 and September 2020. THN was more often provided to methadone (n = 116/225, 52%) and sublingual buprenorphine patients (38/89, 43%) than those treated with depot buprenorphine (20/101, 20%; χ 2 = 28.89, P < 0.001). THN was also more common in patients accessing TADs during follow‐up (111/210, 53%) than those not accessing TADs (63/205, 31%; χ 2 = 20.86, P < 0.001), but no significant differences by age, sex or Indigenous status.
Patient outcomes
ATOP scores pre‐COVID and at follow‐up are shown in Table 3. The proportion of patients reporting any alcohol, amphetamines, cocaine use and mean days used in the past 28 days (for those reporting any use) remained stable. Significantly fewer patients reported using opioids (McNemar's test, χ 2 = 4.563, P = 0.033) or benzodiazepines (McNemar's test, χ 2 = 6.017, P = 0.014). Median days use of opioids among patients using pre‐COVID or at follow‐up decreased from 4 to 2 days (n = 132, Wilcoxon signed‐rank test, Z = −3.445, P = 0.001). Although the proportion reporting any injecting drug use remained stable, the median number of days injected reduced from 5 to 4 days (n = 124, Wilcoxon signed‐rank test, Z = −2.577, P = 0.010). Cannabis was the only substance reported in which the proportion of patients with past‐month use increased from 33% to 38% (McNemar's test, χ 2 = 4.817, P = 0.028). The median frequency of use among those using cannabis also increased, from 12 to 16 days (n = 156, Wilcoxon signed‐rank test, Z = −2.331, P = 0.020).
Table 3.
Changes in substance use, social conditions and health ratings (ATOP) for the n = 366 patients with ATOPs available both pre‐COVID and during follow up
| ATOP item | Pre‐COVID | Follow up | Paired tests a |
|---|---|---|---|
| Alcohol | |||
| Used, n/N (%) | 100/363 (27%) | 88/359 (24%) | P = 0.215 |
| Days used, b mean (SD); median | 8.6 (9.3); 4 | 10.1 (9.7); 7.5 | — |
| N (%) increased use by ≥4 days c | — | 30 (8%) | — |
| Cannabis | |||
| Used, n/N (%) | 120/356 (33%) | 139/358 (38%) | P = 0.028 |
| Days used, b mean (SD); median | 18.1 (10.8); 21 | 18.0 (11.0); 26 | — |
| N (%) increased use by ≥4 days | — | 58 (17%) | — |
| Benzodiazepines | |||
| Used, n/N (%) | 102/358 (28%) | 80/355 (22%) | P = 0.014 |
| Days used, b mean (SD); median | 14.6 (11.7); 12 | 16.9 (11.4); 20 | — |
| N (%) increased use by ≥4 days | — | 23 (7%) | — |
| Stimulants | |||
| Used, n/N (%) | 73/349 (20%) | 60/350 (16%) | P = 0.120 |
| Days used, b mean (SD); median | 6.5 (8.2); 3 | 5.9 (7.4); 3 | — |
| N (%) increased use by ≥4 days | — | 12 (4%) | — |
| Opioids | |||
| Used, n/N (%) | 110/360 (30%) | 87/346 (24%) | P = 0.033 |
| Days used, b mean (SD); median | 12.2 (10.7); 8 | 7.9 (9.1); 4 | — |
| N (%) increased use by ≥4 days | — | 21 (6%) | — |
| Injecting | |||
| Used, n/N (%) | 105/346 (29%) | 82/339 (22%) | P = 0.077 |
| Days used, b mean (SD); median | 10.7 (10.5); 5 | 8.1 (8.9); 4 | — |
| Employment | |||
| Any days, n/N (%) | 50/357 (14%) | 49/338 (13%) | P = 1.000 |
| Days, b mean (SD); median | 15.3 (7.7); 17 | 16.0 (6.7); 20 | — |
| Study/training | |||
| Any days, n/N (%) | 6/356 (2%) | 10/335 (3%) | P = 0.549 |
| Days, b mean (SD); median | 12.0 (6.8);10.5 | 16.8 (7.7); 18 | — |
| Homeless, n/N (%) | 44/366 (12%) | 18/366 (5%) | P < 0.001 |
| At risk of eviction, n/N (%) | 17/366 (5%) | 14/366 (4%) | P = 0.678 |
| Any housing risk, n/N (%) | 54/366 (14%) | 24/366 (7%) | P < 0.001 |
| Caring for children | |||
| <5 years, n/N (%) | 24/366 (7%) | 21/366 (6%) | P = 0.629 |
| 5–15 years, n/N (%) | 26/366 (7%) | 20/366 (6%) | P = 0.286 |
| Arrests, n/N (%) | 12/366 (3%) | 13/366 (4%) | P = 1.000 |
| Violence to you, n/N (%) | 17/366 (5%) | 9/366 (3%) | P = 0.152 |
| Violence to others, n/N (%) | 3/366 (1%) | 5/366 (2%) | P = 0.688 |
| Psychological health | |||
| N, mean (SD); median | 328, 6.3 (1.8); 7 | 312, 6.5 (1.6); 7 | P = 0.181 |
| n (%) deteriorated by ≥2 points d | — | 42 (12%) | — |
| Physical health | |||
| N, mean (SD); median | 328, 6.6 (1.8); 7 | 311, 6.5 (1.6); 7 | P = 0.229 |
| n (%) deteriorated by ≥2 points | — | 55 (15%) | — |
| Quality of life | |||
| N, mean (SD); median | 324, 6.7 (1.8); 7 | 308, 6.8 (1.6); 7 | P = 0.157 |
| n (%) deteriorated by ≥2 points | — | 38 (14%) | — |
Tests: McNemar's test for categorical variables; paired t‐tests for psychological health, physical health and quality of life.
For patients who reported any use. cMinimum statistically reliable change at 95% significance level, calculated using Jacobson and Truax [20]. dMinimum statistically reliable change at the 80% significance level, calculated using Jacobson and Truax [20]. Most variables had some missing data; data points available are indicated by the denominator N. ATOP, Australian Treatment Outcome Profile.
Homelessness reduced significantly in the follow‐up period—from 12% to 5% (McNemar's test, χ 2 = 18.225, P < 0.001), otherwise there were no significant changes in social conditions (employment, study, child care, arrests or violence). Similarly, mean patient ATOP ratings of psychological, physical health and quality of life remained unchanged across the group over the study period.
Table 3 shows the proportion of patients who deteriorated in one of the global health scores (physical health, psychological health or quality of life) by 2 or more points, and the proportion of patients who increased their use of each substance by 4 or more days. With regards to overall health scores changes—32% (91/282) deteriorated in one or more of the three health scales; 35% (123/356) increased their substance use by ≥4 days in one or more drug class, and 22% (80/358) had a clinically relevant increase in substance use (alcohol, opioids, benzodiazepines and stimulants).
Factors associated with clinically relevant increases in substance use
Table 4 includes the unadjusted and adjusted odds ratios for a clinically relevant increase in substance use. Note that depot buprenorphine patients (n = 89) are excluded for this table as dosing site and TAD variables were not relevant to this medication—although data for all patients (n = 358) at follow‐up is provided in Table S5 (Supporting Information).
Table 4.
Unadjusted and adjusted likelihoods and 95% confidence intervals for independent variables versus clinically relevant increases in composite substance use for n = 269 opioid agonist treatment patients (excludes depot buprenorphine patients and those with missing data at follow up)
| All n = 269 | Clinically relevant increase in substance use (n = 62) | No clinically relevant increase in substance use (n = 206) | Unadjusted ORs | Adjusted ORs | |
|---|---|---|---|---|---|
| Age, mean (SD) | 44 (11) | 42 (10) | 44 (11) | 0.985 (0.96–1.01) | 0.985 (0.96–1.02 |
| Sex | |||||
| Female | 85 (32%) | 22 (35%) | 63 (31%) | 1.218 (0.67–2.21) | 1.489 (0.78–2.83) |
| Male | 184 (68%) | 41 (65%) | 143 (69%) | (Ref) | (Ref) |
| Indigenous status | |||||
| Indigenous | 53 (20%) | 9 (14%) | 44 (21%) | 0.614 (0.28–1.34) | 0.386 (0.17–0.90) |
| Non‐indigenous | 216 (80%) | 54 (86%) | 162 (79%) | (Ref) | (Ref) |
| Any housing risk at follow up | |||||
| No housing risk | 249 (93%) | 56 (89%) | 193 (94%) | 0.539 (0.21–1.42) | 0.632 (0.22–1.78) |
| Homeless or at risk | 20 (7%) | 7 (11%) | 13 (6%) | ||
| Medication type at follow up | |||||
| Methadone | 195 (72%) | 47 (75%) | 148 (72%) | 1.151 (0.60–2.19) | 0.684 (0.31–1.52) |
| Sublingual buprenorphine | 74 (28%) | 16 (22%) | 58 (28%) | (Ref) | (Ref) |
| Takeaway category at follow up | |||||
| None | 83 (31%) | 27 (43%) | 56 (27%) | (Ref) | (Ref) |
| 1–5 per week | 99 (37%) | 25 (40%) | 74 (36%) | 0.701 (0.37–1.34) | 0.854 (0.39–1.87) |
| 6+ per week | 87 (32%) | 11 (17%) | 76 (37%) | 0.300 (0.14–0.66) | 0.273 (0.10–0.77) |
| Dosing site at follow up | |||||
| Public clinic | 128 (48%) | 38 (60%) | 90 (44%) | (Ref) | (Ref) |
| Community | 141 (52%) | 25 (40%) | 116 (56%) | 0.510 (0.29–0.91) | 0.705 (0.34–1.45) |
OR, odds ratio.
In the unadjusted analysis, clinically relevant increases in composite substance use were related to dosing site [17.7% (25/141) of pharmacy‐dosed patients compared to 29.7% (38/128) of clinic‐dosed patients (odds ratio [OR] 0.510, 0.29–0.91)] and access to TADs: with clinically relevant increases seen in 32.5% (27/83) of patients with no TADs, 25.3% (25/99) with 1–5 weekly TADs (OR 0.701, 0.37–1.34) and 12.6% (11/87) with 6+ TADs (OR 0.300, 0.14–0.55).
The multivariate model included independent variables of age, sex, Indigenous status, any housing risk, type of OAT medication, OAT dosing site and level of TAD provided at follow‐up. In the multivariate analysis, Aboriginal or Torres Strait Islander status was the only significant patient characteristic (OR 0.39, 0.17–0.90), and TAD access was the only significant treatment condition, with those accessing 6+ TADs/week having lower odds of deteriorating than those accessing no TADs/week (OR 0.27, 0.10–0.77).
Discussion
The global COVID‐19 pandemic caused significant disruption to the provision of health services and required rapid action to ensure continuity of care, especially for vulnerable populations. A number of changes to OAT service provision were implemented in SESLHD in late March–early April 2020, particularly increased TAD provision, reduced clinic dosing and telehealth services. Additionally, the fortuitous availability of depot buprenorphine provided a new treatment option that was consistent with social distancing requirements.
The outcomes identified in this evaluation are generally positive. There were high rates of treatment retention over the 5‐month follow‐up period [only 21 patients (<5%) ‘dropping out’ of OAT altogether], two unrelated deaths and only one case of COVID‐19 infection amongst patients or staff. At a cohort level, the results suggest reductions in opioid and benzodiazepine use and increases in cannabis use. These are similar to findings from other evaluations of substance use in the Australian community since the pandemic, and may reflect changes in the availability of specific illicit drugs, motivations and social contexts for substance use during a period of ‘lockdown’ [21, 22].
On an individual level, increases in the use of substances considered to be higher risk for TADs (opioids, alcohol, benzodiazepines, stimulants), were observed in 22% of patients. These ‘deteriorations’ were more prominent in patients with fewer TADs. This can be interpreted as either: (i) the decision‐framework used for determining suitability for TADs appropriately identified those at greater risk of clinically relevant increases in substance use; or (ii) restricting TADs ‘caused’ increased substance use. While our study design does not allow us to definitively answer this, previous randomised trials of unsupervised buprenorphine treatment suggest providing or restricting TADs does not result in significant differences in substance use [23, 24]—suggesting the clinical framework employed was effective in identifying patients suited to TADs. The increased use of TADs is an important departure from our historical emphasis upon supervised dosing in Australia, and our findings suggest that we can consider reducing levels of supervised dosing for many patients. However, some caution is required in this interpretation as we have no data regarding other risks of increased TADs such as non‐medical use (e.g. injecting) of TADs by patients, or evidence of harms from diverted TADs. Further (see limitations below), this evaluation examines outcomes during a period of ‘social lockdown’ and further research is required in ‘post‐pandemic’ conditions.
One risk mitigation strategy when providing TAD to patients was the offer of THN, with 41% of patients accessing THN from the services during the study period. The uptake of THN was appropriately greater amongst those patients receiving methadone and those receiving TADs. THN is available from a range of health services and pharmacies in Sydney, and those who declined the intervention may have already received THN prior to the study period. Further research is required to better understand barriers to THN uptake amongst OAT populations.
The increased telehealth activity also reduced face‐to‐face patient contact. The number of contacts using telehealth increased from 14% to 24%, and the total average number of monthly (non‐dosing) contacts reduced by 25% (2.42–1.83). The vast majority of these telehealth contacts were made by telephone rather than audio‐visual technologies reflecting this population's limited access to the data, technology and ‘tech literacy’ requirements of audio‐visual platforms.
This is amongst the first attempts in Australian alcohol and other drugs services to utilise routinely collected clinical information extracted from electronic medical records to evaluate a service model. A strength of analysing routinely collected service‐level data is that it allows for rapid before and after comparisons without significant delays or costs in establishing research procedures, and includes all patients of the service—not only those who consent to participate—addressing problems of recruitment bias. However, the approach uses information reported by patients to clinicians, and as such may introduce recall and/or responder bias.
There are other limitations to this evaluation. Data linkage with hospitals would allow for the identification of other potential harms (e.g. overdoses, injecting related harms); however, time and resource constraints in establishing data linkage procedures prevent us from reporting such findings at this time. Further, the 5‐month follow‐up period is relatively short and ongoing evaluation is required, particularly as many of the additional social supports temporarily implemented by governments in response to COVID‐19 (such as increased housing and financial support) have since been reversed after our evaluation period. Indeed, the evaluation covers a period when many social restrictions (‘lockdown’) and social supports (e.g. enhanced welfare benefits) were in place—which are possible confounding variables that may have affected patterns of substance use and health status of participants. Ongoing evaluation of service changes (e.g. increased TAD availability) and their impact upon client outcomes is required following pandemic conditions in order to assess their ongoing suitability.
The treatment services look very different in the ‘post’ period, with over two‐thirds (69%) of non‐depot patients accessing any TADs (vs. 24% pre‐COVID), one‐third (31%) accessing 6+ TADS per week (vs. 7%) and 52% of patients dosed at community pharmacy (vs. 24%). While we cannot determine whether the increase in the use of depot buprenorphine is due to expected uptake of a new product or the COVID‐19 policy, it is now one of the standard treatment options.
These changes are an opportunity to reflect on the Australian OAT model of care whereby supervised dosing (few TADs) remains the normative approach. The data from this evaluation provide preliminary support for future models of care, and within SESLHD, a co‐design process is underway with clinician and consumer input. The risks of COVID‐19 will likely remain for years to come and in Australia, recent environmental events (e.g. bushfires, floods) also intermittently disrupt access to health services. It is important that we continue to evaluate the effectiveness of OAT models of care to better ensure continuity of treatment under pandemic and other environmental challenges.
Conflict of Interest
NL has received research funding from Camurus for unrelated research. VH has received money from Camurus and Viiv for providing education and training sessions. TC has received money from Camurus for providing education sessions.
Supporting information
Table S1. Changes in opioid agonist treatment (OAT) service delivery in South Eastern Sydney Local Health District (SESLHD) due to the COVID‐19 pandemic.
Table S2. Pre‐COVID and COVID‐19 response frameworks for determining take‐away doses (TAD) in South Eastern Sydney Local Health District Drug and Alcohol services.
Table S3. Medical record data sources accessed for the study.
Table S4. Breakdown of patient movement between opioid treatment conditions (medication prescribed—methadone, sublingual buprenorphine or depot buprenorphine—and number of take‐away doses (TAD) permitted for methadone and sublingual buprenorphine) between patients attending treatment in the pre‐COVID period and at follow up. Greyed‐out boxes indicate no patients moved between those two conditions.
Table S5. Demographics and opioid agonist treatment dosing conditions for patients (n = 358, excluding those with missing data at follow up), by whether they had clinically relevant increases in composite substance use (increasing by 4+ days their use of alcohol, benzodiazepines, opioids and/or stimulants).
Acknowledgements
We thank patients and clinicians of Drug and Alcohol Services, South Eastern Sydney Local Health District. We particularly acknowledge the assistance of Dr Libby Topp, Sarah Hutchinson, Anni Ryan, Miriam van Zanten, Julie Fagan and Veronica Ganora.
Nicholas Lintzeris BMedSci, MBBS, PhD, FAChAM, Director and Conjoint Professor, Rachel M. Deacon PhD, Research Associate, Victoria Hayes MBBS, MPH, FAFPHM, FAChAM, Staff Specialist, Conjoint Lecturer, Tracy Cowan BSc Nursing (Mental Health), Nurse Unit Manager, Llewellyn Mills PhD, Research Fellow, Laila Parvaresh MD, MPH, AFRACMA, FAFPHM, Staff Specialist and Affiliated Lecturer, Lucy Harvey Dodds MBBS, FAChAM, MRCGP, MSc, Staff Specialist and Conjoint Associate Lecturer, Louisa Jansen, Consumer Representative, Raelene Dojcinovic BSc Nursing, Grad Cert Drug Alcohol, MPH, Clinical Nurse Consultant, Man C. Leung, MClinPharm, Acting Senior Pharmacist, Apo Demirkol MD, MSc, MMed (Pain Mgt), PhD, FAFPHM, FAChAM, Medical Unit Manager, Senior Staff Specialist, Conjoint Associate Professor and Senior Staff Specialist, Therese Finch Dip Health Services Mgt, Grad Cert Health Informatics, Health Information Manager, Kristie Mammen, BPsych (Hons), MAPS, Program Manager.
References
- 1. Degenhardt L, Grebely J, Stone J et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet 2019;394:1560–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet 2019;393:1760–72. [DOI] [PubMed] [Google Scholar]
- 3. Australian Institute of Health Welfare . National opioid pharmacotherapy statistics annual data collection. Canberra: AIHW, 2021. [Google Scholar]
- 4. Gowing L, Ali R, Dunlop A, Farrell M, Lintzeris N. National guidelines for medication‐assisted treatment of opioid dependence, 2014.
- 5. NSW Ministry of Health . NSW clinical guidelines for opioid dependence. North Sydney: NSW Ministry of Health, 2018. [Google Scholar]
- 6. Lintzeris N, Dunlop A, Masters D. Clinical guidelines for use of depot buprenorphine (Buvidal® and Sublocade) in the treatment of opioid dependence. Sydney: NSW Ministry of Health, 2019. [Google Scholar]
- 7. Australian Product Information: Buvidal® weekly (buprenorphine) solution for injection. Therapeutic Goods Administration, 2018.
- 8. Australian Product Information: Buvidal® monthly (buprenorphine) solution for injection. Therapeutic Goods Administration, 2018.
- 9. Australian Product Information: Sublocade (Buprenorphine). Therapeutic Goods Administration, 2019.
- 10. Lintzeris N, Hayes V & Arunogiri S. Interim guidance for the delivery of medication assisted treatment of opioid dependence in response to COVID‐19: a national response. Royal Australasian College of Physicians, 2020. Available at: https://www.racp.edu.au//docs/default‐source/news‐and‐events/covid‐19/interim‐guidance‐delivery‐of‐medication‐assisted‐treatment‐of‐opiod‐dependence‐covid‐19.pdf?sfvrsn=e36eeb1a_4 Accessed August 2021.
- 11. Dunlop A, Lokuge B, Masters D et al. Challenges in maintaining treatment services for people who use drugs during the COVID‐19 pandemic. Harm Reduct J 2020;17:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scheibein F, Stowe MJ, Arya S et al. Responding to COVID‐19: emerging practices in addiction medicine in 17 countries. Front Psych 2021;12:634309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Storen R & Corrigan N. COVID‐19: a chronology of state and territory government announcements (up until 30 June 2020): Commonwealth of Australia, 2020. Available at: https://www.aph.gov.au/About_Parliament/Parliamentary_Departments/Parliamentary_Library/pubs/rp/rp2021/Chronologies/COVID‐19StateTerritoryGovernmentAnnouncements. Accessed July 2021.
- 14. Ryan A, Holmes J, Hunt V et al. Validation and implementation of the Australian Treatment Outcomes Profile in specialist drug and alcohol settings. Drug Alcohol Rev 2014;33:33–42. [DOI] [PubMed] [Google Scholar]
- 15. Deacon RM, Mammen K, Bruno R et al. Assessing the concurrent validity, inter‐rater reliability and test‐retest reliability of the Australian Treatment Outcomes Profile (ATOP) in alcohol and opioid treatment populations. Addiction 2021;116:1245–55. [DOI] [PubMed] [Google Scholar]
- 16. eHealth NSW Government CHOC Electronic Medical Record. Available at: https://www.ehealth.nsw.gov.au/programs/clinical/emr-connect/choc. Accessed August 2021.
- 17. Lintzeris N, Mammen K, Holmes J, et al. Australian Treatment Outcomes Profile (ATOP) manual 1: using the ATOP with individual clients, 2020.
- 18. Deacon RM, Mammen K, Holmes J et al. Assessing the validity of the Australian Treatment Outcomes Profile for telephone administration in drug health treatment populations. Drug Alcohol Rev 2020;39:441–6. [DOI] [PubMed] [Google Scholar]
- 19. IBM Corporation . IBM SPSS Statistics for Windows, version 24.0. Armonk, NY: IBM Corporation, released 2016. [Google Scholar]
- 20. Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 1991;59:12–9. [DOI] [PubMed] [Google Scholar]
- 21. Dietze PM, Peacock A. Illicit drug use and harms in Australia in the context of COVID‐19 and associated restrictions: anticipated consequences and initial responses. Drug Alcohol Rev 2020;39:297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sutherland R, Baillie G, Memedovic S, et al. Key findings from the ‘Australians' Drug Use: Adapting to Pandemic Threats’ (ADAPT) study, 2020.
- 23. Bell J, Shanahan M, Mutch C. A randomized trial of effectiveness and cost‐effectiveness of observed versus unobserved administration of buprenorphine–naloxone for heroin dependence. Addiction 2007;102:1899–907. [DOI] [PubMed] [Google Scholar]
- 24. Dunlop A, Brown A, Oldmeadow C et al. Effectiveness and cost‐effectiveness of unsupervised buprenorphine‐naloxone for the treatment of heroin dependence in a randomized waitlist controlled trial. Drug Alcohol Depend 2017;174:181–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Changes in opioid agonist treatment (OAT) service delivery in South Eastern Sydney Local Health District (SESLHD) due to the COVID‐19 pandemic.
Table S2. Pre‐COVID and COVID‐19 response frameworks for determining take‐away doses (TAD) in South Eastern Sydney Local Health District Drug and Alcohol services.
Table S3. Medical record data sources accessed for the study.
Table S4. Breakdown of patient movement between opioid treatment conditions (medication prescribed—methadone, sublingual buprenorphine or depot buprenorphine—and number of take‐away doses (TAD) permitted for methadone and sublingual buprenorphine) between patients attending treatment in the pre‐COVID period and at follow up. Greyed‐out boxes indicate no patients moved between those two conditions.
Table S5. Demographics and opioid agonist treatment dosing conditions for patients (n = 358, excluding those with missing data at follow up), by whether they had clinically relevant increases in composite substance use (increasing by 4+ days their use of alcohol, benzodiazepines, opioids and/or stimulants).


