Dear Editor,
The widespread diffusion of severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), a member of the coronavirus family responsible for Coronavirus disease (COVID‐19), is still in the headlines for the impressive health and economic burden worldwide. The immune response against this novel virus is still undergoing extensive research, and the possible long‐term outcomes remain basically unknown. Immunity plays a key role in the defense against several microorganisms, and SARS‐CoV‐2 is no different. The infections can result in a hyperactive immune response with excessive inflammatory reactions. 1 These are generally accompanied by the release of impressive amounts of proinflammatory cytokines, an event known as “cytokine storm,” that is correlated with lung damage, organ failure, and a negative prognosis. 2 , 3 The possibility that these immunologic derangements could result in the induction and/or the exacerbation of other immune‐mediated diseases, including cutaneous ones, has not been completely investigated.
We report the case of a 61‐year‐old female patient referred to our dermatologic department for the development of asymptomatic bilateral sclerotic cutaneous lesions on the forearms which appeared 2 months before. The physical examination revealed the presence of several brownish and violaceous plaques with mild erythematous borders on forearms, with sclerotic appearance, partially tending to confluence. The lesions showed a symmetrical and bilateral distribution and were about 5–10 cm in diameter (Fig. 1a). The patient referred that the skin manifestations started about 1 month after the dismissal from hospitalization for COVID‐19 pneumonia (confirmed through RT‐PCR). Her familiar and personal history was negative for autoimmune and chronic inflammatory skin disorders. On suspicion of localized morphea, blood examination tests and skin biopsy were performed. Blood tests, including antinuclear antibodies (ANA), antibodies to single‐stranded DNA (a‐ssDNA), autoantibodies to extractable nuclear antigens (ENAs), and COVID‐19 swab test, were negative excluding systemic involvement. Histological examination revealed the presence of thin epidermis with moderate dermal sclerosis and thickening of collagen fibers, confirming the diagnosis of morphea.
Figure 1.
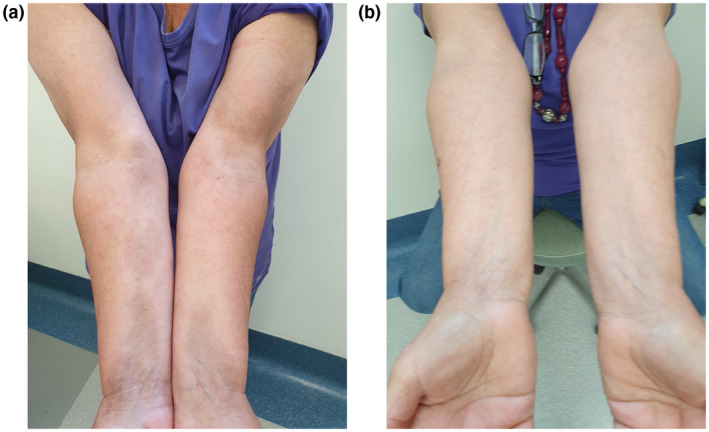
Clinical manifestations: (a) the presence of several brownish and violaceous plaques with mild erythematous borders on forearms, with sclerotic appearance, partially tending to confluence. (b) Complete clinical resolution after 16 weeks of treatment with clobetasol ointment and vitamin E emollient
A topical therapy with a high‐potency steroid (clobetasol ointment) and vitamin E emollient was started, with a remarkable improvement after 8 weeks and a complete clinical resolution after 16 weeks of treatment (Fig. 1b).
Morphea, also known as localized scleroderma, is an inflammatory skin condition that is characterized by the presentation of single or multiple inflammatory or sclerotic plaques. 4 While the pathogenesis remains largely unknown, several factors can contribute to the emergence of autoimmune disease including the genetic predisposition, environmental triggers such as bacterial and viral infections, and intrinsic factors, such as hormonal and immunologic dysregulation. 5
In this case, the negative personal anamnesis for autoimmune disorders and the development of cutaneous manifestations after COVID‐19 infection suggested a possible link between autoimmune disease and SARS‐CoV‐2. Some authors proposed that SARS‐CoV‐2 could act as a trigger in the development of organ‐specific autoimmune disorders, in genetic predisposed subjects. 6 In particular, a molecular mimicry phenomenon between virus and human protein has been hypothesized. Hence, the exaggerated activation of immune system against the virus could induce a cross‐reaction with auto‐antigens in common with viral peptides, leading to an autoimmune dysfunction. 7
Recently, some authors reported the development and/or worsening of inflammatory skin diseases after COVID‐19 infection, such as atopic dermatitis 8 and psoriasis, 9 induced by abnormal activation of the immune system in response to virus and consequent inflammatory pathways.
To the best of our knowledge, in the literature, there are no reports of morphea triggered by COVID‐19 infection. We report this case to underline the importance of this clinical condition during the COVID‐19 pandemic for the dermatologists, in order to provide a proper diagnosis and a correct therapeutic management.
Acknowledgment
The patient in this manuscript has given written informed consent.
Conflict of interest: None.
Funding source: None.
References
- 1. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID‐19 cytokine storm; what we know so far. Front Immunol 2020;11:1446. 10.3389/fimmu.2020.01446. Published 2020 Jun 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 2020; 395: 497–506. doi: 10.1016/S0140-6736(20)30183-5. Epub 2020 Jan 24. Erratum in: Lancet. 2020: PMID: 31986264; PMCID: PMC7159299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46: 846–848. doi: 10.1007/s00134-020-05991-x. Epub 2020 Mar 3. Erratum in: Intensive Care Med. 2020 Apr 6; PMID: 32125452; PMCID: PMC7080116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laxer RM, Zulian F. Localized scleroderma. Curr Opin Rheumatol 2006; 18: 606–613. 10.1097/01.bor.0000245727.40630.c3. PMID: 17053506. [DOI] [PubMed] [Google Scholar]
- 5. Cohen A, Shoenfeld Y. The viral autoimmunity relationship. Viral Immunol 1995; 8: 1–9. [DOI] [PubMed] [Google Scholar]
- 6. Ehrenfeld M, Tincani A, Andreoli L, et al. Covid‐19 and autoimmunity. Autoimmun Rev 2020; 19: 102597. doi: 10.1016/j.autrev.2020.102597. Epub 2020 Jun 11. PMID: 32535093; PMCID: PMC7289100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oldstone MB. Molecular mimicry: its evolution from concept to mechanism as a cause of autoimmune diseases. Monoclon Antib Immunodiagn Immunother 2014; 33: 158–165. 10.1089/mab.2013.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. COVID‐19‐associated surge of atopic dermatitis. EBioMedicine 2021; 64: 103268. doi: 10.1016/j.ebiom.2021.103268. PMID: 33641743; PMCID: PMC7910672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ozaras R, Berk A, Ucar DH, Duman H, Kaya F, Mutlu H. Covid‐19 and exacerbation of psoriasis. Dermatol Ther 2020; 33: e13632. doi: 10.1111/dth.13632. Epub 2020 Jun 2. PMID: 32436303; PMCID: PMC7280710. [DOI] [PMC free article] [PubMed] [Google Scholar]


