Dear Editor,
A healthy 48‐year‐old woman presented to hospital with a worsening erythematous eruption, with onset 14 days after receiving her first dose of the ChAdOx1 nCoV‐19 (AZD1222) vaccine. Aside from minor discomfort at the injection site that spontaneously resolved, she denied any other post‐vaccination symptoms. A pruritic eruption initially appeared on her chest, extending rapidly to the trunk, limbs and orogenital mucosa, with florid exfoliation. There were associated fevers, nausea and skin tenderness. Past medical history was unremarkable, with no other medications preceding the eruption. She was prescribed betamethasone valerate 0.02% cream with twice daily topical application, as well as fexofenadine 180 mg once daily per orally by her GP for her pruritic eruption with no relief. Paracetamol 1 g four times daily and ibuprofen 300 mg three times daily per orally were taken for her fever with no improvement. The patient had taken paracetamol, ibuprofen and fexofenadine previously with no issues.
Examination showed a confluent erythematous maculopapular eruption with a positive Nikolsky sign and orogenital mucosal involvement (Fig. 1). Skin biopsies demonstrated full‐thickness epidermal necrosis, pauci‐inflammatory dermis, and negative direct immunofluorescence (Fig. 2). Serology did not reveal any active infections with mycoplasma pneumoniae, herpes simplex virus or adenovirus. Screening tests were performed for HIV, hepatitis B, hepatitis C, tuberculosis and Strongyloides infection, which all returned negative results. Aside from mildly increased liver transaminases, blood differential and chemistry were within normal limits.
Figure 1.
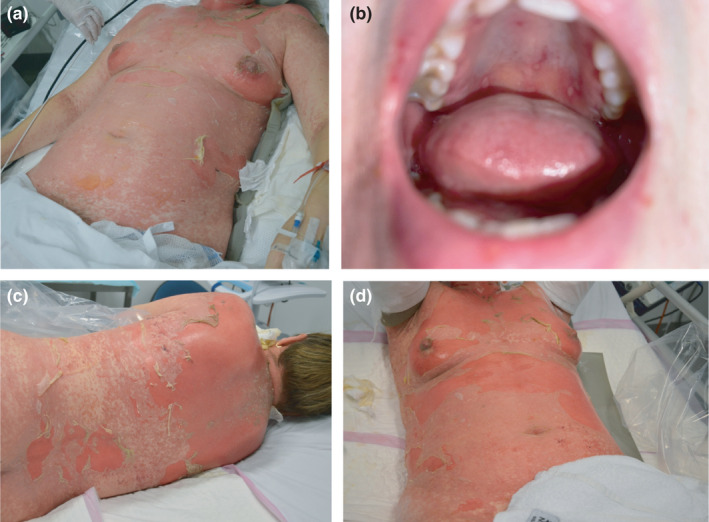
(a) & (b) show clinical images taken on day 4 of admission, showing denuded skin on the anterior trunk (a) with flaccid bullae measuring up to 40mm in diameter and with a total BSA of epidermally detached skin to 15%. Also present were erosions of the hard palate (b) and labia majora (not shown); there was no involvement of the vaginal mucosa or conjunctiva. (c) & (d) show clinical images taken on day 6 of admission, demonstrating progression of exfoliation on the trunk.
Figure 2.
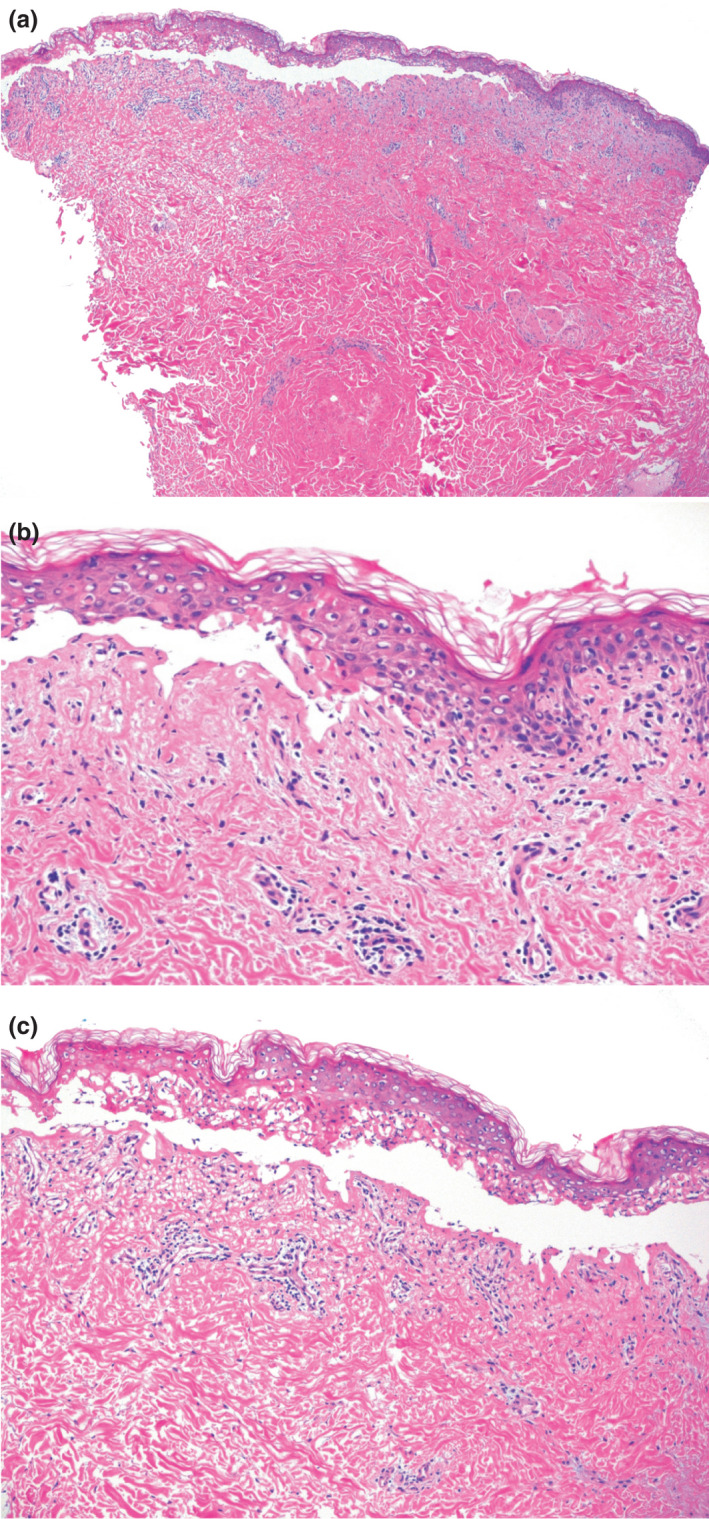
Shows histopathology of skin biopsy demonstrating partial‐ to full‐thickness epidermal necrosis, subepidermal split, basal vacuolation and scattered superficial dermal perivascular lymphocytes (x40, x200 & x100, H&E). Direct immunofluorescence was negative for fibrinogen, C3, IgA, IgG or IgM deposition.
She was admitted to a burns unit for management of Toxic Epidermal Necrolysis (TEN) due to ChAdOx1 nCoV‐19 vaccine. Her SCORTEN at time of admission was 2, scoring for age (>40 years) and more than 10% body surface area (BSA) of epidermal detachment. Treatment with adalimumab, a human IgG1 monoclonal antibody to Tumour Necrosis Factor‐alpha (TNF‐α), was given as an 80mg stat dose via subcutaneous injection. Two further doses were administered on days 3 and 5 after which progression of the eruption ceased. Detachment of involved skin continued up to 28 days of admission, with 90% of BSA involved. She made a full recovery and was discharged home after 35 days.
TEN is a severe cutaneous adverse reaction and a medical emergency, with a mortality rate of up to 30%. 1 SJS/TEN have a combined incidence between 2‐7 cases per million annually, whilst vaccine‐induced SJS/TEN is even rarer with less than 0.1 cases of SJS/TEN per million doses of influenza vaccine administered between 2010 to 2017. 2 , 3
The ChAdOx1 nCoV‐19 adenoviral vector vaccine induces a Th1‐polarised response to confer protection against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) via clonal expansion of CD8+ cytotoxic T‐lymphocytes (CTL), which play a pivotal role in the pathogenesis of TEN via granulysin and granzyme B release to induce keratinocyte apoptosis. 2 , 4 TEN following ChAdOx1 nCoV‐19 vaccination may relate to its immunogenicity to stimulate a robust CD8+ CTL response, which peaks between 7 and 28 days after vaccination, consistent with our patients' onset of exfoliative bullous eruptions 14 days after first exposure. 4
TNF‐α is elevated in serum and skin samples of TEN patients, although the extent TNF‐α induces keratinocyte apoptosis in TEN is unknown. 2 The efficacy of TNF‐α inhibitors to abrogate TEN has been demonstrated previously with Etanercept. 5 Adalimumab may prove to be a highly effective treatment for TEN, although larger randomised control studies are required to validate this.
We raise awareness of the possibility of TEN following ChAdOx1 nCoV‐19 vaccination and encourage clinicians to have a high index of suspicion for individuals with a rapidly progressive cutaneous eruption following ChAdOx1 nCoV‐19 vaccination to minimise delay in diagnosis and referral to a burns unit. However, we caution that this is a very rare event, the risk of which is minimal compared to the morbidity and mortality of SARS‐CoV‐2. It should not deter the wider community from receiving this or any other vaccine.
The authors do not declare any conflicts of interest, disclosures or funding sources.
Patient consent was obtained for this manuscript as per Australasian Journal of Dermatology author guidelines and was prepared in accordance with the CARE guidelines.
No ethics approval was required for this manuscript.
REFERENCES
- 1. Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J. Am. Acad. Dermatol. 2013; 69: 187.e1–187.e16: quiz 203–4. [DOI] [PubMed] [Google Scholar]
- 2. Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J. Am. Acad. Dermatol. 2013; 69: 173.e1–173.e13; quiz 85‐6. [DOI] [PubMed] [Google Scholar]
- 3. Su JR, Haber P, Ng CS et al. Erythema multiforme, Stevens Johnson syndrome, and toxic epidermal necrolysis reported after vaccination, 1999–2017. Vaccine 2020; 38: 1746–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ewer KJ, Barrett JR, Belij‐Rammerstorfer S et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV‐19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021; 27: 270–8. [DOI] [PubMed] [Google Scholar]
- 5. Paradisi A, Abeni D, Bergamo F et al. Etanercept therapy for toxic epidermal necrolysis. J. Am. Acad. Dermatol. 2014; 71: 278–83. [DOI] [PubMed] [Google Scholar]


