Summary
Background
Older age and comorbidities are the main risk factors for adverse COVID‐19 outcomes in patients with inflammatory bowel disease (IBD). The impact of IBD medications is still under investigation.
Aims
To assess risk factors for adverse outcomes of COVID‐19 in IBD patients and use the identified risk factors to build risk indices.
Methods
Observational cohort study. Univariable and multivariable logistic regression was used to identify risk factors associated with pneumonia, hospitalisation, need for ventilatory support, and death.
Results
Of the 937 patients (446 with ulcerative colitis [UC]) evaluated, 128 (13.7%) had asymptomatic SARS‐CoV‐2 infection, 664 (70.8%) had a favourable course, and 135 (15.5%) had moderate or severe COVID‐19. In UC patients, obesity, active disease and comorbidities were significantly associated with adverse outcomes. In patients with Crohn's disease (CD), age, obesity, comorbidities and an additional immune‐mediated inflammatory disease were identified as risk factors. These risk factors were incorporated into two indices to identify patients with UC or CD with a higher risk of adverse COVID‐19 outcomes. In multivariable analyses, no single IBD medication was associated with poor COVID‐19 outcomes, but anti‐TNF agents were associated with a lower risk of pneumonia in UC, and lower risks of hospitalisation and severe COVID‐19 in CD.
Conclusion
The course of COVID‐19 in patients with IBD is similar to that in the general population. IBD patients with active disease and comorbidities are at greater risk of adverse COVID‐19 outcomes. IBD medications do not pose additional risks. The risk indices may help to identify patients who should be prioritised for COVID‐19 re‐vaccination or for therapies for SARS‐CoV‐2 infection.
The course of COVID‐19 in patients with IBD patients is similar to that in the general population. IBD patients with active disease and comorbidities are at greater risk of adverse COVID‐19 outcomes. IBD medications do not pose additional risks.

1. BACKGROUND
Over the past year, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has rapidly spread around the world. 1 Research into the pathology of SARS‐CoV‐2‐induced coronavirus disease 2019 (COVID‐19) has revealed that the immune system plays two crucial roles in the infection: it controls viral replication in the early stage, and it overproduces pro‐inflammatory cytokines in patients who go on to develop severe disease. 2 Thus, physicians who care for patients with immune‐mediated inflammatory diseases (IMID) are concerned about the impact that COVID‐19 could have on these patients.
Like all IMIDs, the two main types of inflammatory bowel disease (IBD), namely ulcerative colitis (UC) and Crohn's disease, are characterised by an abnormal functioning of the innate and adaptive immune systems, leading to a chronic, progressive condition. From emerging data, the incidence and prevalence of SARS‐CoV‐2 infection in IBD patients are not different from those in the general population. 3 , 4 , 5 , 6 , 7 Additionally, the prevalence of adverse COVID‐19 outcomes, namely pneumonia, hospitalisation, intensive care unit admission and death, appears to be similar to that in the general population. 4 , 5 , 6 , 7 , 8 , 9 Numerous studies that investigated risk factors for adverse COVID‐19 outcomes in IBD patients concordantly found similarities with those in the general population, namely older age 6 , 7 , 8 , 9 , 10 , 11 , 12 and presence of comorbidities. 3 , 6 , 8 , 9 , 10 , 12 , 13 However, in the assessment of IBD‐related factors, discordant results were obtained. For example, some studies reported that disease activity was a risk factors for adverse outcomes of SARS‐CoV‐2 infection, 9 , 12 while other studies identified treatment with corticosteroids, 8 , 11 , 14 aminosalicylates, 11 , 13 and thiopurines (alone or in combination with anti‐TNF‐α agents) 13 as risk factors. These discrepancies may be due to insufficient numbers of cases or differences in epidemiological methodology.
The ongoing SARS‐CoV‐2 vaccination program has also raised an important clinical question about the eligibility of IBD patients for priority access to primary vaccination and booster doses. On one hand, this decision could be supported by evidence of a lower serological response to vaccines or natural infection, related to either IBD itself 15 or IBD therapies, namely anti‐TNF agents. 16 , 17 On the other hand, if priority access is granted to people at higher risk of adverse COVID‐19 outcomes, should this apply to some or all IBD patients? To answer this question, this observational study investigated risk factors for adverse COVID‐19 outcomes in a large, nation‐wide cohort of IBD patients, and used multivariable analyses to build indices to identify patients at the relatively highest risk.
2. METHODS
This observational cohort study was supported by the Italian Group for the Study of Inflammatory Bowel Disease (IG‐IBD). The primary objective was to identify risk factors for adverse outcomes of COVID‐19 in IBD patients. The secondary objective was to develop an index to identify IBD patients at increased risk of severe COVID‐19. The study protocol was approved by the IG‐IBD Scientific Committee and the Coordinating Ethical Committee of ASST Rhodense. All 96 IBD centres affiliated with IG‐IBD were invited to participate in the study.
Patients at participating IBD centres were consecutively included in this study if they had an established diagnosis of Crohn's disease (CD) or UC for at least 6 months and confirmed SARS‐CoV‐2 infection plus follow‐up for at least 1 month. A confirmed SARS‐CoV‐2 infection was defined as either the polymerase chain reaction‐confirmed presence of SARS‐CoV‐2 genome in a nasopharyngeal swab or the ELISA‐confirmed presence of anti‐SARS‐CoV‐2 spike protein antibodies in serum, or both. Data collection was done from March 11 to December 31, 2020.
For all patients, the following data were collected from medical charts and, when possible, patient interviews: age, sex, current smoking habit (yes or no), IBD type, IBD duration, IBD activity (classified as in remission, mild, moderate or severe according to the partial Mayo score for UC 18 and the Harvey‐Bradshaw index for CD 19 ) in the 3 months before the diagnosis of SARS‐CoV‐2 infection, IBD treatments, comorbidities (including other concomitant IMIDs), Charlson's comorbidity index (CCI), 20 symptoms of SARS‐CoV‐2 infection and COVID‐19 outcome (favourable, moderate or severe). Symptoms of COVID‐19 were classified as: gastrointestinal (diarrhoea, nausea or abdominal pain), systemic (fever, arthralgia, myalgia, asthenia or hyporexia), respiratory (pharyngodynia, cough, rhinitis and dyspnoea) and dysgeusia or anosmia. A moderate COVID‐19 outcome was defined as a diagnosis of pneumonia (demonstrated by chest CT or radiography) or the need for hospitalisation, while a severe outcome was defined as ventilatory assistance use (continuous positive airway pressure, non‐invasive mechanical ventilation or intubation) or death. These data were entered into an electronic database accessible to participating centres.
2.1. Statistical analysis
Analyses were conducted using SPSS Statistical Software (v. 13.0, IBM). Differences in quantitative variables at baseline between UC and CD patients were tested for significance using the t test. Associations between the type of IBD and categorical variables (baseline characteristics and symptoms of COVID‐19) were tested for significance using the chi‐square test. A two‐tailed P < 0.05 was indicative of statistical significance.
Risk factors for moderate‐to‐severe COVID‐19 outcomes were identified by logistic regression with univariable and multivariable analyses. Multivariable analyses were adjusted for confounding factors that could simultaneously impact upon another variable and the outcome (eg elderly patients are uncommonly prescribed anti‐TNF agents, so the use of these drugs was adjusted for age). Confounding factors were selected according to the literature 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 and to reports by the Italian national health service on causes of death from COVID‐19 in the general population and in IBD patients. 21 The confounding factors selected to adjust the regression analyses were therefore age, comorbidities, IMID and disease activity. Logistic regression was also used to assess the impact of medications on COVID‐19 outcomes.
Variables that were retained in the multivariable analyses at P < 0.05 were used to build an index for the risk of moderate‐to‐severe COVID‐19 in IBD patients. The coefficients from the multivariable analysis were used to assess the weight of each variable retained in the model and to calculate the index. We tested the sensitivity, specificity, and positive and negative likelihood ratios of this index using the clinical data from our population. ROC curve analysis was used to set the most sensitive and specific cut‐off to identify IBD patients with infection by SARS‐CoV‐2 at risk of moderate‐to‐severe COVID‐19.
3. RESULTS
We enrolled 937 IBD patients with a confirmed SARS‐CoV‐2 infection (Table 1) from 47 centres. The study group comprised 446 patients with UC (47.6%) and 491 with CD (52.4%), including 79 patients whom we had already studied. 9 The UC and CD groups differed significantly for age (CD patients were younger), disease activity, and therapies, with salicylates and vedolizumab used more frequently by UC patients and TNF antagonists and ustekinumab used more frequently by CD patients. At least one comorbidity was present in 376 (40.1%) IBD patients [89 (9.5%) had arterial hypertension and 21 were obese (2.2%)]; 116 patients (12.4%) had an IMID other than IBD.
TABLE 1.
Baseline characteristics of IBD patients with SARS‐CoV‐2 infection
| IBD (n = 937) | UC (n = 446) | CD (n = 491) | P value | |
|---|---|---|---|---|
| Age, years, median (range) | 44 (10‐86) | 47 (10‐86) | 43 (12‐84) | <0.001 |
| Age ≥60 years, n (%) | 185 (19.7) | 106 (23.7) | 75 (15.2) | 0.003 |
| Males, n (%) | 513 (54.7) | 252 (56.5) | 261 (53.1) | 0.43 |
| Current smokers, n (%) | 148 (15.8) | 59 (13.2) | 89 (18.1) | 0.05 |
| Disease duration, years, median (range) | 11 (0‐71) | 11 (0‐61) | 11 (0‐71) | 1.00 |
| Disease activity, n (%) | ||||
| Remission | 559 (59.6) | 254 (56.9) | 305 (62.1) | 0.002 |
| Mild | 211 (22.5) | 99 (22.1) | 112 (22.8) | 0.002 |
| Moderate | 124 (13.2) | 63 (14.1) | 61 (12.4) | 0.89 |
| Severe | 41 (4.3) | 20 (4.4) | 21 (4.2) | 1.00 |
| Therapy, n (%) a | ||||
| None | 56 (6.0) | 20 (4.4) | 36 (7.3) | 0.11 |
| Salicylates | 492 (52.5) | 333 (74.6) | 159 (32.3) | <0.001 |
| Corticosteroids | 122 (13.0) | 70 (15.6) | 52 (10.5) | 0.04 |
| Immunomodulators | 101 (11.2) | 47 (10.5) | 54 (10.9) | 0.75 |
| TNF antagonists | 346 (36.9) | 114 (25.5) | 232 (47.2) | <0.001 |
| TNF antagonists + immunomodulators | 22 (2.3) | 9 (2.0) | 13 (2.6) | 0.61 |
| Vedolizumab | 115 (12.3) | 71 (15.9) | 44 (8.9) | <0.001 |
| Ustekinumab | 51 (5.4) | 4 (0.9) | 47 (9.5) | <0.001 |
| Other | 13 (1.4) | 8 (1.7) | 5 (1.0) | 0.50 |
| Comorbidities, n (%) | 376 (40.1) | 188 (42.1) | 188 (38.2) | 0.32 |
| IMID | 116 (12.4) | 47 (10.5) | 69 (14.1) | 0.11 |
| Arterial hypertension b | 89 (9.5) | 55 (12.3) | 34 (6.9) | 0.01 |
| Obesity c | 21 (2.2) | 12 (2.6) | 9 (1.8) | 0.55 |
| Charlson's comorbidity index, n (%) | ||||
| 0 | 538 (57.4) | 234 (52.5) | 311 (63.3) | <0.001 |
| 1 | 178 (20.7) | 96 (21.5) | 84 (17.1) | 0.34 |
| 2 | 102 (21.9) | 57 (12.8) | 68 (13.8) | 0.08 |
| 3 | 59 (6.2) | 32 (7.2) | 27 (5.4) | 0.06 |
| 4 | 18 (1.9) | 9 (2.0) | 9 (1.8) | 0.05 |
| 5 | 15 (1.6) | 10 (2.2) | 5 (1.0) | 0.25 |
| 6 | 9 (0.9) | 6 (1.3) | 3 (0.6) | 0.21 |
| 7 | 1 (0.1) | 1 (0.2) | 0 (0) | 1.00 |
| 8 | 1 (0.1) | 1 (0.2) | 0 (0) | 1.00 |
Abbreviations: CD, Crohn's disease; IMID, immune‐mediated inflammatory disease; UC, ulcerative colitis.
The data reflect the fact that some patients were taking more than one therapy simultaneously.
Defined as >140/90 mm Hg.
Body mass index >30 kg/m2.
Overall, 809 patients (86.3%) had symptomatic SARS‐CoV‐2 infection, including 392 UC patients (87.9% of all UC patients) and 417 CD patients (84.9%) (Table S1). The most prevalent class of symptoms was respiratory, registered in 60.8% of UC patients and 48.9% of CD patients. The least prevalent class of symptoms was gastrointestinal in both groups. A favourable course of infection with symptoms resolution was recorded in 664 cases (70.8%).
For the IBD patients with moderate or severe outcomes of COVID‐19, 145 (15.5%) had pneumonia, 130 (13.9%) required hospitalisation, 65 (6.9%) required ventilatory support, and 25 (2.7%) died. The rates of all adverse COVID‐19 outcomes, but death, were significantly higher in UC patients than in CD patients (Figure S1).
3.1. Risk factors for moderate or severe COVID‐19 outcomes
In UC patients, the variables found to be predictive of moderate or severe outcomes at univariable analysis were age (per unit increase, P < 0.001), active disease of any severity (P = 0.001), obesity (P < 0.001), arterial hypertension (P = 0.01), and CCI (per unit increase, P < 0.001) (Table S2). At multivariable analysis, active disease (P < 0.001), obesity (P < 0.001), and CCI (P < 0.001) were significantly associated with a moderate or severe outcome. Univariable analyses in CD patients identified age (P < 0.001), obesity (P = 0.008), IMID (P < 0.001), and CCI (P < 0.001) as associated with moderate or severe outcomes, while multivariable analysis indicated age (P = 0.01), obesity (P = 0.02), and IMID (P = 0.01).
Regression analyses were repeated looking specifically at the risk of COVID‐19‐related pneumonia (Table 2). For UC patients, univariable analyses identified age ≥60 years, active disease of any severity, obesity, hypertension and CCI ≥2 as associated with the risk of pneumonia, and multivariable analyses found that active disease (OR = 1.88), obesity (OR = 23.76), and CCI ≥2 were associated with the risk of pneumonia. For CD patients, age ≥60 years, obesity, concomitant IMID, and a CCI ≥2 were significantly associated with pneumonia in the univariable analysis, whereas in the multivariable analysis only obesity (OR = 6.54) and concomitant IMID (OR = 3.52) were associated with the risk of pneumonia.
TABLE 2.
Risk factors for COVID‐19‐related pneumonia, hospitalisation and severe outcomes a in IBD patients
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Pneumonia | ||||||
| Ulcerative colitis | ||||||
| Age ≥60 years | 4.66 | 2.85‐7.61 | <0.001 | — | — | — |
| Male sex | 1.17 | 0.76‐1.81 | 0.47 | — | — | — |
| Current smoking | 1.47 | 0.78‐2.75 | 0.22 | — | — | — |
| Active disease (any severity) | 1.76 | 1.11‐2.78 | 0.01 | 1.88 | 1.12‐3.13 | <0.001 |
| Obesity | 20.83 | 4.48‐96.86 | <0.001 | 23.76 | 4.67‐120.6 | <0.001 |
| Arterial hypertension | 2.22 | 1.20‐40.9 | 0.01 | — | — | — |
| IMID | 1.01 | 0.48‐2.12 | 0.97 | — | — | — |
| CCI ≥2 | 5.93 | 3.63‐9.68 | <0.001 | 5.76 | 2.04‐16.31 | <0.001 |
| Crohn's disease | ||||||
| Age ≥60 years | 3.76 | 2.00‐7.05 | <0.001 | — | — | — |
| Male sex | 0.77 | 0.43‐1.37 | 0.38 | — | — | — |
| Current smoking | 0.69 | 0.30‐1.59 | 0.39 | — | — | — |
| Active disease (any severity) | 1.23 | 0.90‐1.67 | 0.18 | — | — | — |
| Obesity | 7.40 | 1.92‐28.52 | 0.003 | 6.54 | 1.48‐28.85 | 0.01 |
| Arterial hypertension | 1.96 | 0.77‐4.99 | 0.15 | — | — | — |
| IMID | 3.45 | 1.82‐6.52 | <0.001 | 3.52 | 1.81‐6.87 | <0.001 |
| CCI ≥2 | 3.82 | 2.07‐7.08 | <0.001 | |||
| Hospitalisation | ||||||
| Ulcerative colitis | ||||||
| Age ≥60 years | 5.32 | 3.20‐8.84 | <0.001 | — | — | — |
| Male sex | 1.21 | 0.77‐1.92 | 0.40 | — | — | — |
| Current smoking | 1.11 | 0.56‐2.21 | 0.93 | — | — | — |
| Active disease (any severity) | 1.76 | 1.09‐2.84 | 0.01 | 1.92 | 1.12‐3.31 | 0.01 |
| Obesity | 24.32 | 5.22‐113.3 | <0.001 | 22.8 | 4.40‐118.9 | <0.001 |
| Arterial hypertension | 2.39 | 1.28‐4.46 | 0.006 | — | — | — |
| IMID | 0.87 | 0.39‐1.94 | 0.73 | — | — | — |
| CCI ≥2 | 6.59 | 3.95‐10.99 | <0.001 | 3.97 | 1.34‐11.77 | 0.01 |
| Crohn's disease | ||||||
| Age ≥60 years | 4.51 | 2.36‐8.62 | <0.001 | — | — | — |
| Male sex | 1.40 | 0.76‐2.56 | 0.28 | — | — | — |
| Current smoking | 0.94 | 0.42‐2.10 | 0.89 | — | — | — |
| Active disease (any severity) | 1.52 | 0.82‐2.80 | 0.17 | — | — | — |
| Obesity | 5.10 | 1.23‐21.13 | 0.02 | — | — | — |
| Arterial hypertension | 1.74 | 0.64‐4.76 | 0.27 | — | — | — |
| IMID | 2.30 | 1.15‐4.60 | 0.01 | 2.26 | 1.09‐4.67 | 0.02 |
| CCI ≥2 | 4.19 | 2.21‐7.92 | <0.001 | — | — | — |
| Severe outcomes | ||||||
| Ulcerative colitis | ||||||
| Age ≥60 years | 5.44 | 2.96‐9.99 | <0.001 | 2.57 | 1.00‐6.60 | 0.04 |
| Male sex | 1.92 | 1.04‐3.57 | 0.03 | 2.85 | 1.35‐6.00 | 0.005 |
| Current smoking | 1.47 | 0.67‐3.22 | 0.32 | — | — | — |
| Active disease (any severity) | 1.78 | 0.99‐3.21 | 0.049 | 2.03 | 1.03‐3.97 | 0.03 |
| Obesity | 12.49 | 3.77‐40.75 | <0.001 | 20.34 | 4.83‐85.6 | 0.01 |
| Arterial hypertension | 2.19 | 1.04‐4.58 | 0.03 | — | — | — |
| IMID | 0.49 | 0.14‐1.66 | 0.25 | — | — | — |
| CCI ≥2 | 6.23 | 3.36‐11.55 | <0.001 | 1.53 | 1.17‐1.99 | 0.001 |
| Crohn's disease | ||||||
| Age ≥60 years | 5.90 | 2.57‐13.84 | <0.001 | — | — | — |
| Male sex | 1.36 | 0.59‐3.12 | 0.46 | — | — | — |
| Current smoking | 0.39 | 0.09‐1.72 | 0.21 | — | — | — |
| Active disease (any severity) | 1.63 | 0.72‐3.72 | 0.23 | — | — | — |
| Obesity | 10.97 | 2.56‐46.94 | 0.001 | 12.07 | 2.51‐58.01 | 0.002 |
| Arterial hypertension | 2.91 | 0.93‐9.06 | 0.06 | — | — | — |
| IMID | 2.29 | 0.91‐5.73 | 0.07 | — | — | — |
| CCI ≥2 | 5.06 | 2.19‐11.69 | <0.001 | 1.77 | 1.18‐2.65 | 0.005 |
Abbreviations: CCI, Charlson's comorbidity index; CI, confidence interval; IMID, immune‐mediated inflammatory disease; OR, odds ratio.
Ventilatory assistance or death.
Regarding hospitalisation (Table 2), in UC patients, age ≥60 years, active disease of any severity, obesity, hypertension and CCI ≥2 were found to be associated with risk in univariable analyses. In multivariable analyses adjusted for confounders, only active disease (OR = 1.92), obesity (OR = 22.8) and CCI ≥2 points (OR = 3.97) were found to be associated with the risk of hospitalisation. In CD patients, age ≥60 years, obesity, a concomitant IMID and CCI ≥2 were significantly associated with hospitalisation at the univariable analysis; at the multivariable analysis adjusted for confounders, only concomitant IMID (OR = 2.26) was found to be associated with the risk of hospitalisation.
A final analysis investigated the risk of severe COVID‐19 outcomes (need for ventilatory support or death; Table 2). In UC patients, age ≥60 years, male sex, active disease of any severity, obesity, hypertension and CCI ≥2 were found to be associated with the risk of severe COVID‐19; at the multivariable analyses adjusted for confounders, age ≥60 years (OR = 2.57), male sex (OR = 2.85), active disease (OR = 2.03), obesity (OR = 20.34), and CCI ≥2 (OR = 1.53) were associated with the risk of severe COVID‐19. In CD patients, age ≥60 years, obesity and CCI ≥2 were significantly associated with severe COVID‐19 at univariable analyses; at multivariable analyses adjusted for confounders, only obesity (OR = 12.07) and CCI ≥2 (OR = 1.77) were associated with the risk of severe COVID‐19.
3.2. Impact of medications on COVID‐19 outcomes
These results are shown in Table 3.
TABLE 3.
Impact of IBD medications on SARS‐CoV‐2 infections in IBD patients
| OR (95% CI) a | ||||
|---|---|---|---|---|
| Symptoms b | Pneumonia | Hospitalisation | Severe COVID‐19 c | |
| Univariable analysis | ||||
| Ulcerative colitis | ||||
| TNF antagonists | 0.51 (0.28‐0.90) | 0.39 (0.20‐0.73) | — | 0.34 (0.14‐0.84) |
| Crohn's disease | ||||
| TNF antagonists | 0.43 (0.24‐0.78) | 0.38 (0.20‐0.73) | 0.41 (0.21‐0.80) | 0.28 (0.10‐0.76) |
| Salicylates | 2.16 (1.25‐3.74) | 2.16 (1.20‐3.89) | 2.44 (1.32‐4.52) | 2.78 (1.21‐6.37) |
| Multivariable analysis | ||||
| Ulcerative colitis | ||||
| TNF antagonists | — | 0.44 (0.22‐0.86) | — | — |
| Crohn's disease | ||||
| TNF antagonists | — | — | 0.46 (0.23‐0.92) | 0.31 (0.11‐0.88) |
| Salicylates | — | — | — | — |
Only significant results (univariable analysis, P < 0.05) are reported.
Any symptoms of COVID‐19.
Ventilatory support or death.
In the UC population, at univariable analysis, the use of anti‐TNF agents was associated with significantly lower risks of COVID‐19, pneumonia, and severe COVID‐19. Similarly, in CD patients, the use of anti‐TNF agents was associated with significantly lower risks of COVID‐19, pneumonia, hospitalisation, and severe COVID‐19. In contrast, in CD patients, the use of salicylates was associated with higher risks of COVID‐19 symptoms, pneumonia, hospitalisation and severe outcomes. The other medications did not have any impact on COVID‐19 outcomes.
After adjusting for confounding factors, at multivariable analyses, the use of anti‐TNF agents remained significantly associated with a lower risk of pneumonia in UC patients (OR = 0.44; 95% CI, 0.22‐0.86) and with lower risks of hospitalisation (OR = 0.46; 95% CI, 0.23‐0.92) and severe COVID‐19 (OR = 0.31; 95% CI, 0.11‐0.88) in CD patients. Salicylates were not confirmed to be associated with the risk of adverse outcomes in CD patients.
3.3. Risk assessment for moderate‐severe COVID‐19 in IBD patients
Based on the multivariable analyses for moderate‐severe COVID‐19 outcomes, we built two risk indices that sum the contributions of significant variables. For UC patients, the index was calculated with the formula (where yes = 1 and no = 0):
ROC curve analysis (Figure 1A) identified a cut‐off ≥0.90 for increased risk of any adverse outcome of SARS‐CoV‐2 (area under the curve [AUC] = 0.79; 95% CI, 0.75‐0.83), with 0.71 sensitivity (95% CI, 0.61‐0.80), 0.74 specificity (95% CI, 0.69‐0.79), a positive likelihood ratio (+LR) of 2.79, and a negative likelihood ratio (−LR) of 0.39. The same cut‐off was found to be the best predictor for the risk of pneumonia (AUC = 0.79; 95% CI, 0.75‐0.83), hospitalisation (AUC = 0.81; 95% CI, 0.77‐0.85), and severe COVID‐19 (AUC = 0.80; 95% CI, 0.76‐0.84).
FIGURE 1.
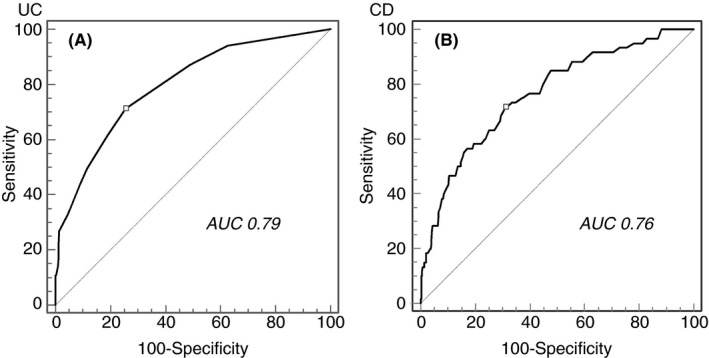
ROC curve analysis of the IG‐IBD COVID‐19 index for the risk of moderate‐to‐severe COVID‐19. A, UC patients. B, CD patients
For CD patients, the index was calculated with the formula (where yes = 1 and no = 0):
ROC curve analysis for CD patients identified a cut‐off ≥2.20 for increased risk of any adverse outcome of SARS‐CoV‐2 (AUC = 0.76; 95% CI, 0.72‐0.80), with 0.71 sensitivity (95% CI, 0.58‐0.82), 0.69 specificity (95% CI, 0.64‐0.73), +LR = 2.31, and –LR = 0.41 (Figure 1B). A score of ≥2.50 was the best predictor for the risk of pneumonia (AUC = 0.76; 95% CI, 0.72‐0.80) and hospitalisation (AUC = 0.77; 95% CI, 0.73‐0.80), whereas a score ≥2.44 was the best predictor for severe COVID‐19 (AUC = 0.78; 95% CI, 0.74‐0.81).
4. DISCUSSION
In this study, the majority of IBD patients had an asymptomatic (13.7%) or favourable course (70.8%) of SARS‐CoV‐2 infection. However, UC patients were more likely than CD patients to have symptoms and to develop moderate or severe COVID‐19. Among the 145 patients who had moderate or severe COVID‐19, age ≥60 years, male sex, obesity, arterial hypertension and another concomitant IMID were risk factors for an adverse outcome. Considering variables related to IBD, disease activity of any severity was an additional risk factor, but no single IBD medication was found at multivariable analysis to be associated with adverse COVID‐19 outcomes. On the contrary, anti‐TNF agents were associated with a lower risk of pneumonia in UC patients and with lower risks of hospitalisation and severe COVID‐19 in CD patients.
Overall, our findings confirm that the course of COVID‐19 in IBD patients is similar to that observed in the general population. 3 , 4 , 5 , 6 , 7 Moreover, they confirm that the general risk factors for worse outcomes of COVID‐19 (age, male sex and comorbidities) in IBD patients are similar to those in the general population, as already observed in other studies of IBD patients. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 Among IBD‐related risk factors, the finding of a negative role of disease activity agrees with previously reported studies. 9 , 12
Understanding if IBD therapies affect the evolution of SARS‐CoV‐2 infection is crucial because of the possible consequences on the management of these patients. Indeed, stopping, postponing or avoiding therapies due to the fear of a negative impact on the infection could lead to IBD flares with detrimental consequences. This study found that patients taking anti‐TNF agents had lower risks of pneumonia (UC patients), hospitalisation and severe COVID‐19 (CD patients); this protective role against severe COVID‐19 confirms findings of previous studies. 8 , 11 , 13 Due to the small subgroup of patients in combination therapy with anti‐TNF agents and immunosuppressants, we are not able to draw conclusions about its reported potential negative impact. 13 The potential protective role of anti‐TNF agents could be due to their downregulation of ACE2 expression in the intestine, 22 their known effects on cytokine storms, 23 or to a general dampening of the inflammatory response during the hyperimmune phase of the infection. 2
Our study did not find an association between salicylates and COVID‐19 outcomes, once confounders were eliminated through multivariable analysis. This finding is in apparent contrast to reports of an association between salicylate use and a negative course of SARS‐CoV‐2 infection from the SECURE‐IBD international registry. 11 , 13 This potential negative role of salicylates is a matter of debate: besides the theoretical plausibility, 24 it is possible that several biases led to this finding, including the fact that anti‐TNF agents were used as a comparator. 25 Our study also did not find any impact of corticosteroids, in contrast to earlier studies where these drugs were negative factors for a worse outcome of COVID‐19 with a possible dose‐dependent effect. 8 , 11 , 14 However, the close association with disease activity and the lack of complete information about doses, reasons for taking these drugs and duration of therapy do not allow us to draw firm conclusions.
Using the risk factors identified in multivariable analysis for moderate‐to‐severe COVID‐19, we developed two indices of the risk for adverse outcomes in UC and CD patients. In our models, scores ≥0.9 for UC patients and ≥2.20 for CD patients were the best cut‐offs to identify those at higher risk. These indices could be useful in identifying IBD patients who could be prioritised for vaccination, especially in contexts where vaccines are scarce and when boosters will become necessary. However, due to the relatively low absolute number of IBD patients at higher risk of adverse outcomes, these indices could have a relatively poor diagnostic accuracy. Moreover, they need to be validated before they are used in clinical practice.
This study reports the findings from a large cohort of consecutive IBD patients with COVID‐19 from a single country, where the management of IBD is relatively homogeneous. The participation of many IG‐IBD centres across Italy makes our study a reliable portrait of the Italian situation of IBD patients infected by SARS‐CoV‐2. Importantly, we evaluated UC and CD patients separately since UC and CD are different diseases, with different therapies and different indices to assess disease activity. Indeed, in our study, the UC and CD populations differed significantly in terms of baseline characteristics, including therapies, and symptoms of SARS‐CoV‐2 infection, so we analysed them separately for COVID‐19 risk factors. Instead, none of the prior studies on COVID‐19 outcomes in IBD patients distinguished between UC and CD patients. Finally, the fact that we adjusted our risk factor analyses for several confounders should clarify some of the conflicting results reported so far.
Our study has some limitations. First, we cannot exclude the under‐reporting of cases: some participating centres may not have included all their patients due to the voluntary referral, and asymptomatic and mild forms of SARS‐CoV‐2 infection may have been missed (mainly patients in remission or not under strict monitoring). On the contrary, we also cannot exclude the over‐reporting of asymptomatic and mild forms of SARS‐CoV‐2 infection in patients under close follow‐up (ie, patients in biological therapy). Our findings can be extended only to other settings similar in social, cultural, political and economic backgrounds. Finally, the proposed indices to identify IBD patients at higher risk of adverse COVID‐19 outcomes should be validated before clinical implementation.
In conclusion, this study confirms that SARS‐CoV‐2 generally has a mild course in IBD patients, and that the risk factors for moderate‐to‐severe COVID 19 are the same as observed in the general population. On the basis of these results, we feel that no additional or different precautions should be adopted for IBD patients. In particular, because we did not find any evidence for a negative effect of IBD therapies on the outcome of SARS‐CoV‐2 infection, our study provides support for the recommendations of the European Crohn´s and Colitis Organisation, 26 which state to not stop in advance medications needed to maintain IBD in remission and to avoid flares due to discontinuation. The indices created here to identify UC and CD patients with a higher risk of adverse COVID‐19 outcomes may boost the effectiveness of ongoing and future vaccination programs and of future specific therapies for SARS‐CoV‐2 by identifying a subset of IBD patients who merit prioritisation.
AUTHORSHIP
Guarantor of the article: Cristina Bezzio.
Author contributions: CB, GF and SS: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; GF statistical analysis; All the remaining listed: acquisition of data; critical revision of the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Funding interests: None.
Declaration of personal interests: CB received lecture fees from Takeda, MSD, AbbVie and Janssen. AA served as a consultant for AbbVie, Allergan, Amgen, Biogen, Bristol Myers Squibb, Celgene, Celltrion, Ferring, Gilead, Janssen, Lilly, MSD, Mylan, Pfizer, Roche, Samsung Bioepis, Sandoz, and Takeda. FF, SA and MM received lecture fees from Janssen. AO served as a consultant for AbbVie, MSD, Janssen, Pfizer, Takeda, Sofar and Chiesi. FAC served as consultant and a member of advisory board for Mundipharma, AbbVie, MS&D, Takeda, Janssen, Roche and Celgene, received lecture fees from AbbVie, Amgen, Ferring, Takeda and Allergy Therapeutics and received unrestricted research grants from Giuliani, Sofar, MSD, Takeda and AbbVie. FC served as consultant and a member of the advisory board for Mundipharma, AbbVie, MSD, Takeda, Janssen, Roche and Celgene and received lecture fees from AbbVie, Amgen, Ferring, Takeda and Allergy Therapeutics. DR served as consultant and received lecture fees from Janssen, Ferring and Errekappa. FZ received lecture fees from Alphasigma, Takeda and Janssen. SF received consultancy fees from Sofar, Zambon, AbbVie and Takeda and is a member of advisory boards for Janssen Pharmaceuticals. MD served as a speaker, consultant and advisory board member for AbbVie, Takeda, Janssen, Norgine, Pfizer, MSD, Celltrion, Roche, Gilead, Bioclinica, Ferring, SOFAR, Chiesi and Zambon. LP served as a speaker, consultant and advisory board member for AbbVie, Takeda, Janssen, Biogen, Sandoz, Fresenius‐Kabi and Eli Lilly. PB served as a speaker, consultant or advisory board member for Takeda and Janssen. CR reports personal fees from AbbVie, Janssen Cilag, MSD, Recordati, Takeda and Vifor. MC served as a speaker or advisory board member for Takeda, MSD, AbbVie, Shire, Fresenius and Janssen. CF served as consultant for Amgen. GF received consultancy fees from Ferring, MSD, AbbVie, Takeda, Janssen, Amgen, Sandoz, Samsung Bioepis and Celltrion. SS received lecture fees from Takeda Pharmaceuticals and Janssen Pharmaceuticals and served as a consultant and advisory board member for AbbVie and Janssen Pharmaceuticals.
Supporting information
Figure S1
Tables S1‐S2
ACKNOWLEDGEMENTS
We gratefully thank Stefanos Bonovas for his contribution to the statistical analyses. Valerie Matarese provided scientific editing.
APPENDIX 1.
COVID‐19 IG‐IBD Study Group
Francesca Coppini, Patrizia Alvisi, Viviana Gerardi, Angela Variola, Silvia Mazzuoli, Marco Vincenzo Lenti, Daniela Pugliese, Mariangela Allocca, Francesca Ferretti, Jenny Roselli, Fabrizio Bossa, Alessandra Giuliano, Nicole Piazza, Gianpiero Manes, Alessandro Sartini, Andrea Buda, Federica Micheli, Valeria Ciardo, Giovanni Casella, Angelo Viscido, Giorgia Bodini, Valentina Casini, Alessandra Soriano, Arnaldo Amato, Laurino Grossi, Sara Onali, Matteo Rottoli, Rocco Spagnuolo, Stefania Baroni, Claudio Cortelezzi, Monia Baldoni, Marta Vernero, Franco Scaldaferri, Giovanni Maconi, Alessia Dalila Guarino, Andrea Palermo, Renata D'Incà, Maria Lia Scribano, Livia Biancone, Lucio Carrozza, Marta Ascolani, Francesco Costa, Antonio Di Sabatino, Irene Zammarchi, Matteo Gottin, Francesco Simone Conforti.
APPENDIX 2.
The complete list of author affiliations
Cristina Bezzio, Gastroenterology Unit, Rho Hospital, ASST Rhodense, Rho, Milan, Italy; Alessandro Armuzzi, CEMAD, IBD Unit, Internal Medicine and Gastroenterology Unit, Department of Medical and Surgical Sciences, Fondazione Policlinico Universitario Gemelli IRCCS, Rome, Italy and University Department of Translational Medicine and Surgery, Catholic University of Sacro Cuore, Rome, Italy; Federica Furfaro, IBD Unit, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy; Sandro Ardizzone, Gastroenterology Unit, ASST Fatebenefratelli Sacco, Milan, Italy; Department of Biomedical and Clinical Sciences, University of Milan, Milan, Italy; Monica Milla, IBD Referral Center, Gastroenterology Department, Azienda Ospedaliero‐Universitaria Careggi, Florence, Italy; Sonia Carparelli, Gastroenterology and Endoscopy Unit, Fondazione IRCCS Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy; Ambrogio Orlando, IBD Unit, Villa Sofia Cervello Hospital, Palermo, Italy; Flavio Andrea Caprioli, Gastroenterology and Endoscopy Unit, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy; Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy; Fabiana Castiglione, Gastroenterology Unit, Department of Clinical Medicine and Surgery, University Federico II of Naples, Naples, Italy; Chiara Viganò, Division of Gastroenterology and Center for Autoimmune Liver Diseases, Department of Medicine and Surgery, University of Milano‐Bicocca, and European Reference Network on Hepatological Diseases (ERN RARE‐LIVER), San Gerardo Hospital, Monza, Milan, Italy; Davide G. Ribaldone, Division of Gastroenterology, Department of Medical Sciences, Università di Torino, Turin; Fabiana Zingone, Department of Surgical, Oncological and Gastroenterological Sciences ‐ DISCOG, University Hospital, Padua, Italy; Rita Monterubbianesi, Gastroenterology and Endoscopy Unit, Azienda Ospedaliera San Camillo Forlanini, Rome, Italy; Nicola Imperatore, Gastroenterology and Endoscopy Unit, Antonio Cardarelli Hospital, Naples; Stefano Festa, IBD Unit, San Filippo Neri Hospital, Rome, Italy; Marco Daperno, Gastroenterology Unit, Mauriziano Hospital, Turin, Italy; Ludovica Scucchi, Department of Systems Medicine, University Tor Vergata, Rome, Italy; Antonio Ferronato, UOSD Endoscopia Digestiva, Ospedale Alto Vicentino, AULSS 7 Pedemontana, Santorso, VI, Italy; Luca Pastorelli, Gastroenterology and Liver Unit, ASST Santi Paolo e Carlo, and School of Medicine at Ospedale San Paolo, Department of Health Sciences, University of Milan, Milan, Italy; Paola Balestrieri, Gastroenterology and Endoscopy Unit, Policlinico Campus Bio Medico, Rome, Italy; Chiara Ricci, Gastroenterology Unit, Clinical and Experimental Sciences Department, Spedali Civili Hospital, University of Brescia, Brescia, Italy; Maria Cappello, Gastroenterology and Hepatology Section, Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties, University of Palermo, Palermo, Italy; Carla Felice, Department of Internal Medicine, University of Padua, Padua, Italy; Gionata Fiorino, IBD Unit, IRCCS Humanitas Research Hospital, Rozzano, MI, Italy; Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, MI, Italy and Simone Saibeni, Gastroenterology Unit, Rho Hospital, ASST Rhodense, Rho, MI, Italy on behalf of the Italian Group for the Study of Inflammatory Bowel Disease (IG‐IBD)
Bezzio C, Armuzzi A, Furfaro F, et al. Therapies for inflammatory bowel disease do not pose additional risks for adverse outcomes of SARS‐CoV‐2 infection: an IG‐IBD study. Aliment Pharmacol Ther. 2021;54:1432–1441. 10.1111/apt.16663
Gionata Fiorino and Simone Saibeni are equal contributors.
The complete list of author affiliations are listed in Appendix 2.
The Handling Editor for this article was Dr Rohit Loomba, and it was accepted for publication after full peer‐review.
Contributor Information
Cristina Bezzio, Email: bezzioc@gmail.com.
the Italian Group for the Study of Inflammatory Bowel Disease (IG‐IBD):
Francesca Coppini, Patrizia Alvisi, Viviana Gerardi, Angela Variola, Silvia Mazzuoli, Marco Vincenzo Lenti, Daniela Pugliese, Mariangela Allocca, Francesca Ferretti, Jenny Roselli, Fabrizio Bossa, Alessandra Giuliano, Nicole Piazza, Gianpiero Manes, Alessandro Sartini, Andrea Buda, Federica Micheli, Valeria Ciardo, Giovanni Casella, Angelo Viscido, Giorgia Bodini, Valentina Casini, Alessandra Soriano, Arnaldo Amato, Laurino Grossi, Sara Onali, Matteo Rottoli, Rocco Spagnuolo, Stefania Baroni, Claudio Camillo Cortelezzi, Monia Baldoni, Marta Vernero, Franco Scaldaferri, Giovanni Maconi, Alessia Dalila Guarino, Andrea Palermo, Renata D'Incà, Maria Lia Scribano, Livia Biancone, Lucio Carrozza, Marta Ascolani, Francesco Costa, Antonio Di Sabatino, Irene Zammarchi, Matteo Gottin, and Francesco Simone Conforti
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request.
REFERENCES
- 1. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neurath MF. COVID‐19 and immunomodulation in IBD. Gut. 2020;69:1335‐1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ludvigsson JF, Axelrad J, Halfvarson J, et al. Inflammatory bowel disease and risk of severe COVID‐19: a nationwide population‐based cohort study in Sweden. United Eur Gastroenterol J. 2021;9:177‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taxonera C, Alba C, Olivares D. What is the incidence of COVID‐19 in patients with IBD in western countries? Gastroenterology. 2021;160:1901‐1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allocca M, Chaparro M, Gonzalez HA, et al. Patients with inflammatory bowel disease are not at increased risk of COVID‐19: a large multinational cohort study. J Clin Med. 2020;9:3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Derikx LAAP, Lantinga MA, de Jong DJ, et al. Clinical outcomes of Covid‐19 in patients with inflammatory bowel disease: a nationwide cohort study. J Crohns Colitis. 2021;15:529‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Attauabi M, Poulsen A, Theede K, et al. Prevalence and outcomes of COVID‐19 among patients with inflammatory bowel disease – a Danish prospective population‐based cohort study. J Crohns Colitis. 2021;15:540‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lukin DJ, Kumar A, Hajifathalian K, et al.; Roberts Center Study Group Study Group; Weill Cornell Medicine‐Gastrointestinal Study Group . Baseline disease activity and steroid therapy stratify risk of COVID‐19 in patients with inflammatory bowel disease. Gastroenterology. 2020;159:1541‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bezzio C, Saibeni S, Variola A, et al. Outcomes of COVID‐19 in 79 patients with IBD in Italy: an IG‐IBD study. Gut 2020;69:1213‐1217. [DOI] [PubMed] [Google Scholar]
- 10. Axelrad JE, Malter L, Hong S, Chang S, Bosworth B, Hudesman D. From the American epicenter: coronavirus disease 2019 in patients with inflammatory bowel disease in the New York City metropolitan area. Inflamm Bowel Dis. 2021;27:662‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID‐19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481‐491.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh S, Khan A, Chowdhry M, Bilal M, Kochhar GS, Clarke K. Risk of severe coronavirus disease 2019 in patients with inflammatory bowel disease in the United States: a multicenter research network study. Gastroenterology. 2020;159:1575‐1578.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ungaro RC, Brenner EJ, Gearry RB, et al. Effect of IBD medications on COVID‐19 outcomes: results from an international registry. Gut. 2021;70:725‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khan N, Mahmud N, Trivedi C, Reinisch W, Lewis JD. Risk factors for SARS‐CoV‐2 infection and course of COVID‐19 disease in patients with IBD in the Veterans Affair Healthcare System. Gut. 2021;70:1657‐1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pozdnyakova V, Botwin GJ, Sobhani K, et al. Decreased antibody responses to Ad26.COV2.S relative to SARS‐CoV‐2 mRNA vaccines in patients with inflammatory bowel disease. Gastroenterology. 2021: S0016–5085(21):03360–3366. doi: 10.1053/j.gastro.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chanchlani N, Lin S, Chee D, et al. Adalimumab and infliximab impair SARS‐CoV‐2 antibody responses: results from a therapeutic drug monitoring study in 11422 biologic‐treated patients. J Crohns Colitis 2021. doi: 10.1093/ecco-jcc/jjab153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kennedy NA, Lin S, Goodhand JR, et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV‐19 SARS‐CoV‐2 vaccines in patients with IBD. Gut. 2021;70:1884‐1893. [DOI] [PubMed] [Google Scholar]
- 18. D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763‐786. [DOI] [PubMed] [Google Scholar]
- 19. Harvey RF, Bradshaw JM. A simple index of Crohn's disease activity. Lancet. 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 20. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 21. Official site of the Istituto Superiore di Sanità Italiano, Italian Health Superior Institute. https://www.epicentro.iss.it/en/coronavirus/sars‐cov‐2‐analysis‐of‐deaths. Accessed September 22, 2021.
- 22. Li XZ, Qiu Y, Jeffery L, et al. Down‐regulation of colonic ACE2 expression in patients with inflammatory bowel disease responding to anti‐TNF therapy: implications for COVID‐19. Front Med (Lausanne). 2021;7:613475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Deventer SJ. Review article: targeting TNF alpha as a key cytokine in the inflammatory processes of Crohn's disease–the mechanisms of action of infliximab. Aliment Pharmacol Ther. 1999;13(Suppl 4):3‐8. [DOI] [PubMed] [Google Scholar]
- 24. Rousseaux C, Lefebvre DL, Lefebvre P, et al. Intestinal antiinflammatory effect of 5‐aminosalicylic acid is dependent on peroxisome proliferator‐activated receptor‐gamma. J Exp Med. 2005;201:1205‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magro F, Dias CC, Morato M. Aminosalicylates and COVID‐19: facts or coincidences? Gastroenterology. 2021;160:1884‐1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Magro F, Rahier JF, Abeu C, et al. Inflammatory bowel disease management during the COVID‐19 outbreak: the 10 do's and don'ts from the ECCO‐COVID Taskforce. J Crohns Colitis 2020;14(14 Suppl 3):S798‐S806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Tables S1‐S2
Data Availability Statement
Data are available upon reasonable request.


