Summary
Background
Poor immune responses are frequently observed in patients with inflammatory bowel disease (IBD) receiving established vaccines; risk factors include immunosuppressants and active disease.
Aims
To summarise available information regarding immune responses achieved in patients with IBD receiving established vaccines. Using this information, to identify risk factors in the IBD population related to poor vaccine‐induced immunity that may be applicable to vaccines against COVID‐19.
Methods
We undertook a literature review on immunity to currently recommended vaccines for patients with IBD and to COVID‐19 vaccines and summarised the relevant literature.
Results
Patients with IBD have reduced immune responses following vaccination compared to the general population. Factors including the use of immunomodulators and anti‐TNF agents reduce response rates. Patients with IBD should be vaccinated against COVID‐19 at the earliest opportunity as recommended by International Advisory Committees, and vaccination should not be deferred because a patient is receiving immune‐modifying therapies. Antibody titres to COVID‐19 vaccines appear to be reduced in patients receiving anti‐TNF therapy, especially in combination with immunomodulators after one vaccination. Therefore, we should optimise any established risk factors that could impact response to vaccination in patients with IBD before vaccination.
Conclusions
Ideally, patients with IBD should be vaccinated at the earliest opportunity against COVID‐19. Patients should be in remission and, if possible, have their corticosteroid dose minimised before vaccination. Further research is required to determine the impact of different biologics on vaccine response to COVID‐19 and the potential for booster vaccines or heterologous prime‐boost vaccinations in the IBD population.
Vaccination for patients with Inflammatory Bowel Disease during the COVID‐19 pandemic.

1. INTRODUCTION
A novel coronavirus referred to as SARS‐CoV‐2 (severe acute respiratory syndrome coronavirus 21) was identified in late 2019 as the causative agent of a respiratory syndrome named coronavirus disease (COVID‐19) and has subsequently resulted in a worldwide pandemic. As of summer 2021, COVID‐19 has been confirmed in 184 324 026 people worldwide and has resulted in 3 992 680 deaths. 1 It is clear that risk factors such as older age, obesity and underlying conditions such as heart disease, diabetes and immune suppression can increase mortality. The Centres for Disease Control and Preventions (CDC) definition of immunocompromised individuals includes patients on prolonged courses of corticosteroids or other immunosuppressive medications, a group which includes a high proportion of patients with IBD. 2
The aim of the SECURE‐IBD database established during this current pandemic is to determine the risk of patients with IBD developing severe outcomes from COVID‐19. To date, 6328 cases of COVID‐19 have been reported in patients with IBD with 103 deaths. 3 From cases reported to SECURE‐IBD 15% of patients with IBD have been hospitalised and 3% have required ICU admission. 3 A recent meta‐analysis found that reassuringly the risk of contracting severe COVID‐19 in patients with IBD is not higher than the general population and the use of biologics may be associated with better outcomes for patients who contract COVID‐19. 4 Managing the risks of COVID‐19 in patients with IBD has been the subject of much effort. Given the development of numerous vaccines against COVID‐19, attention has turned to the role of vaccination as a key tool to manage the risks associated with COVID‐19.
Effective vaccines generate an immune response that mimics that induced by natural infection. Vaccinated individuals can produce large quantities of high‐affinity antibodies or effector T cells quickly, thus protecting them from severe disease if subsequently exposed to the pathogen. Vaccine‐induced protective immune responses are especially important in vulnerable cohorts especially those considered immunocompromised which include a sub‐cohort of patients with IBD. There is evidence that patients with IBD remain at significant risk of vaccine‐preventable infections, suggesting vaccines confer suboptimal protection in this cohort. 5 , 6
Several vaccines against COVID‐19 have recently been approved for use and are being deployed in widespread immunisation programmes. In this review article, we aim to address several key questions which will help inform our approach to COVID‐19 vaccination in patients with IBD. Firstly, we will discuss whether patients with IBD show altered vaccine responses and which disease characteristics contribute to modulating vaccine‐induced immunity. Secondly, we will review what can be learnt from the existing data on the impact of IBD therapies on response to vaccination by focusing on a number of established anti‐viral vaccines. Finally, we will examine how this informs our approach to the delivery of the current and upcoming COVID‐19 vaccines to maximise their impact in the IBD community.
2. METHODOLOGY
2.1. Study selection
A comprehensive literature search was conducted for relevant literature (published articles and abstracts) by performing a systematic search of two databases: PubMed and Cochrane Library CENTRAL. No restrictions were applied to language or publication date. Keywords used were “inflammatory bowel disease” or “crohn's disease” or “ulcerative colitis” and/or “vaccine response” or “Influenza” or “Hepatitis B” or “Varicella” or “COVID‐19 vaccination” or “vaccine uptake.” Current European and American guidelines on current vaccinations in patients with IBD and guidelines on vaccination against COVID‐19 infection were also reviewed. Eligible articles were reviewed and the quality was assessed by two independent reviewers.
2.2. Inclusion/exclusion criteria
Studies pertaining to or referencing the following topics were eligible for inclusion: (a) vaccine uptake in patients with IBD; (b) differences in the innate and adaptive immunity in patients with IBD; (c) vaccine response rates in patients with IBD; (d) COVID‐19 vaccines; (e) response rates to COVID‐19 vaccines in the IBD community. Case series or case reports were excluded due to high risk of publication bias. Studies that reported insufficient data on the outcomes of interest were also excluded.
3. DO PATIENTS WITH IBD HAVE SUBOPTIMAL RESPONSES TO VACCINATION?
IBD is characterised by chronic inflammation arising from an abnormal host immune response to dietary and microbial antigens. The pathogenesis of both Crohn's disease (CD) and ulcerative colitis (UC) is complex and is thought to be secondary to the interplay between genetic susceptibility, environmental factors and an altered gut microbiota leading to aberrant innate and adaptive immune responses. 7 , 8 Multiple immune pathways are dysregulated in both CD and UC. 7 , 8 There have been several reports suggesting IBD may arise from a fundamentally inadequate rather than excessive gut immune response with one study showing a defective neutrophil recruitment and bacterial clearance in patients with CD. 9
There is a body of evidence highlighting immune‐system dysfunction in patients with IBD. Toll‐like receptors (TLRs) and Nod‐like receptors (NLRs) are pathogen recognition receptors (PRRs) that alert the innate immune system to the presence of microbes by detecting conserved molecular patterns (eg bacterial lipopolysaccharide or viral nucleic acids). Ligation of TLRs/NLRs triggers innate immune responses and pro‐inflammatory cytokine production that drives the subsequent adaptive immune response. PRRs play a critical role in maintaining gut homeostasis, controlling immune responses along with shaping the microbiota. Patients with IBD exhibit differential expression of TLRs in comparison to healthy controls. 10 Mutations in NLRs have been identified in CD, with NOD2 mutations the most common mutation. 11 Vaccine formulations contain adjuvants that activate innate immunity via PRRs resulting in local inflammation at the injection site. Antigen‐presenting cells (APCs) traffic to the site of injection in response to these inflammatory signals and are enabled to process and present antigens and prime both the humoral and cellular arms of the adaptive immune response (Figure 1). Thus, the inherent defects in microbial sensing that underpin IBD pathogenesis may also impact a patient's response to vaccination.
FIGURE 1.
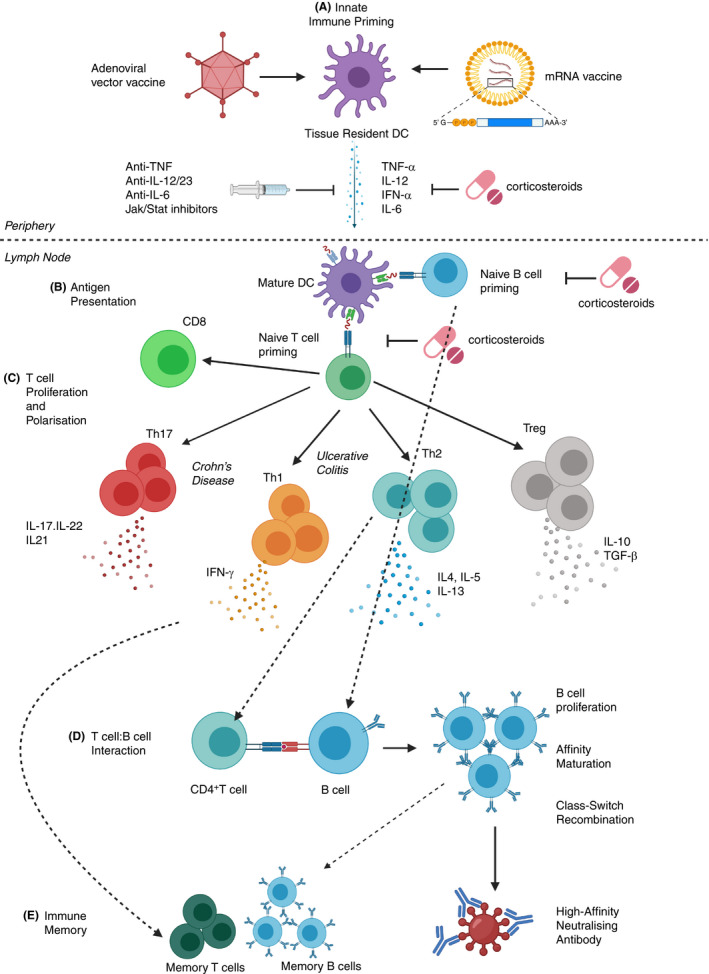
Impact of having IBD and IBD medications on the immune response to COVID‐19 vaccines. A, Innate immune priming: IBD is associated with SNPs in genes regulating the innate immune response (eg innate immune sensors such as TLRs), therefore tissue resident antigen presenting cells (eg DCs) in patients with IBD may respond differently to the vaccines. Inflammatory cytokines produced in response to the vaccines may be blunted by anti‐inflammatory medications (eg corticosteroids or biologic agents such as anti‐TNF). B, Antigen presentation: Mature DC migrate to the local LN where they present antigen to naïve CD4/CD8+ T and B lymphocytes, providing co‐stimulation and driving polarisation by secreting cytokines. IBD medications can limit antigen presentation. C, T cell proliferation and polarisation: Patients with CD tend to have immune responses polarised towards inflammatory Th1/Th17 cells, while patients with UC have a bias towards Th2 cells. D, CD4+ T cell: B cell interaction: Antigen specific CD4+ T cells interact with B cells providing co‐stimulation via CD40:CD40L interaction to drive B cell proliferation, affinity maturation and class switch recombination. T cell‐derived cytokines (eg IL‐4) are key to determining the antibody isotype and function. E, Immune Memory: Antigen‐specific T and B cell clones expanded due to vaccination should give rise to long‐lived memory cells. Patients with IBD frequently display an exhausted T cell phenotype (due to constant immune activation) and this may impact the phenotype and function of immune memory cells. Image created by BioRender.com.
Dendritic cells (DC) are an important population of APCs expressing high levels of PRRs. They respond to microbial signals, traffic to local lymph nodes where they process and present antigens to naïve T‐cells. Once in the lymph node, they upregulate co‐stimulatory molecules such as CD40/CD80/CD86 and secrete cytokines such as IL‐12 that are required for T‐cell polarisation. The plasmacytoid DC subset plays an important role in anti‐viral immunity as they are a potent source of type I Interferon (IFN). 12 Thus, DCs are key mediators of response to vaccination. Patients with IBD have significantly lower levels of circulating DC during disease flares compared to healthy controls. 11 Even patients with the inactive disease have shown reduced frequencies of circulating DC. 13 A significantly higher frequency of plasmacytoid DC in the inflamed colonic mucosa and mesenteric lymph nodes of IBD patients compared to healthy controls has also been reported. 14 It appears that in IBD especially when the disease is active, DC migrate from the bloodstream to the gut.
Macrophages are another population of APCs critical in the initiation of vaccine‐induced immunity and protection against viral infection. Macrophages have the ability to destroy virally infected cells and produce IFN. However, these effects are evident only if the virus is destroyed or contained by macrophages. If a virus replicates in macrophages, the infected macrophages may aid viral transmission. The permissiveness of macrophages for viral replication depends on factors including the age and host genetics. 15 In CD, macrophages are compromised and produce subnormal amounts of pro‐inflammatory cytokines. 16 Whether these defects in DC, macrophages and PRRs in patients with IBD could impact systemic immunogenicity and aspects such as a patient's response to vaccination is still unknown. One recent review of 14 590 patients with IBD reported an elevated risk of opportunistic infections (OI) but no increased risk was evident for patients on biologic therapy. 17 This observation supports the hypothesis that an immune‐system dysfunction in these patients may contribute to poorer vaccine response. Despite the indirect evidence outlined above, it remains the case that there is little direct evidence that patients with IBD, even if experiencing active disease, should be considered significantly immunosuppressed and therefore less likely to respond to vaccination. The statement from the ECCO guidelines on OI in IBD still, therefore, remains broadly true when it states “Patients with IBD should not be routinely considered to have altered immunocompetence.” 5 This topic should, however, remain a focus for investigation and efforts made to evaluate whether patients with IBD, not receiving systemic immunosuppressive therapies show any difference in the degree in initial response and durability of response to COVID‐19 vaccination.
4. ARE WE COMPLYING WITH CURRENT VACCINATION RECOMMENDATIONS FOR PATIENTS WITH IBD?
Expert recommendations promoting the efficacy and safety of vaccinations are widespread in the IBD literature. 5 , 18 , 19 , 20 Both ECCO and BSG guidelines advocate screening for OI and vaccinating where possible, prior to commencing immunomodulatory therapy. 5 , 18 Given 80% of patients will require corticosteroids, 40% thiopurines and 20% anti‐TNF therapy over their disease course, 5 , 16 the following vaccinations should be considered for patients with IBD: Varicella‐zoster, Human Papilloma Virus (HPV), Influenza (yearly), Hepatitis B (HBV) and Pneumococcal vaccines. 5
Despite current guidelines, multiple studies have shown suboptimal vaccination levels amongst patients with IBD. Reasons include lack of patient education and the importance of vaccination being overlooked by gastroenterologists or general practitioners. 21 , 22 , 23 , 24 , 25 One observational study found implementation of a screening and vaccination proforma significantly improved gastroenterologists’ compliance with vaccination guidelines. 26
From the patient's perspective, lack of awareness (49%) and fear of side effects (18%) are the most common reasons for not having the influenza vaccine. 6 Uptake of the COVID‐19 vaccine worldwide has been promising. Several studies have looked at attitudes to COVID‐19 vaccine uptake and reasons for vaccine hesitancy but none to date specifically in the IBD community. One study of 1000 people online in Ireland and the UK revealed 75% of participants intend to get a COVID‐19 vaccine, 11% said they would not be vaccinated and 14% were unsure regarding vaccination. Women and younger people were significantly less likely to report an intention to avail of a COVID‐19 vaccine. The survey revealed that peer influences are strongly associated with young women's intentions on vaccination. 27 A separate polish study questioned 1427 people on COVID‐19 vaccine uptake. Interesting predictors for acceptance of the vaccination included being talked through the importance of vaccination and potential side‐effects by a medical professional and suffering from chronic illnesses. Those who opted not to be vaccinated were most frequently concerned about the vaccine efficacy or side‐effects. 28 Both these studies highlight the importance and need for members of the IBD multidisciplinary team to inform and counsel our patients to ensure optimal uptake of COVID‐19 vaccines. Involvement of patient organisations is also necessary with clear and concise patient information available which clinicians can refer their patients to. 29
5. DO IBD THERAPIES REDUCE VACCINE RESPONSES IN PATIENTS WITH IBD?
Immunosuppressive treatment is a significant driver of the increased susceptibility to infection observed in patients with IBD. 5 One recent study reported a threefold increase of serious systemic viral infections in patients with IBD compared to the general population. The main risk factors for contracting infection were clinically active IBD and exposure to thiopurines. 30 Data on rates of immunogenicity to vaccines against COVID‐19 are limited in patients with IBD but we can extrapolate data from other vaccination programmes focusing mainly on vaccinations against viruses including influenza, HBV and Varicella‐zoster.
5.1. Influenza vaccine
Approximately 300 000‐650 000 people die worldwide from influenza each year. 31 The risk of contracting influenza and requiring hospitalisation is significantly greater in the IBD population, 32 therefore, yearly influenza vaccination is recommended. 5 , 18 , 19 The influenza vaccine comes in two forms, an inactivated vaccine and a live vaccine. The live vaccine is not recommended for use in immunocompromised patients. 5 , 18 , 19 Guidelines do not advise whether immunocompromised patients should receive standard dosage (SD) or high dosage (HD) of the trivalent inactivated influenza vaccine. One systematic review highlighted the fact that patients who received a HD vaccination had increased rates of seroconversion compared to those who received the SD in both immunocompromised individuals and adults aged 50‐64 years. 33
The emergence of the novel influenza A (H1N1) virus in 2009 stimulated research activity in the field of influenza vaccination. In the general population, serological protection rates of greater than 85% were reported with the H1N1 influenza vaccine. 34 , 35 However, rates of serological protection to influenza in patients with IBD tend to differ depending on treatment strategies. One study found influenza vaccine yielded high seroprotection rates in patients with IBD, however, patients receiving anti‐TNF treatment had lower rates of persistent seroprotection at 6 months post‐vaccination. 36 Cullen et al found serological protection rates against the H1N1 influenza vaccine in the IBD community was much less than that of the general population at only 50%. 37 Levels of seroprotection were significantly lower in patients receiving immunosuppression (glucocorticoids, immunomodulators or biologic treatments) compared with patients not on these drugs (44% versus 64%). 37 A prospective randomised control trial (RCT) examined serologic response to the inactivated trivalent influenza vaccine in patients receiving infliximab (IFX) and found despite patients mounting an initial immune response to vaccination, response rates ranged between 25% and 40%. 38 Interestingly, vaccine administration at the time of infusion, or between infusions, did not impact response. 38 Furthermore, a 2018 Japanese study assessed the immunogenicity of the quadrivalent influenza vaccine for patients with IBD on immunosuppression and found patients receiving IFX had lower seroprotection rates than those on 5‐ASA or azathioprine. 39
The addition of a booster vaccine does not appear to improve response rates in patients with IBD. 39 , 40 However, the HD quadrivalent influenza vaccine seems to improve immunogenicity in patients on immunosuppressive therapy. 41 , 42 One RCT found patients with IBD on anti‐TNF monotherapy receiving the HD influenza vaccine had significantly higher post‐immunisation antibody levels compared with SD vaccine, 41 with similar results seen in patients with rheumatoid arthritis on immunosuppressants. 42
Overall, the inactivated influenza vaccine is safe to administer to patients with IBD, including patients on immunosuppressants with no association with increased IBD activity. 43 , 44
Patients on immunosuppressants have reduced seroconversion rates compared to the general population. The ideal time to vaccinate patients is prior to starting immunosuppressive therapy where possible, to improve response rates. It is unclear whether patients on immunosuppressants would have higher response rates and be better protected with the HD vaccination protocol and this could be an area for further research if similar response rates are seen with the COVID‐19 vaccines.
5.2. Hepatitis B vaccination
The prevalence of HBV infection varies throughout the world, with <1% of the population of Northern Europe being infected. 45 ECCO and BSG guidelines recommend all patients should be screened for HBV at diagnosis of IBD to help expedite necessary vaccinations and reduce delays initiating therapy. 5 , 18
In the healthy, general population 10% of HBV vaccine recipients fail to mount an adequate antibody response. 46 Andrade et al 47 found patients receiving IFX, azathioprine or combination therapy had lower anti‐HBsAg levels indicating an inadequate vaccine response. A 2017 meta‐analysis found response rate to the HBV vaccine in patients with IBD, regardless of therapy was 61% 48 compared to 90% in the general population. 46 Younger patients and those vaccinated during remission had higher response rates. Use of immunosuppressive agents was associated with reduced rates of immunogenicity (Table 1). 48 Loras et al 49 found seroconversion rates against Hepatitis B were only 44% in adults with IBD on anti‐TNF therapy (Table 1). A second metanalysis evaluated the efficacy of the HBV vaccine in patients with IBD and found patients with IBD were significantly less likely to respond to the HBV vaccination compared with healthy controls. Overall, the pooled proportion of adequate response to the Hepatitis B vaccine in patients with IBD was 61% and the odds ratio of HBV response in patients with IBD was 0.13 (95% confidence interval 0.05‐0.33, P = 0.001). 50 Patients with IBD on immunosuppressants had significantly lower serological response rates to the HBV vaccine compared to the general population. 50
TABLE 1.
Impact of medications on seroprotection rates for Hepatitis B vaccination
| Medications | No immunosuppression (5‐asa or no medication) | Immunosuppression (Immunomodulator or biologic therapy) | Anti‐TNF therapy only |
|---|---|---|---|
| Seroprotection rates | 77% a | 52% a | 44% b |
Gisbert J et al Aliment Pharmacol Ther. 2012.
Loras C et al J Crohns Colitis. 2014.
The standard HBV vaccination of three doses is given at 0, 1 and 6 months with an accelerated schedule for “rapid protection” with dosing at 0, 1, 2 and 12 months. 51 A randomised prospective study found patients with IBD had a significantly higher response rate to the accelerated dosing schedule compared to standard dosing (75% vs 41%) (Table 2). 52 A recent RCT from Chaparro et al found a 4‐dose schedule was more effective than a 3‐dose regimen with significantly higher response rates for Hepatitis B vaccination in patients with IBD. As seen in other studies older age and treatment with immunomodulators or anti‐TNFs impaired response to vaccination. 53
TABLE 2.
Impact of accelerated dosing versus standard dosing on seroprotection rates for Hepatitis B
| Vaccine schedule (mo) | General population | IBD cohort | |
|---|---|---|---|
| Standard dosing | 0, 1, 6 (1.0 ml, 20 µg recombinant HBsAg) | >90% a | 41% b |
| Accelerated double dosing | 0, 1, 2, 12 (2 × 1.0 ml, 20 µg recombinant HBsAg) | >90% a | 75% b |
Kubba A et al Commun Dis Public Health. 2013.
Gisbert J et al Aliment Pharmacol Ther. 2012.
ECCO guidelines recommend all IBD patients receive an accelerated vaccination schedule using a double‐dose protocol, whilst the ACG guidelines recommend the standard vaccination schedule. 5 , 19 Once further data are available on response rates in patients with IBD to the COVID‐19 vaccines the use of accelerated or double‐dose vaccine schedules for sub‐cohorts of patient with IBD that have impaired response to the vaccine may be an option.
5.3. Varicella zoster vaccine
Varicella‐zoster virus (VZV) causes chickenpox and herpes zoster (shingles). In most European countries there is close to universal VZV seroconversion by late childhood. 54 Primary VZV infection is more severe in adults than children. 55 Patients with IBD on immunosuppression appear to be at increased risk of complications with primary varicella infection. 56 , 57 A retrospective review of 20 patients with IBD on immunosuppression found a 20% mortality from primary VZV infection, with three of these patients on corticosteroids at the time of infection. 58 A separate retrospective study found a strong association with the requirement of hospitalisation for primary VZV and IBD in a paediatric cohort. 59 Given the high risk of complications with primary varicella infection in patients with IBD, the ACG and ECCO recommend screening for prior exposure to varicella in all patients with IBD and vaccination if naïve. 5 , 19 The varicella vaccine is a live vaccine, therefore, cannot be given to patients receiving immunosuppressants. Both ECCO and the ACG recommends vaccination at least 3‐4weeks prior to commencing immunosuppressants. 5 , 19 In a systematic review of 40 observational studies in patients with immune‐mediated disorders (IBD n = 20 556) investigators found although seroconversion following the varicella vaccine was high, it was reduced by immunosuppressive therapies. 60
6. NOVEL VACCINES AGAINST COVID‐19
Vaccines against SARS‐CoV‐2 that elicit protective immune responses are crucial for the prevention and mitigation of the morbidity and mortality associated with severe COVID‐19. Various strategies have been employed to rapidly develop vaccines including standard inactivated virus vaccines, live attenuated vaccines, and newer technologies such as nucleic acid vaccines and viral‐vectored vaccines. To date, multiple vaccines against COVID‐19 have entered pre‐clinical and clinical trials. 61 The four lead vaccines to date available are two viral‐vector and two mRNA‐based vaccines. Here we will provide a short summary of each vaccine focusing on results from current trials and briefly discuss the current data on response rates in patients with IBD to the COVID‐19 vaccines and the gaps in knowledge regarding patients with IBD and vaccination.
6.1. Viral vector‐based vaccines
Viral‐vectored vaccines rely on the delivery of one or more antigens encoded in the context of an unrelated modified virus. Prior to the COVID‐19 pandemic only one viral‐vectored vaccine called Dengvaxia (Sanofi‐Pasteur), a recombinant Dengue vaccine has been licensed for human use. 62
Given the large amount of different viral vectors available and the vast knowledge gathered about their manipulation and function as immunogens, viral vector‐based vaccines represent a highly versatile platform for vaccine development. The viral vectors themselves are detected as foreign as they trigger PRRs and initiate innate immune responses, thus mimicking natural viral infection inducing potent immune responses. Strong antigen‐specific cellular and humoral immune responses against the target antigen can be induced by these vaccines (Figure 1). One study looking at the Canarypox‐virus vaccine vector ALVAC found this viral‐vector acts as an adjuvant through a mechanism requiring natural killer cells derived IFN‐γ, DC activation and chemokine secretion. 63 We have recently demonstrated that NK cells isolated from the blood of IBD patients produce markedly reduced levels of IFNγ and this suboptimal NK cell response may impact on the ability of patients with IBD to respond to this class of vaccine. 64
Two viral vector‐based vaccines against COVID‐19 have been approved to date.
AstraZeneca has developed a chimpanzee adenovirus‐vectored vaccine that encodes the spike glycoprotein of SARS‐CoV‐2 (ChAdOx1 nCoV‐19 vaccine). 65 Phase 1/2 trial showed the induction of humoral responses after the first dose of the vaccine and an additional increase in humoral immune outcomes after the second dose. 66 Subsequently a large, randomised placebo control phase 3 trial of the ChAdOx1 nCoV‐19 vaccine involving 23,848 adults reported this vaccine is highly effective in preventing COVID‐19. No hospitalisations or severe cases of COVID‐19 were reported in participants receiving the vaccine. There was a total of 131 COVID‐19 cases reported, 30 (0.5%) in the vaccinated group and 101 (1.7%) in the control group. 67 In this study overall vaccine efficacy was 70% which was statistically significant compared to placebo. No serious safety events related to the vaccine were reported. 67 The vaccine generated similarly robust immune responses against the SARS‐CoV‐2 virus across all age groups. 67 The UK Medicines and Healthcare products Regulatory Agency (MHRA) and EMA have provided authorisation for emergency supply of the ChAdOx1 nCoV‐19 vaccine. 68 , 69
Since the approval of the AstraZeneca vaccine, both EU and UK regulators have investigated reports of unusual blood clots after receiving the ChAdOx1 nCoV‐19 vaccine. The EMA’s investigating committee reviewed 62 cases of cerebral venous sinus thrombosis and 24 cases of splanchnic vein thrombosis reported in the EU’s drug safety database as of March 2021, 18 of which were fatal. At that point, around 25 million people in the EU and UK had received the AstraZeneca vaccine. The agency said that most cases occurred in women aged under 60 within two weeks of vaccination. 70 , 71 Overall, it was found 1 in 250 000 people with the AstraZeneca vaccine will develop blood clots with low platelets. However, the risk of developing a clot from COVID‐19 infection is much higher with a prevalence of 7.8% in one study for pulmonary embolism and 1.6% for a stroke. 72 The MHRA have advised offering an alternative vaccine where possible to those under 30 years of age given risk‐benefit calculation. 71 Both the EMA and the UK’s MHRA have advised that unusual blood clots with low blood platelets should be listed as a very rare side effect of the AstraZeneca vaccine but overall, the vaccine is very safe and effective. 70 , 71
The Janssen Pharmaceutical Companies of Johnson & Johnson have developed a viral vector‐based vaccine, the Ad26.COV2‐S vaccine, after preclinical studies demonstrated a single dose provides protection against SARS‐CoV‐2 infection in rhesus macaques. 73 Results from phase 1/2 trials found a single dose of this adenovirus serotype 26‐vectored vaccine induced strong neutralising antibody responses. 74 Given these promising results a randomised, double‐blind, placebo‐controlled, phase 3 trial called the ENSEMBLE trial of the replication‐defective Ad26.COV2‐S vaccine was initiated. 43 783 participants were recruited with 468 symptomatic cases of COVID‐19 identified during the study. Results reported Janssen's Ad26.COV2‐S vaccine was 66% effective in preventing moderate‐to‐severe COVID‐19, 28 days after vaccination. 75 The level of protection against moderate‐to‐severe COVID‐19 infection was 72% in the United States, 66% in Latin America and 57% in South Africa. 75 The ENSEMBLE trial was the first to include efficacy against the newly emerging strains of coronavirus. The Ad26.COV2‐S vaccine was 85% effective in preventing severe disease across all regions studied. 41% of participants had comorbidities associated with an increased risk for progression to severe COVID‐19. 75 Efficacy against severe disease appeared to increase over time with no reported COVID‐19 cases in vaccinated participants reported after day 49. 75 Overall, this vaccine was well tolerated. EUA of this vaccine has been approved by the EMA. 76 As seen with the AstraZeneca vaccine blood clots have been reported post‐vaccination with this vaccine. The EMA’s safety committee advised a warning about unusual blood clots with low blood platelets should be added to the product information for the Janssen COVID‐19 vaccine. Of seven cases, blood clots occurred mostly at unusual sites such cerebral venous sinus thrombosis or splanchnic vein thrombosis as seen with the AstraZeneca vaccine. 77
A third viral vector‐based vaccine called the Sputnik V vaccine is currently in phase 3 clinical trials. This vaccine is a human adenoviral vector‐based vaccine using a heterologous recombinant adenovirus approach using adenovirus 26 (Ad26) and adenovirus 5 (Ad5) as vectors. The use of two varying serotypes is intended to overcome any pre‐existing adenovirus immunity in the population. The second interim analysis (n = 21 977) for this trial of the Sputnik V vaccine reported an efficacy of 95% 21 days after the second dose. 78 So far, 78 confirmed cases of COVID‐19 have been identified with 62 cases in the placebo group and 12 in the vaccine group. Although EMA approval is still pending, the Sputnik V vaccine received approval from the Russian Ministry of Health in August 2020 and under emergency rules has been approved for use to vaccinate the population of Russia.
6.2. mRNA‐based vaccines
An alternative novel technology deployed for rapid COVID‐19 vaccine development involves nucleic acid vaccines. Nucleic acid‐based vaccine technologies employ either antigen encoding plasmid DNA or RNA, as messenger RNA or viral replicons. mRNA vaccines can induce both humoral and cellular immune responses, encode any antigen of choice and allow a high degree of adaptability. A major advantage of mRNA vaccines is they offer a flexible one‐for‐all large‐scale, rapid and cost‐effective manufacturing process. A variety of preclinical studies have demonstrated the ability of non‐replicating mRNA vaccines to induce immune responses and confer protection against pathogens with pandemic potential, such as Zika virus, Ebola virus and influenza. 79 , 80 , 81
The first mRNA vaccine to receive approval by both the EMA and FDA for EUA was the mRNA‐based COVID‐19 vaccine launched by Pfizer and BioNTech, BNT162b2. Early results from phase 1/2 trials found that these lipid nanoparticle‐formulated, nucleoside‐modified mRNA vaccines, elicited receptor‐binding domain‐specific neutralising IgG and antibodies. 82 Of two vaccine candidates, the BNT162b2 vaccine produced a higher T‐cell response and progressed to phase 3 clinical trials. 82 A total of 43 448 participants were recruited for this trial. 21 720 received BNT162b2 vaccine and 21 728 received placebo. Results demonstrated the BNT162b2 vaccine was 95% effective against COVID‐19 28 days after vaccination. In subgroup analysis, the observed efficacy of the vaccine in adults over 65 years was over 94%. 83 In this trial, 172 confirmed cases of COVID‐19 were observed in the placebo group vs 9 in the vaccine group. 83 No serious safety concerns were reported. The most commonly reported systemic events were fatigue and headache. The incidence of serious adverse events was low and was similar in the vaccine and placebo groups. 83 Although there were no reports of anaphylaxis in the clinical trial since approval severe allergy‐like reactions have been reported in at least 21 people who received the BNT162b2 vaccine. 84 It is thought this anaphylaxis may be due to polyethylene glycol that has been included in vaccine formulation as a stabiliser. 84 The FDA has advised individuals with severe allergic reactions to vaccines or ingredients in the vaccine should avoid this vaccine. 85 The UK MHRA advised individuals with a history of anaphylaxis to medicine or food not to receive the vaccine. 86
A second mRNA vaccine, the Moderna vaccine, completed a phase three trial called the COVE trial after promising results from phase 1/2 clinical trials. 87 Positive results from the phase 3 trial showed a vaccine efficacy against COVID‐19 of 94% and vaccine efficacy against severe COVID‐19 was 100%. 88 In this study, 7000 participants were over the age of 65 and over 5000 participants under the age of 65 had high‐risk chronic diseases. In total 42% of participants were defined as a medically high‐risk group. 88 One hundred and ninety‐six cases of COVID‐19 occurred, of which 30 cases were severe. All 30 cases occurred in the placebo group and none in the vaccinated group. 88 No serious safety concerns have been identified to date. The most common adverse reactions reported include injection site pain, fatigue, myalgia, arthralgia, headache, and erythema at the injection site. 88 EUA of this vaccine by the EMA has been granted.
In addition to these leading vaccines, numerous other potential COVID‐19 vaccines are in phase 3 clinical trial at present. 89 A summary of potential vaccines is summarised in Table 3 and we will hopefully see results for several other vaccines on the horizon using numerous different mechanisms of action in the next 12 months.
TABLE 3.
SARS‐CoV2 vaccines currently approved and/or in phase 3 clinical trials
| Vaccine developer | Vaccine candidate | Vaccine platform | Phase | Route of administration | Doses | Dosing schedule |
|---|---|---|---|---|---|---|
| Pfizer, BioNTech | BNT162b2 | RNA‐based vaccine |
Phase 2/3 Approved by EMA and FDA |
IM | 2 | Day 0 + 28 |
| Janssen Pharmaceutical | Ad26.COV2‐S | Viral vector |
Phase 3 Approved by FDA |
IM | 1‐2 | Day 0 or Day 0 + 56 |
| AstraZeneca, University of Oxford | AZD1222 | Viral vector |
Phase 3 Approved by EMA and FDA |
IM | 1‐2 | Day 0 + 28 |
| Moderna, NIAID | mRNA‐1273 | RNA‐based vaccine |
Phase 3 Approved by EMA and FDA |
IM | 2 | Day 0 + 28 |
| Gamaleya Research Institute | Sputnik V | Viral vector | Phase 3 | IM | 2 | Day 0 + 21 |
| CanSino Biological Inc | Ad5‐nCoV | Viral vector | Phase 3 | IM | 1 | Day 0 |
| Sinovac Biotech | Coronoa Vac | Inactivated virus | Phase 3 | IM | 2 | Day 0 + 14 |
| Sinopharm + Wuhan Institute of Biological Products | Vero cell | Inactivated virus | Phase 3 | IM | 2 | Day 0 + 21 |
| Sinopharm + Beijing Institute of Biological Products | Vero cell | Inactivated virus | Phase 3 | IM | 2 | Day 0 + 21 |
| Novavax | NVX‐CoV‐2373 | Protein subunit | Phase 3 | IM | 2 | Day 0 + 21 |
| Anhui Zhifei Longcom Biopharmaceutical | CHO Cell | Protein subunit | Phase 3 | IM | 2‐3 |
Day 0 + 28 ±56 |
| CureVac AG | CVnCoV | RNA‐based vaccine | Phase 2/3 | IM | 2 | Day 0 + 28 |
| Inovio Pharmaceuticals | INO‐4800 electroporation | DNA‐based vaccine | Phase 2/3 | ID | 2 | Day 0 + 28 |
| Bharat Biotech International Limited | BBV152 | Inactivated virus | Phase 3 | IM | 2 | Day 0 + 14 |
These up‐and‐coming vaccines have brought about hope and relief worldwide that there is a possible end in sight to the current pandemic however numerous questions remain unanswered. One key unanswered question is how long the vaccine's effectiveness will last which can only be answered with longitudinal observational studies. Vaccine effect can wane over time because of declining immunologic memory or changing antigenicity of the pathogen. A vaccination can be followed with booster doses to maintain a protective level of immunity among susceptible individuals, but the nature of the protection over time must be understood so that an effective vaccination and boosting schedule can be determined.
One possible option to improve immunogenicity to COVID‐19 especially in immunocompromised cohorts such as patients with IBD is mixing vaccines.
The main bottleneck in developing vaccines for intracellular infections is the ability to induce strong and long‐lasting cell‐mediated immunity. Stimulation of a functional CD8 response is often crucial in addition to a Th1‐type CD4 T cell response. Over the past decade, studies have shown that prime–boost immunisations can be given with unmatched vaccine delivery methods while using the same antigen, in a “heterologous” prime–boost format. In many cases, heterologous prime–boost can be more immunogenic than homologous prime–boost. 90 , 91 One study in humans looking at heterologous prime–boost immunisation schedules showed promising results. A DNA prime‐modified vaccinia virus Ankara (MVA) boost vaccine encoding thrombospondin‐related adhesion protein partially protected healthy malaria‐naive adults against Plasmodium falciparum sporozoite. 92 In a separate study conducted in calves, DNA prime with Ag85B, MPT64 and MPT83 antigens followed by a BCG boost was able to elicit higher immune responses and better protection than BCG alone against Mycobacterium bovis. 93 A heterologous onetime DNA prime and one‐time inactivated influenza vaccine boost was also found to be more immunogenic than twice administered homologous prime–boost using either DNA or inactivated influenza vaccine alone. 94 The use of heterologous prime–boost vaccination schedules is currently being looked at for the COVID‐19 vaccines and results are promising. In mice models following vaccination with a self‐amplifying RNA vaccine and an adenoviral vectored vaccine (ChAdOx1 nCoV‐19/AZD1222) against SARS‐CoV‐2 investigators found antibody response was higher in two‐dose heterologous vaccination regimens than single‐dose regimens. Interestingly, the cellular immune response after a heterologous regimen is dominated by cytotoxic T cells and Th1+ CD4 T cells, which is superior to the response induced in homologous vaccination regimens in mice. 95 In one small study in humans 26 individuals received a ChAdOx1 nCoV‐19 prime followed by a BNT162b2 boost after an 8‐week interval. Antibody titres increased significantly over time resulting in strong neutralisation titres 2 weeks after the BNT162b2 boost. Neutralising activity against the prevalent strain B.1.1.7 was 3.9‐fold higher than in individuals receiving homologous BNT162b2 vaccination, only 2‐fold reduced for variant of concern B.1.351, and similar for variant B.1.617. No adverse outcomes were noted. 96
A second key question is how effective are the current vaccines against the numerous new variants of COVID‐19 emerging? The COVID‐19 vaccines currently approved are expected to provide at least some protection against new virus variants because these vaccines elicit a broad immune response involving a range of antibodies and effector immune cells. Therefore, changes or mutations in the virus should not make vaccines completely ineffective. To date, multiple different variants of the COVID‐19 virus have been identified and the four main variants of concern are the alpha, beta, gamma and delta variants. The beta variant was first detected in South Africa and contains the E484K mutation that is thought to help the virus partially evade antibodies. Studies do suggest two doses of COVID‐19 vaccination offer strong protection against infection. One study looked at the effectiveness of the Pfizer/BioNTech vaccine to two strains of the beta variant in Qatar. This study found the vaccine was 89.5% effective against the B.1.1.7 variant of COVID‐19 and 75% against the B.1.351 variant. Overall vaccine effectiveness against severe COVID‐19 for either of these strains was 97%. 97 Janssens viral‐vector vaccine was also still in clinical trials when the beta strain emerged and vaccine effectiveness against severe COVID‐19 was robust with 82% efficacy at preventing severe disease. 75 Recently the delta variant has become the dominant variant of COVID‐19 virus. Reassuringly current vaccines are effective against this strain. After a full course vaccine effectiveness against the delta strain was 88% with the Pfizer/BioNTech vaccine and 67% with the AstraZeneca vaccine. 98 In the event that current vaccines prove to be less effective against one or more variants, it will be possible to change the composition of the vaccines to protect against these variants. WHO has recommended that all countries increase the sequencing of the COVID‐19 virus where possible to identify different variants. 99
For patients with IBD, one of the most pertinent questions is the efficacy of these new vaccines for patients on immunosuppressive medications. To date two studies have looked at response rates to the COVID‐19 vaccine in patients with IBD on immunosuppressants. The ICARUS study recently published looked at antibody response to the mRNA COVID‐19 vaccines (Pfizer and Moderna) in patients with IBD (n = 48) compared to a control group without IBD (n = 43). 100 There was no significant difference in anti‐Spike IgG levels between patients with IBD and the control group at any time points. 85% of patients were receiving biologic therapy at the time of vaccination, all on monotherapy. At the time of this study 33 patients had received one dose of the mRNA vaccine, 15 patients had received both vaccines and 3 patients with IBD had a history of previous COVID‐19 infection. All 15 patients with IBD who completed two‐dose vaccine schedules seroconverted. Although numbers were small investigators found patients treated with vedolizumab (n = 9) had no significant differences in index values for anti‐RBD IgG but had significantly lower anti‐S IgG levels compared to patient receiving anti‐TNF therapy (n = 5). Reassuringly in this study authors reported a 100% seroconversion rate to complete Pfizer‐BioNTech and Moderna mRNA COVID‐19 vaccines in IBD patients on biologic monotherapy with robust serological responses. 100 A second larger study from the UK called the CLARITY study compared antibody response rates post one dose of a COVID‐19 vaccine. This was a large multicentre study including patients from 92 hospitals. 101 Patients with IBD were vaccinated with either the viral vector AstraZeneca vaccine or the mRNA Pfizer/BioNTech vaccine. Patients included were either receiving vedolizumab (n = 428) or IFX (n = 865) at the time of vaccination. Investigators found mean antibody concentrations were lower in patients treated with IFX than vedolizumab both with the mRNA vaccine and viral‐vector vaccine (6.0 U/ml vs 28.8 U/ml). 100 Amongst patients receiving IFX mean antibody concentrations were lower if patients were on concomitant immunomodulators. On multivariate analysis, age over 60 years, immunomodulator use, non‐white ethnicity and smoking were independently associated with lower antibody concentrations for either vaccine. Seroconversion rates varied significantly between patients treated with IFX and vedolizumab after one vaccine dose. The lowest rates of seroconversion were observed in participants treated with IFX in combination with an immunomodulator for both the Pfizer (27%) and AstraZeneca (20.2%) vaccines. Highest rates of seroconversion were seen in patients treated with vedolizumab monotherapy. 101 A smaller subset of patients (n = 27) had completed the two‐dose vaccination schedule with seroconversion rates of 86% for those on IFX and 86% for those on vedolizumab. In both IFX and vedolizumab treated patient's antibody levels and seroconversion rates were higher after two doses than after one primary vaccine. Seroconversion rates were also higher in patients with IBD who received one vaccination but had a previous history of COVID‐19 infection. 82% of patients with previous COVID‐19 infection treated with IFX seroconverted and 97% treated with vedolizumab seroconverted after one vaccine dose. 101 Overall to date the CLARITY study is the largest study looking at antibody response to the COVID‐19 vaccine in patients with IBD. This study showed anti‐SARS‐CoV‐2 spike antibody levels and rates of seroconversion are lower following vaccination with a single‐dose of either COVID‐19 vaccine in patients with IBD treated with IFX compared with vedolizumab. Combination therapy with an immunomodulator further reduced immunogenicity to both vaccines in IFX‐treated patients. From both the CLARITY and ICARUS studies we can see overall either by vaccination after infection or a second dose of vaccine with either the mRNA or viral vector COVID‐19 vaccines rates of seroconversion are high in patients with IBD. 100 , 101 Both the mRNA and viral vector vaccines appear to induce similar rates of immunity with neither appearing to be more effective than the other in patients with IBD on immunosuppressants. 101 Delayed second dosing of the COVID‐19 vaccine should be avoided in patients treated with IFX. 101 A separate study by Ehmsen et al investigated the impact of cancer on antibody response to the COVID‐19 vaccines and found antibody titres rapidly decreased from 36 days to 3‐month for most patients with cancer, resulting in seroconversion of approximately 10% of the seropositive to seronegative, most prominently for patients with haematologic cancer. 102 For patients with haematologic cancer, seronegativity was significantly associated with certain diagnoses, remission statuses, and treatments, but the lack of T cell responses was only significantly associated with steroid use. 102 Further research is required to confirm the results of the above studies in patients with IBD and better understand whether alterations in the innate immune response in patients with IBD impact vaccine response or whether the impact of different medications such as different biologic therapies or immunomodulators impacts the adaptive immune response and vaccine efficacy. Once the impact of IBD itself, different disease‐related factors and medications are determined on vaccine response, observational studies will help determine if manipulation of the timing of biologic therapies in relation to vaccination or use of booster vaccines or heterologous prime–boost vaccination schedules could improve antibody response in sub‐cohorts of patients with IBD.
Overall, to date we can be guided by advice provided by the International Organisation for the Study of Inflammatory Bowel Disease and the COVID‐19 ECCO taskforce both of which advise patients with IBD should be vaccinated against SARS‐CoV‐2 at the earliest opportunity possible and vaccination should not be deferred because a patient with IBD is receiving immune‐modifying therapies. 103 , 104 , 105 Although data are minimal, the ECCO Taskforce cautiously recommends to use the mRNA vaccine to vaccinate IBD patients on immunomodulatory medication since the vaccine's efficacy to protect against the mild and severe disease was shown to be higher for mRNA vaccines (94%‐95%) compared to the viral vector‐vaccines, where the mild disease still occurs in about 30%‐40% of the vaccinated persons. 104
7. CONCLUSION
From observations related to the use of established vaccines in patients with IBD, we can conclude that patients with IBD tend to have poorer vaccine‐induced immunity than the general population. The use of immunosuppressants and disease activity are both implicated in causing lower rates of seroconversion. For certain vaccines including the hepatitis B vaccine and influenza vaccines accelerated protocolls and higher dosing have shown potential to improve the immunity achieved for patient with IBD. To date, data are limited in the IBD population on response rates to the COVID‐19 vaccine but similar issues seem to be arising as seen with established vaccines. There is clearly a requirement for large scale collaborative research efforts to gather larger datasets on the response rates to different vaccines amongst patients with IBD and examine the impact of both disease activity and different IBD therapies on the immunity generated by vaccination. It is recommended all patients with IBD can be vaccinated against COVID‐19 with the currently available vaccines. To improve immunogenicity, it seems prudent to take a few elementary precautions prior to patients being vaccinated; patients should ideally be in disease remission and if possible, corticosteroids doses should be minimised. Preferably, the vaccination should be given prior to newly commencing potentially immunosuppressant medications where possible (though IBD treatments should not be unduly delayed to allow vaccination). Research into the benefits of double dose vaccines, additional booster dosing or use of heterologous prime–boost vaccination schedules for immunosuppressed patients’ needs to be considered. Finally, the potential for short drug holidays (with oral agents), vaccination at the trough level for biologic agents or antibody testing in vulnerable cohorts may be an area that would benefit from prospective evaluation.
AUTHORSHIP
Guarantor of the article: Jayne Doherty.
Author contributors: Authors contributions as submitted on original submission are correct. No funding was required for this review article. All authors have reviewed and approve the final version of this paper.
ACKNOWLEDGEMENT
Declaration of personal interest: None.
Doherty J, Fennessy S, Stack R, et al. Review Article: vaccination for patients with inflammatory bowel disease during the COVID‐19 pandemic. Aliment Pharmacol Ther. 2021;54:1110–1123. 10.1111/apt.16590
The Handling Editor for this article was Dr Mike Burkitt, and this uncommissioned review was accepted for publication after full peer‐review.
Funding information
The authors have no funding related to this work to disclose.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Coronavirus disease (COVID‐19) – World Health Organization. World Health Organisation. 2021. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019. Accessed July 7, 2021. [Google Scholar]
- 2. COVID‐19 and Your Health. Centres for Disease Control and Prevention. 2019. https://www.cdc.gov/coronavirus/2019‐ncov/need‐extra‐precautions/people‐with‐medical‐conditions.html. Accessed January 3, 2021. [Google Scholar]
- 3. Brenner EJ, Ungaro RC, Colombel JF & Kappelman MD. SECURE‐IBD Database Public Data Update. 2021. https://covidibd.org/current‐data/. Accessed July 7, 2021.
- 4. Singh AK, Jena A, Kumar‐M P, Sharma V, Sebastian S. Risk and outcomes of coronavirus disease in patients with inflammatory bowel disease: a systematic review and meta‐analysis. United European Gastroenterol J. 2021;9:159‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rahier Jf, Magro F, Abreu C, et al. Second European evidence‐based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443‐468. [DOI] [PubMed] [Google Scholar]
- 6. Melmed GY, Ippoliti AF, Papadakis KA, et al. Patients with inflammatory bowel disease are at risk for vaccine‐preventable illnesses. Am J Gastroenterol. 2006;101:1834‐1840. [DOI] [PubMed] [Google Scholar]
- 7. Torres J, Mehandru S, Colombel J‐F, Peyrin‐Biroulet L. Crohn's disease. Lancet. 2017;389:1741‐1755. [DOI] [PubMed] [Google Scholar]
- 8. Ungaro R, Mehandru S, Allen PB, Peyrin‐Biroulet L, Colombel J‐F. Ulcerative colitis. Lancet. 2017;389:1756‐1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marks DJ, Harbord MW, MacAllister R, et al. Defective acute inflammation in Crohn's disease: a clinical investigation. Lancet. 2006;25:668‐678. [DOI] [PubMed] [Google Scholar]
- 10. Lu Y, Li X, Liu S, Zhang Y, Zhang D. Toll‐like receptors and inflammatory bowel disease. Front Immunol. 2018;9:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3‐10. [DOI] [PubMed] [Google Scholar]
- 12. Ye Y, Gaugler B, Mohty M, Malard F. Plasmacytoid dendritic cell biology and its role in immune‐mediated diseases. Clin Transl Immunology. 2020;9:e1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baumgart DC, Metzke D, Scheffold J, et al. Patients with active inflammatory bowel disease lack immature peripheral blood plasmacytoid and myeloid dendritic cells. Gut. 2005;54:228‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baumgart DC, Metzke D, Guckelberger O, et al. Aberrant plasmacytoid dendritic cell distribution and function in patients with Crohn's disease and ulcerative colitis. Clin Exp Immunol. 2011;166:46‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klimpel GR. Immune defenses. In: Baron S, ed. Medical microbiology. 4th edition. University of Texas Medical Branch at Galveston: 1996. Chapter 50. https://www.ncbi.nlm.nih.gov/books/NBK8423/ [PubMed] [Google Scholar]
- 16. Segal AW. Studies on patients establish Crohn's disease as a manifestation of impaired innate immunity. J Intern Med. 2019;286:373‐388. [DOI] [PubMed] [Google Scholar]
- 17. Bonovas S, Fiorino G, Allocca M, et al. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: a systematic review and network meta‐analysis. Clin Gatroenterol Hepatol. 2016;14:1385‐1397. [DOI] [PubMed] [Google Scholar]
- 18. Lamb CA, Kennedy NA, Raine T, et al; IBD guidelines eDelphi consensus group . British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1‐s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Farraye F, Melmed GY, Lichtenstein GR, Kane SV. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017;112:241‐258. [DOI] [PubMed] [Google Scholar]
- 20. Rubin LG, Levin MJ, Ljungman P, et al; Infectious Disease Society of America . IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2013;2014:309‐318. [DOI] [PubMed] [Google Scholar]
- 21. Selby L, Kane S, Wilson J, et al. Receipt of preventive health services by IBD patients is significantly lower than by primary care patients. Inflamm Bowel Dis. 2008;14:253‐258. [DOI] [PubMed] [Google Scholar]
- 22. Desalermos AP, Farraye FA, Wasan SK. Vaccinating the inflammatory bowel disease patient. Expert Rev Gastroenterol Hepatol. 2015;9:91‐102. [DOI] [PubMed] [Google Scholar]
- 23. Gurtej Malhi AR, Thanabalan R, Croitoru K, Silverberg MS, Hillary Steinhart A, Nguyen GC. Vaccination in inflammatory bowel disease patients: attitudes, knowledge, and uptake. J Crohns Colitis. 2015;9:439‐444. [DOI] [PubMed] [Google Scholar]
- 24. Yeung JH, Goodman KJ, Fedorak RN. Inadequate knowledge of immunization guidelines: a missed opportunity for preventing infection in immunocompromised IBD patients. Inflamm Bowel Dis. 2012;18:34‐40. [DOI] [PubMed] [Google Scholar]
- 25. Malhi G, Rumman A, Thanabalan R, et al. Vaccination in inflammatory bowel disease patients: attitudes, knowledge, and uptake. J Crohns Colitis. 2015;9:439‐444. [DOI] [PubMed] [Google Scholar]
- 26. Aj W, Weltman M, Burger D, et al. Implementing guidelines on the prevention of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2013;7:449‐456. [DOI] [PubMed] [Google Scholar]
- 27. NUI Galway Researcher Sheds New Light on Vaccine Hesticancy in Ireland and the UK. 2021. https://www.nuigalway.ie/about‐us/news‐and‐events/news‐archive/2021/may/research‐sheds‐new‐light‐on‐vaccine‐hesitancy‐in‐ireland‐and‐the‐uk.html. Accessed June 7, 2021.
- 28. Malesza M, Bozym M. Factors influencing COVID‐19 vaccination uptake in an elderly sample in Poland. medRxiv. 10.1101/2021.03.21.21254047 [DOI] [Google Scholar]
- 29. Coronavirus vaccine for people with Crohn’s or Colitis. Crohn’s and Colitis UK, 2021. https://www.crohnsandcolitis.org.uk/news/latest‐coronavirus‐vaccine‐for‐people‐with‐crohns‐or‐colitis. Accessed January 1, 2021.
- 30. Wisniewski A, Kirchgesner J, Seksik PH, et al; the Saint‐Antoine IBD Network . Increased incidence of systemic serious viral infections in patients with inflammatory bowel disease associates with active disease and use of thiopurines. United Eur Gastroenterol J. 2020;8:303‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza‐associated respiratory mortality: a modelling study. Lancet. 2018;391:1285‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tinsley A, Navabi S, Williams E, et al. Increased risk of influenza and influenza‐related complications among 140,480 patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:369‐376. [DOI] [PubMed] [Google Scholar]
- 33. Samson S, Leventhal P, Salamand C, et al. Immunogenicity of high‐dose trivalent inactivated influenza vaccine: a systematic review and meta‐analysis. Expert Rev Vaccines. 2019;18:295‐308. [DOI] [PubMed] [Google Scholar]
- 34. Greenberg ME, Lai MH, Hartel GF, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361:2405‐2413. [DOI] [PubMed] [Google Scholar]
- 35. Plennevaux P, Sheldon E, Blatter M, Reeves‐Hoche M, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet. 2010;375:41‐48. [DOI] [PubMed] [Google Scholar]
- 36. Launay O, Abitbol V, Krivine A, et al; MICIVAX Study Group . Immunogenicity and safety of influenza vaccine in inflammatory bowel disease patients treated or not with immunomodulators and/or biologics: a two‐year prospective study. J Crohns Colitis. 2015;9:1096‐1107. [DOI] [PubMed] [Google Scholar]
- 37. Cullen G, Bader C, Korzenik J, Sands B. Serological response to the 2009 H1N1 influenza vaccination in patients with inflammatory bowel disease. Gut. 2012;61:385‐391. [DOI] [PubMed] [Google Scholar]
- 38. deBruyn J, Fonseca K, Ghosh S, et al. Immunogenicity of influenza vaccine for patients with inflammatory bowel disease on maintenance infliximab therapy: a randomized trial. Inflamm Bowel Dis. 2016;22:638‐647. [DOI] [PubMed] [Google Scholar]
- 39. Shirai S, Hara M, Sakata Y, et al. Immunogenicity of quadrivalent influenza vaccine for patients with inflammatory bowel disease undergoing immunosuppressive therapy. Inflamm Bowel Dis. 2018;24:1082‐1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matsumoto H, Ohfuji S, Kea W. Booster influenza vaccination does not improve immune response in adult inflammatory bowel disease patients treated with immunosuppressives: a randomized controlled trial. J Gastroenterol. 2015;50:876‐886. [DOI] [PubMed] [Google Scholar]
- 41. Caldera F, Hillman L, Saha S, et al. Immunogenicity of high dose influenza vaccine for patients with inflammatory bowel disease on anti‐TNF monotherapy: a randomized clinical trial. Inflamm Bowel Dis. 2020;26:593‐602. [DOI] [PubMed] [Google Scholar]
- 42. Colmegna I, Useche ML, Rodriguez K, et al. Immunogenicity and safety of high‐dose versus standard‐dose inactivated influenza vaccine in rheumatoid arthritis patients: a randomised, double‐blind, active‐comparator trial. Lancet Rheum. 2020;2:e14–e23. 10.1016/s2665-9913(19)30094-3 [DOI] [PubMed] [Google Scholar]
- 43. Mamula P, Markowitz J, Piccoli D, Klimov A, Cohen L, Baldassano RN. Immune response to influenza vaccine in pediatric patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:851‐856. [DOI] [PubMed] [Google Scholar]
- 44. Lu Y, Jacobson D, Ashworth L, et al. Immune response to influenza vaccine in children with inflammatory bowel disease. Am J Gastroenterol. 2009;104:444‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Epidemiological assessment of hepatitis B and C among migrants in the EU/EEA. European Centre for Disease Control and Prevention. 2016. https://www.ecdc.europa.eu/en/publications‐data/epidemiological‐assessment‐hepatitis‐b‐and‐c‐among‐migrants‐eueea. Accessed January 11, 2021. [Google Scholar]
- 46. Kubba A, Taylor P, Greneek B, Strobel S. Non‐responders to hepatitis B vaccination: a review. Commun Dis Public Health. 2003;6:106‐112. [PubMed] [Google Scholar]
- 47. Andrade P, Santos‐Antunes J, Rodrigues S, Lopes S, Macedo G. Treatment with infliximab or azathioprine negatively impact the efficacy of hepatitis B vaccine in inflammatory bowel disease patients. J Gastroenterol Hepatol. 2015;30:1591‐1595. [DOI] [PubMed] [Google Scholar]
- 48. Jiang H‐Y, Wang S‐Y, Deng M, et al. Immune response to hepatitis B vaccination among people with inflammatory bowel diseases: a systematic review and meta‐analysis. Vaccine. 2017;35:2633‐2641. [DOI] [PubMed] [Google Scholar]
- 49. Loras C, Gisbert JP, Saro MC, et al; REPENTINA Study, GETECCU Group . Impact of surveillance of hepatitis b and hepatitis c in patients with inflammatory bowel disease under anti‐TNF therapies: Multicenter prospective observational study (REPENTINA 3). J Crohns Colitis. 2014;8:1529‐1538. [DOI] [PubMed] [Google Scholar]
- 50. Kochhar GS, Mohan BP, Khan SR, et al. Hepatitis‐B vaccine response in inflammatory bowel disease patients: A systematic review and meta‐analysis. Inflam Bowel Dis. 2021; 10.1093/ibd/izaa353 [DOI] [PubMed] [Google Scholar]
- 51. Hepatitis B—Vaccine Introduced to Primary Schedule. Chapter 9. Health Service Excecutive. 2008. https://www.hse.ie/eng/health/immunisation/hcpinfo/guidelines/chapter9.pdf [Google Scholar]
- 52. Gisbert J, Menchen L, Garcia‐Sanchez V, Marin I, Villagrasa J, Chaparro M. Comparison of the effectiveness of two protocols for vaccination (standard and double dosage) against hepatitis B virus in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:1379‐1385. [DOI] [PubMed] [Google Scholar]
- 53. Chaparro M, Gordillo J, Domènech E, et al. Fendrix vs engerix‐B for primo‐vaccination against hepatitis B infection in patients with inflammatory bowel disease: a randomized clinical trial. Am J Gastroenterol. 2020;115:1802‐1811. [DOI] [PubMed] [Google Scholar]
- 54. Bollaerts K, Riera‐Montes M, Heininger U, et al. A systematic review of varicella seroprevalence in European countries before universal childhood immunization: deriving incidence from seroprevalence data. Epidemiol Infect. 2017;145:2666‐2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Arbeter AM. Clinical trials of varicella vaccine in healthy adolescents and adults. Infect Dis Clin North Am. 1996;10:609‐615. [DOI] [PubMed] [Google Scholar]
- 56. Leung VS, Nguyen MT, Bush TM. Disseminated primary varicella after initiation of infliximab for Crohn’s disease. Am J Gastroenterol. 2004;99:2503‐2504. [DOI] [PubMed] [Google Scholar]
- 57. Deutsch DE, Olson AD, Kraker S, Dickinson CJ. Overwhelming varicella pneumonia in a patient with Crohn’s disease treated with 6‐mercaptopurine. J Pediatr Gastroenterol Nutr. 1995;20:351‐353. [DOI] [PubMed] [Google Scholar]
- 58. Cullen G, Baden RP, Cheifetz AS. Varicella zoster virus infection in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2392‐2403. [DOI] [PubMed] [Google Scholar]
- 59. Adams DJ, Nylund CM. Hospitalization for varicella and zoster in children with inflammatory bowel disease. J Pediatr. 2016;171:140‐145. [DOI] [PubMed] [Google Scholar]
- 60. Croce E, Hatz C, Jonker EF, Visser LG, Jaeger VK, Buhler S. Safety of live vaccinations on immunosuppressive therapy in patients with immune‐mediated inflammatory diseases, solid organ transplantation or after bone‐marrow transplantation—a systematic review of randomized trials, observational studies and case reports. Vaccine. 2017;35:1216‐1226. [DOI] [PubMed] [Google Scholar]
- 61. Rauch S, Jasny E, Schmidt KE, Petsch B. New vaccine technologies to combat outbreak situations. Front Immunol. 2018;9: 10.3389/fimmu.2018.01963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dengue Vaccine | Dengue | CDC. Centre for Disease Control. 2019. https://www.cdc.gov/dengue/prevention/dengue‐vaccine.html. Accessed December 4, 2020. [Google Scholar]
- 63. Ryan EJ, Harenberg A, Burdin N. The Canarypox‐virus vaccine vector ALVAC triggers the release of IFN‐gamma by natural killer (NK) cells enhancing Th1 polarization. Vaccine. 2007;25:3380‐3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zaiatz Bittencourt V, Jones F, Tosetto M, Doherty GA, Ryan EJ. Dysregulation of metabolic pathways in circulating natural killer cells isolated from inflammatory bowel disease patients. J Crohns Colitis. 2021;15:1316‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. van Doremalen N, Lambe T, Spencer A, et al. ChAdOx1 nCoV‐19 vaccination prevents SARS‐CoV‐2 pneumonia in rhesus macaques. BioRxiv. 2020. 10.1101/2020.05.13.093195. Published 2020 May 13. [DOI] [Google Scholar]
- 66. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet. 2020;396:467‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Knoll MD, Wonodi C. Oxford–AstraZeneca COVID‐19 vaccine efficacy. The Lancet. 2021;397:72–74. 10.1016/s0140-6736(20)32623-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Oxford University/AstraZeneca COVID‐19 vaccine approved. Medicines and Healthcare products Regulatory Agency. 2021. https://www.gov.uk/government/news/oxford‐universityastrazeneca‐covid‐19‐vaccine‐approved. Accessed January 5, 2021. [Google Scholar]
- 69. EMA recommends COVID‐19 Vaccine AstraZeneca for authorisation in the EU. European Medicines Agency. 2021. https://www.ema.europa.eu/en/news/ema‐recommends‐covid‐19‐vaccine‐astrazeneca‐authorisation‐eu. Accessed February 7, 2021. [Google Scholar]
- 70. AstraZeneca vaccine: blood clots are “extremely rare” and benefits outweigh risks, regulators conclude. BMJ. 2021;373:n931. [DOI] [PubMed] [Google Scholar]
- 71. AstraZeneca’s COVID‐19 Vaccine: EMA Finds Possible Link to Very Rare Cases of Unusual Blood Clots with Low Blood Platelets. European Medicines Agency (europa.eu). [Google Scholar]
- 72. Tan BK, Mainbourg S, Friggeri A, et al. Arterial and venous thromboembolism in COVID‐19: a study‐level meta‐analysis. Thorax. 2021; thoraxjnl. –2020. 10.1136/thoraxjnl-2020-215383 [DOI] [PubMed] [Google Scholar]
- 73. Mercado NB, Zahn R, Wegmann F, et al. Single‐shot Ad26 vaccine protects against SARS‐CoV‐2 in rhesus macaques. Nature. 2020;586:583‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid‐19 vaccine. N Engl J Med. 2021;384:1824‐1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Johnson & Johnson Announces Single‐Shot Janssen COVID‐19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial. Johnson & Johnson. 2021. https://www.jnj.com/johnson‐johnson‐announces‐single‐shot‐janssen‐covid‐19‐vaccine‐candidate‐met‐primary‐endpoints‐in‐interim‐analysis‐of‐its‐phase‐3‐ensemble‐trial. Accessed February 6, 2021. [Google Scholar]
- 76. EMA recommends COVID‐19 Vaccine Janssen for authorisation in the EU. European Medicines Agency (europa.eu). 2021. https://www.ema.europa.eu/en/news/ema‐recommends‐covid‐19‐vaccine‐janssen‐authorisation‐eu [Google Scholar]
- 77. COVID‐19 Vaccine Janssen: EMA finds possible link to very rare cases of unusual blood clots with low platelets. European Medicines Agency (europa.eu). 2021. https://www.ema.europa.eu/en/news/covid‐19‐vaccine‐janssen‐ema‐finds‐possible‐link‐very‐rare‐cases‐unusual‐blood‐clots‐low‐blood#:~:text=At%20its%20meeting%20of%2020,side%20effects%20of%20the%20vaccine. [Google Scholar]
- 78. Sputnik V . Second interim analysis of clinical trial data showed a 91.4% efficacy for the Sputnik V vaccine on day 28 after the first dose; vaccine efficacy is over 95% 42 days after the first dose. Sputnikvaccine.com. 2020. https://sputnikvaccine.com/newsroom/pressreleases/second‐interim‐analysis‐of‐clinical‐trial‐data‐showed‐a‐91‐4‐efficacy‐for‐the‐sputnik‐v‐vaccine‐on‐d/. Accessed January 5, 2021.
- 79. Pardi N, Hogan MJ, Pelc RS, et al. Zika virus protection by a single low‐dose nucleoside modified mRNA vaccination. Nature. 2017;543:248‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Meyer M, Huang E, Yuzhakov O, Ramanathan P, Ciaramella G, Bukreyev A. Modified mRNA‐based vaccines elicit robust immune responses and protect guinea pigs from Ebola virus disease. J Infect Dis. 2018;217:451‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Petsch B, Schnee M, Vogel AB, et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat Biotechnol. 2012;30:1210‐1216. [DOI] [PubMed] [Google Scholar]
- 82. Mulligan MJ, Lyke KE, Kitchin N, et al. Phase 1/2 study of COVID‐19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589‐593. [DOI] [PubMed] [Google Scholar]
- 83. Polack FP, Thomas SJ, Kitchin N, et al; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer‐BioNTech COVID‐19 vaacine—Centre for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2021;70:46‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fact Sheet for Recipients and Caregivers about comirnaty COVID‐19 Vaccine, mRNA) and Pfizer‐Biontech COVID‐19 Vaccine to Prevent Coronavirus Disease 2019 (COVID‐19). FDA. 2021. https://www.fda.gov/media/144414/download. Accessed January 5, 2021. [Google Scholar]
- 86. Medicines and Healthcare products Regulatory Agency . Confirmation of guidance to vaccination centres on managing allergic reactions following COVID‐19 vaccination with the Pfizer/BioNTech vaccine. GOV.UK. 2020. https://www.gov.uk/government/news/confirmation‐of‐guidance‐to‐vaccination‐centres‐on‐managing‐allergic‐reactions‐following‐covid‐19‐vaccination‐with‐the‐pfizer‐biontech‐vaccine. Accessed January 5, 2021. [Google Scholar]
- 87. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS‐CoV‐2—preliminary report. N Engl J Med. 2020;383:1920‐1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Moderna announces primary efficacy analysis in phase 3 COVE study for its COVID‐19 Vaccine candidate and filing today with U.S FDA for emergency use Authorization. Moderna. 2021. https://investors.modernatx.com/news‐releases/news‐release‐details/moderna‐announces‐primary‐efficacy‐analysis‐phase‐3‐cove‐study. Accessed January 5, 2021. [Google Scholar]
- 89. COVID‐19 vaccine tracker and landscape. WHO. 2021. https://www.who.int/publications/m/item/draftlandscape‐of‐covid‐19‐candidate‐vaccines. Accessed January 5, 2021. [Google Scholar]
- 90. Hu SL, Klaniecki J, Dykers T, Sridhar P, Travis BM. Neutralizing antibodies against HIV‐1 BRU and SF2 isolates generated in mice immunized with recombinant vaccinia virus expressing HIV‐1 (BRU) envelope glycoproteins and boosted with homologous gp160. AIDS Res Hum Retroviruses. 1991;7:615‐620. [DOI] [PubMed] [Google Scholar]
- 91. Girard M, Kieny MP, Pinter A, et al. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc Natl Acad Sci U S A. 1991;88:542‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dunachie SJ, Walther M, Epstein JE, et al. A DNA prime‐modified vaccinia virus ankara boost vaccine encoding thrombospondin‐related adhesion protein but not circumsporozoite protein partially protects healthy malarianaive adults against Plasmodium falciparum sporozoite challenge. Infect Immun. 2006;74:5933‐5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cai H, Yu DH, Hu XD, Li SX, Zhu YX. A combined DNA vaccine‐prime, BCG‐boost strategy results in better protection against Mycobacterium bovis challenge. DNA Cell Biol. 2006;25:438‐447. [DOI] [PubMed] [Google Scholar]
- 94. Wang S, Parker C, Taaffe J, et al. Heterologous HA DNA vaccine prime—inactivated influenza vaccine boost is more effective than using DNA or inactivated vaccine alone in eliciting antibody responses against H1 or H3 serotype influenza viruses. Vaccine. 2008;26:3626–3633. 10.1016/j.vaccine.2008.04.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Spencer AJ, McKay PF, Belij‐Rammerstorfer S, et al. Heterologous vaccination regimens with self‐amplifying RNA and adenoviral COVID vaccines induce robust immune responses in mice. Nat Commun. 2021;12:2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Grob R, Zanoni M, Seidel A, et al. Heterologous ChAdOxl nCov‐19 and BNT162b2 prime‐boost vaccination elicits potent neutralizing antibody responses and T cell reactivity. medRxiv. 10.1101/2021.05.30.21257971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Abu‐Raddad LJ, Chemaitelly HB, Adeel A. Effectiveness of the BNT162b2 Covid‐19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021;385:187‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of COVID‐19 vaccine against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. The effects of virus variants on COVID‐19 vaccines. World Health Organisation. 2021. https://www.who.int/news‐room/feature‐stories/detail/the‐effects‐of‐virus‐variants‐on‐covid‐19‐vaccines?gclid=CjwKCAjw‐e2EBhAhEiwAJI5jgx‐mGHz6Bet85sRgnSXQQuBTsBn09k5H_v6NPuQ6XL_7no9ecNo3XhoC5rwQAvD_BwE [Google Scholar]
- 100. Serre‐Yu W, Rebekah D, Pazos VM, Sacha G, Colombel J‐F, Ken C. Serological response to COVID‐19 vaccination in IBD patients receiving biologics. medRxiv. 2021. https://www.medrxiv.org/content/medrxiv/early/2021/03/20/2021.03.17.21253848.full.pdf [Google Scholar]
- 101. Kennedy NA, Goodhand JR, Bewshea C, et al; Contributors to the CLARITY IBD Study . Anti‐SARS‐CoV‐2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut. 2021;70:865‐875. [DOI] [PubMed] [Google Scholar]
- 102. Ehmsen S, Asmussen A, Jeppesen SS, et al. Antibody and T cell immune responses following mRNA COVID‐19 vaccination in patients with cancer. Cancer Cell. 2021;39:1034–1036. 10.1016/j.ccell.2021.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Siegel C A, Melmed G Y, McGovern DPB, et al. SARS‐CoV‐2 vaccination for patients with inflammatory bowel diseases: recommendations from an international consensus meeting. Gut. 2021;70:635–640. 10.1136/gutjnl-2020-324000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. 7th Interview COVID‐19 ECCO Taskforce, published February 12, 2021.
- 105. Abreu MT, Peyrin‐Biroulet L. Providing guidance during a global viral pandemic for the care of patients with inflammatory bowel disease. J Crohns Colitis. 2020;14:767‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


