Abstract
Objective
To investigate the incidence of and factors associated with SARS–CoV‐2 testing and infection in immune‐mediated inflammatory disease (IMID) patients versus matched non‐IMID comparators from the general population.
Methods
We conducted a population‐based, matched cohort study among adult residents from Ontario, Canada, from January 2020 to December 2020. We created cohorts for the following IMIDs: rheumatoid arthritis (RA), psoriasis, psoriatic arthritis, ankylosing spondylitis, systemic autoimmune rheumatic diseases, multiple sclerosis (MS), iritis, inflammatory bowel disease (IBD), polymyalgia rheumatica, and vasculitis. Each patient was matched with 5 patients without IMIDs based on sociodemographic factors. We estimated the incidence of SARS–CoV‐2 testing and infection in IMID patients and non‐IMID patients. Multivariable logistic regressions assessed odds of SARS–CoV‐2 infection.
Results
We studied 493,499 patients with IMIDs and 2,466,946 patients without IMIDs. Patients with IMIDs were more likely to have at least 1 SARS–CoV‐2 test versus patients without IMIDs (27.4% versus 22.7%), but the proportion testing positive for SARS–CoV‐2 was identical (0.9% in both groups). Overall, IMID patients had 20% higher odds of being tested for SARS–CoV‐2 (odds ratio 1.20 [95% confidence interval 1.19–1.21]). The odds of SARS–CoV‐2 infection varied across IMID groups but was not significantly elevated for most IMID groups compared with non‐IMID comparators. The odds of SARS–CoV‐2 infection was lower in IBD and MS and marginally higher in RA and iritis.
Conclusion
Patients across all IMIDs were more likely to be tested for SARS–CoV‐2 versus those without IMIDs. The risk of SARS–CoV‐2 infection varied across disease subgroups.
INTRODUCTION
Immune‐mediated inflammatory diseases (IMIDs) are complex disorders caused by a combination of genetic susceptibility and environmental factors (1). Population‐based studies have shown that >5% of the general population have a diagnosis of at least 1 IMID (2, 3). Patients with IMIDs have unique characteristics that raise concerns regarding their risk of acquiring SARS–CoV‐2 and outcomes associated with the virus. A higher risk of infections, including viral illnesses, has been reported in a variety of IMIDs (4, 5, 6). Both abnormalities in the host immune defense intrinsic to the underlying IMIDs and those due to immune‐modulating treatments contribute to this risk (7, 8). In addition, a biologically susceptible host may have heightened risk depending on sociodemographic factors including individual and societal behaviors and community spread.
SIGNIFICANCE & INNOVATIONS.
Our large population‐based study found that, despite 20% higher rates of SARS–CoV‐2 testing among patients with immune‐mediated inflammatory diseases (IMIDs), the risk of SARS–CoV‐2 infection was not significantly elevated for most IMID groups compared with non‐IMID patients.
The risk of SARS–CoV‐2 varied across IMID subgroups, being lower in inflammatory bowel disease and multiple sclerosis and higher in rheumatoid arthritis and iritis.
SARS–CoV‐2 infection risk was associated with demographic factors including urban living, multimorbidity, lower socioeconomic status, and residing in a long‐term care facility.
The detection of COVID‐19 infection and thus the reported disease incidence rates are directly influenced by SARS–CoV‐2 testing. SARS–CoV‐2 testing is an important public health measure to monitor and control viral spread in the general population (9). Approaches to SARS–CoV‐2 testing have evolved over time and were a limited resource in early phases of the pandemic. Local recommendations and access to testing also varied across jurisdictions and populations. SARS–CoV‐2 testing rates are influenced by demographic characteristics (sex, race/ethnicity), socioeconomic factors, and comorbidities (10, 11).
Despite multiple studies on the risk of COVID‐19 infection and its complications in IMIDs, evidence from population‐based studies is limited (12). Observational studies reliant on self‐report of infection status, hospital admissions, and targeted testing in selected populations have been reported but are prone to selection biases (13). Reported COVID‐19 infection rates are functions of sociodemographic factors and comorbidities, which influence the propensity of individuals to undergo SARS–CoV‐2 testing (11, 14). Previous studies that have restricted their analysis to hospitalized patients, people tested for active infection, or people who volunteered to participate are most susceptible to collider bias, as the relationships between any variables that relate to outcome will be distorted compared to among the general population (13). Since it is challenging to identify all such potential confounders to mitigate this collider bias, accurate estimates of COVID‐19 risk in IMIDs are best obtained from studies involving unselected population‐based data and careful adjustment of factors affecting SARS–CoV‐2 testing. Our study aims were to fill this knowledge gap by investigating the incidence and factors associated with SARS–CoV‐2 testing and positivity (SARS–CoV‐2 infection) in IMID patients versus matched non‐IMID patients using population health administrative data from Ontario, Canada.
PATIENTS AND METHODS
Study design and setting
We conducted a population‐based, matched cohort study (involving patients with IMIDs matched with non‐IMID comparators) using Ontario health administrative data to assess for SARS–CoV‐2 testing and infection between January 1, 2020 to December 17, 2020. The first patient with COVID‐19 in Ontario was reported January 23, 2020. Ontario comprises almost 40% of the Canadian population, with 14.7 million individuals in 2020. Our study included adults ages ≥20 years living in the province. The Ontario Health Insurance Plan (OHIP) is a public, single‐payer system that covers hospital admissions, physician services, and tests for SARS–CoV‐2 for all Ontario residents (15). Health care encounters are recorded in administrative health databases, which are linked using an encoded identifier that is unique to each resident, provider, and facility. The data used in the study are held securely and analyzed in linked, encoded form at ICES (www.ices.on.ca). The use of data in this project was authorized under section 45 of Ontario's Personal Health Information Protection Act, which does not require review by a research ethics board.
IMID case definitions
We used physician service claims and provincial hospitalization databases to assemble 10 cohorts of patients with the following IMIDs: rheumatoid arthritis (RA), psoriasis, psoriatic arthritis, ankylosing spondylitis, systemic autoimmune rheumatic diseases (SARDs) (including systemic lupus erythematosus, systemic sclerosis, Sjögren's syndrome, mixed connective tissue disease, inflammatory myositis, undifferentiated connective tissue disease), multiple sclerosis (MS), iritis, inflammatory bowel disease (IBD), polymyalgia rheumatica (PMR), and vasculitis (including giant cell arteritis and other types of vasculitides). The cohorts were not mutually exclusive, and each patient could belong to >1 IMID cohort.
We used the OHIP physician service claims database to identify diagnosis codes of IMIDs and information about preexisting comorbidities. These diagnoses are coded using a modification of the International Classification of Diseases, Eighth Revision (ICD‐8) (16). We obtained information on physician specialty by linking to the ICES Physician Database. Hospital diagnoses for IMID were also identified using the Canadian Institute for Health Information's Discharge Abstract Database using the ICD‐10. When available, we used validated IMID case definitions (17, 18, 19, 20, 21), most requiring multiple OHIP diagnosis codes, with at least 1 rendered by a relevant specialist, or a hospital discharge diagnosis. For the remaining IMIDs, we used a similar approach that was based on a combination of physician visits for the specific IMID, including at least 1 by a relevant specialist, or at least 1 hospital discharge diagnosis. Case definitions for inclusion in each cohort are shown in Supplementary Appendix A, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24781. Study entry required that a person was diagnosed with at least 1 of the above detailed IMIDs, be alive, older than 20 years of age, and living in Ontario on January 1, 2020. To ensure accurate baseline information, patients were also required to be eligible for OHIP coverage for at least 3 years prior to the study start date.
General population comparators and assessment of covariates
Each patient was matched with 5 non‐IMID comparators based on age (within 5 years), sex, region of residence (Local Health Integration Network) at study entry (January 1, 2020), and residing in a long‐term care (LTC) facility (within 120 days prior to study entry). Information regarding patients’ demographic characteristics, vital status, and health insurance status was obtained from the Registered Persons Database. Information about residential income quintile was identified using Statistics Canada's census data. LTC residence was ascertained using the Complex Continuing Care‐LTC database, which records the mandatory assessments performed at admission and quarterly on all LTC residents. Comorbidities were assessed within the 3 years prior to the study entry. The number of Johns Hopkins Aggregated Diagnosis Groups, System Version 10 (22), was calculated. Patients were also classified based on their resident postal code as living in a rural or urban area.
SARS–CoV‐2 testing and infection
Information about SARS–CoV‐2 testing and positive results on viral RNA polymerase chain reaction testing (SARS–CoV‐2 infection) was available from the Ontario Laboratory Information System (OLIS). OLIS captures ~88% of all laboratory‐identified SARS–CoV‐2 cases reported by the province during the study period (23, 24). For each subject, we identified whether they had had at least 1 test between January 1, 2020 to December 17, 2020, along with the result of each test. During the study period, the testing criteria for SARS–CoV‐2 in Ontario have changed. Due to limited capacity of SARS–CoV‐2 testing in early stages of the pandemic, testing focused on people who presented with COVID‐19 symptoms or signs and who required admission to the hospital or those belonging to high‐risk groups, including returning travelers, people who lived or worked in a congregate living setting and institutions, and those with occupational exposures (e.g., health care workers). A priority in testing was also given to symptomatic patients requiring frequent contact with the health care system due to their underlying medical conditions (25). With increased testing capacity, the recommendations for testing were broadened, and as of the end of May 2020, the Ontario Ministry of Health guidelines recommend performing SARS–CoV‐2 testing in individuals presenting with at least 1 COVID‐19 sign or symptom or in high‐risk asymptomatic individuals, such as those who had contact with confirmed COVID‐19 cases or workers and residents of specific outbreak sites (e.g., LTC homes) (26).
Statistical analysis
We calculated descriptive statistics for patient characteristics among those with and without IMIDs (at time study entry). We calculated standardized differences between the 2 groups, with a standardized difference of >0.10 indicating a clinically meaningful difference (27). Crude and age‐ and sex‐standardized cumulative incidence of SARS–CoV‐2 testing and infection (along with 95% confidence intervals [95% CIs]) were reported for each IMID group and then overall, using the 2016 Ontario population age structure for age and sex standardization. Patients were followed from study entry (January 2020) until the they experienced any of the following outcomes (whichever came first): end of study (December 17, 2020), death, or first SARS–CoV‐2 testing or infection (for the respective analysis).
We compared the cumulative incidence of those with at least 1 SARS–CoV‐2 test, as well as those with SARS–CoV‐2 infection between all study patients with and without IMIDs. We used multivariable logistic regression to compare SARS–CoV‐2 testing and SARS–CoV‐2 infection in IMID versus non‐IMID patients, adjusted for sociodemographic factors and comorbidities and accounting for matching. Each regression model included the specific IMID diagnosis and the following covariates: age, sex, LTC residence, comorbidities (number of Johns Hopkins Aggregated Diagnosis Groups, System Version 10 [22], socioeconomic status (by census neighborhood income quintiles), and rurality (classified as urban and rural based on residents’ postal code). Then, to assess specific risk factors for SARS–CoV‐2 testing and SARS–CoV‐2 infection, we restricted the analysis to patients with IMIDs alone. We assessed all above variables as potential risk factors for each of these 2 outcomes separately (adjusting for IMID type) and reported their estimated adjusted odds ratios (ORs) along with 95% CIs.
RESULTS
A total of 493,499 adults with IMIDs and 2,466,946 matched non‐IMID comparators who lived in Ontario as of January 2020 were included in our study. Details about cohort creation and the demographic and comorbidity profile of the study population are shown in Supplementary Tables 1 and 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24781. The mean ± SD age of IMID patients was 58.3 ± 17 years, and 60.7% were female (comparators were similar with respect to their demographic characteristics due to matching criteria). In all, 90.4% of the patients belonged to a single IMID category, and the remaining had ≥2 IMIDs. Most patients lived in urban areas (91.3%), and only a small minority (1.4%) lived in an LTC facility. The mean ± SD number of comorbidities (by aggregated diagnosis groups [ADG]) was higher in IMID (9.2 ± 4.4) compared to non‐IMID patients (6.8 ± 4.3).
SARS–CoV‐2 testing
During the study period, a total of 134,981 (27.4%) patients with IMIDs were tested for SARS–CoV‐2 compared with 693,796 (23.4%) of patients without IMIDs. No tests were performed in any of the study patients prior to March 2020; therefore, the results are reported as of this month onward. The age‐ and sex‐standardized testing rate was higher among IMID patients compared to non‐IMID patients starting in March 2020 and remained elevated throughout the study period (Figure 1A). The overall age‐ and sex‐standardized cumulative incidence of SARS–CoV‐2 testing was significantly higher among IMID patients than non‐IMID patients (2,691.8 [95% CI 2,676.3–2,707.3] versus 2,245.3 [95% CI 2,239.0–2,251.6] tested patients per 10,000 population, respectively). Testing rates were significantly higher across all IMID groups versus their respective matched non‐IMID comparators, being highest in individuals with vasculitis (3,124.1 per 10,000 population), IBD (2,929.9 per 10,000 population), and SARDs (2,920.9 per 10,000 population) (Figure 1B). The regression analysis showed that IMID patients were more likely to undergo SARS–CoV‐2 testing with a 29% higher odds of being tested (unadjusted OR 1.29 [95% CI 1.28–1.29]) (Figure 2 and Supplementary Table 3, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24781), and this association remained significant in the multivariable analysis (OR 1.20 [95% CI 1.19–1.21]). The association between having IMID and being tested for SARS–CoV‐2 was found across all IMID subgroups and was highest in those with vasculitis (OR 1.42) and PMR (OR 1.40) (Figure 2 and Supplementary Table 3, available at http://onlinelibrary.wiley.com/doi/10.1002/acr.24781).
Figure 1.
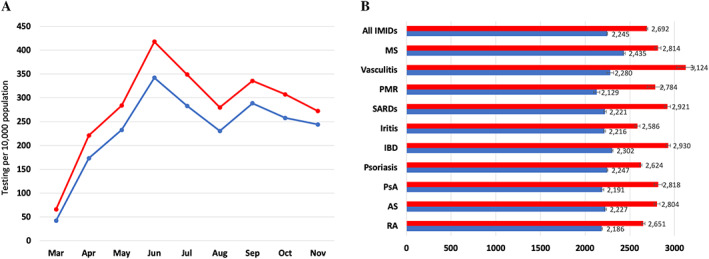
Age‐ and sex‐standardized rates of SARS–CoV‐2 testing in patients with immune‐mediated inflammatory diseases (IMIDs) (red) and in those without IMIDs (blue) for monthly rates of testing (A) and by IMID group (B). Rates are per 10,000 people, standardized to the 2016 Ontario population. AS = ankylosing spondylitis; IBD = inflammatory bowel disease; MS = multiple sclerosis; PMR = polymyalgia rheumatica; PsA = psoriatic arthritis; RA = rheumatoid arthritis; SARDs = systemic autoimmune rheumatic diseases. Error bars indicate the 95% confidence interval.
Figure 2.
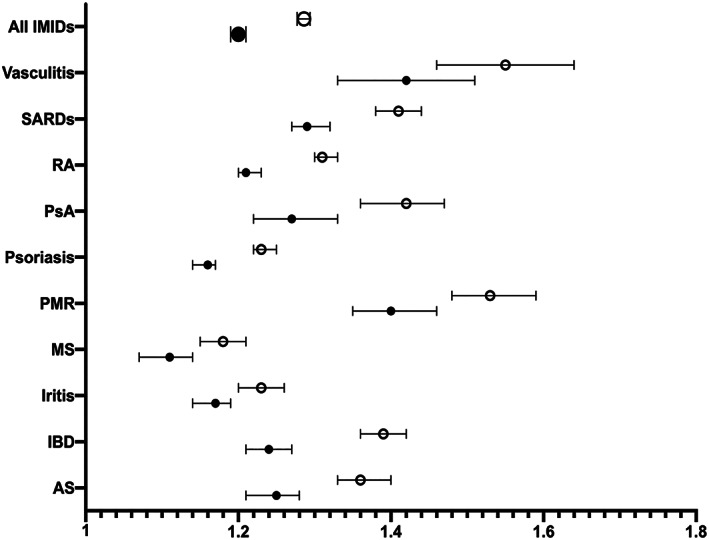
The association between immune‐mediated inflammatory diseases (IMIDs) versus non‐IMIDs and SARS–CoV‐2 testing by logistic regression analysis. Each model is adjusted for age, sex, aggregated diagnosis group score, urban/rural, income, and living in long‐term care. AS = ankylosing spondylitis; IBD = inflammatory bowel disease; MS = multiple sclerosis; PMR = polymyalgia rheumatica; PsA = psoriatic arthritis; RA = rheumatoid arthritis; SARDs = systemic autoimmune rheumatic diseases. Open circles represent unadjusted odds ratios; closed circles represent adjusted odds ratios. Error bars indicate the 95% confidence interval.
Among patients with a diagnosis of IMID, the factor most strongly associated with SARS–CoV‐2 testing was residing in an LTC facility (OR 16.63) (Table 1). Other factors significantly associated with testing among IMID patients included female sex (OR 1.17), higher number of ADGs (OR for higher ADG categories compared to the lowest ADG category ranged from 1.45 to 2.60), and younger age (OR 0.92).
Table 1.
Factors associated with SARS–CoV‐2 testing among patients with immune‐mediated inflammatory disease by multivariable logistic regression*
| Variable | OR (95% CI) |
|---|---|
| Age, per 10‐year increase | 0.92 (0.92–0.93) |
| Sex, female versus male | 1.17 (1.15–1.18) |
| Residing in LTC | 16.63 (15.66–17.76) |
| No. of ADGs | |
| 0–4 | Referent |
| 5–9 | 1.45 (1.42–1.48) |
| 10–14 | 2.05 (2.00–2.10) |
| 15+ | 2.60 (2.53–2.67) |
| Urban versus rural | 0.99 (0.98–1.02) |
| Socioeconomic status by income† | |
| Quintile 1 | Referent |
| Quintile 2 | 1.00 (0.98–1.02) |
| Quintile 3 | 1.00 (0.98–1.02) |
| Quintile 4 | 1.01 (0.99–1.03) |
| Quintile 5 | 1.05 (1.03–1.07) |
N = 493,499; no. of tested = 134,981. The model is adjusted for immune‐mediated inflammatory disease category. 95% CI = 95% confidence interval; ADGs = aggregated diagnosis groups; LTC = long‐term care; OR = odds ratio.
Quintile 1 is the lowest income quintile.
SARS–CoV‐2 infection
A total of 4,541 patients (0.9% of all patients) with IMIDs tested positive for SARS–CoV‐2 compared with 22,157 non‐IMID comparators (0.9%). The incidence of SARS–CoV‐2 infection by month and cumulative incidence by IMID group are shown in Figure 3. The incidence of SARS–CoV‐2 infection over time in IMID patients mirrors that seen in non‐IMID patients, with increased case counts starting in March 2020, reaching a peak in April, and declining through May and June 2020. Another steep increase in cases in September through November 2020 reflects a second wave of the pandemic. Despite higher rates of testing, the standardized cumulative incidence of SARS–CoV‐2 infection in IMID patients was similar to that observed in non‐IMID comparators (90.8 [95% CI 88–93.7] and 90.0 [95% CI 88.7–91.3] per 10,000 population, respectively).
Figure 3.
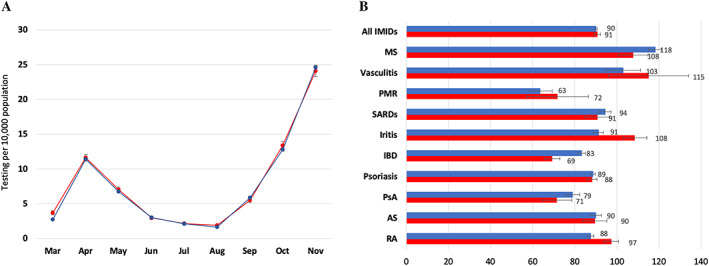
Age‐ and sex‐standardized SARS–CoV‐2 cases in patients with immune‐mediated inflammatory diseases (IMIDs) (red) and in those without IMIDs (blue) for monthly rates of cases (A) and cases by IMID group (B). Rates are per 10,000 people, standardized to the 2016 Ontario population. AS = ankylosing spondylitis; IBD = inflammatory bowel disease; MS = multiple sclerosis; PMR = polymyalgia rheumatica; PsA = psoriatic arthritis; RA = rheumatoid arthritis; SARDs = systemic autoimmune rheumatic diseases. Error bars indicate the 95% confidence interval.
Due to heterogeneity in SARS–CoV‐2 infection risk across the different IMIDs, we do not report the OR for all IMIDs and instead report the individual ORs associated with each individual IMID group (Figure 4 and Supplementary Table 4, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24781). For the majority of IMID groups, SARS–CoV‐2 infection risk was not significantly elevated versus their matched non‐IMID comparators. Lower odds of SARS–CoV‐2 infection were found in IBD (OR 0.75 [95% CI 0.68–0.84]), MS (OR 0.77 [95% CI 0.68–0.87]), and psoriasis (OR 0.94 [95% CI 0.88–0.99]). A significant, yet weaker association was found between iritis (OR 1.13 [95% CI 1.02–1.26]) and RA (OR 1.07 [95% CI 1.01–1.14]) and SARS–CoV‐2 infection.
Figure 4.
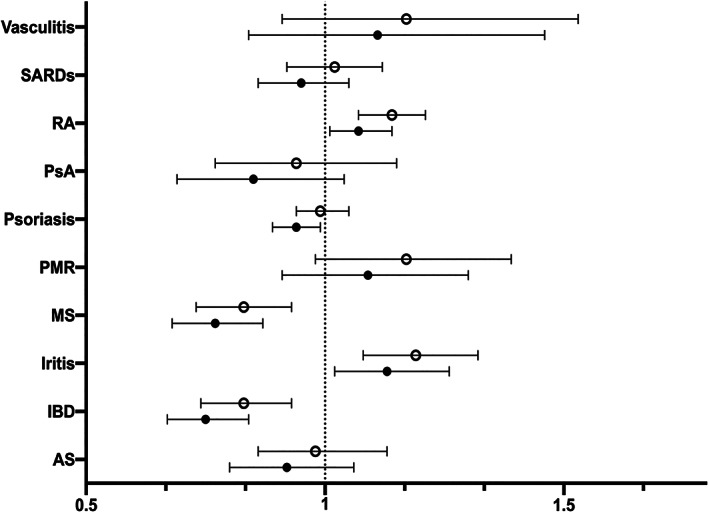
The association between immune‐mediated inflammatory diseases (IMIDs) versus non‐IMIDs and SARS–CoV‐2 infection by logistic regression analysis. Each model is adjusted for age, sex, aggregated diagnosis group score, urban/rural, income quintile, and living in long‐term care. AS = ankylosing spondylitis; IBD = inflammatory bowel disease; MS = multiple sclerosis; PMR = polymyalgia rheumatica; PsA = psoriatic arthritis; RA = rheumatoid arthritis; SARDs = systemic autoimmune rheumatic diseases. Open circles represent unadjusted odds ratios; closed circles represent adjusted odds ratios. Error bars indicate the 95% confidence interval.
Risk factors for SARS–CoV‐2 infection among patients with IMIDs (Table 2) included residing in an LTC facility (OR 16.91), multimorbidity (OR for higher ADG categories compared to the lowest ADG category ranged from 1.35 to 2.05), lower socioeconomic status (OR for the higher income quintiles compared to lowest quintile ranged from 0.60 to 0.86), urban residence (OR 3.52), and younger age (OR 0.89 per 10‐year increase in age).
Table 2.
Factors associated with SARS–CoV‐2 infection among immune‐mediated inflammatory diseases by multivariable logistic regression*
| Variable | OR (95% CI) |
|---|---|
| Age, per 10‐year increase | 0.89 (0.87–0.91) |
| Sex, female versus male | 0.94 (0.88–1.00) |
| Residing in LTC | 16.91 (15.30–18.69) |
| No. of ADGs (range 0–34) | |
| 0–4 | Referent |
| 5–9 | 1.35 (1.20–1.50) |
| 10–14 | 1.75 (1.58–1.95) |
| 15+ | 2.05 (1.82–2.31) |
| Urban versus rural | 3.52 (2.99–4.14) |
| Socioeconomic status by income† | |
| Quintile 1 | Referent |
| Quintile 2 | 0.86 (0.79–0.94) |
| Quintile 3 | 0.90 (0.83–0.99) |
| Quintile 4 | 0.72 (0.65–0.78) |
| Quintile 5 | 0.60 (0.55–0.67) |
N = 493,499; no. of events = 4,541. The model is adjusted for immune‐mediated inflammatory disease category. 95% CI = 95% confidence interval; ADGs = aggregated diagnosis groups; LTC = long‐term care; OR = odds ratio.
Quintile 1 is the lowest income quintile.
DISCUSSION
The COVID‐19 pandemic remains a major global health crisis, with continued efforts to identify populations most at risk of critical and mortal COVID‐19 infection. Our study filled an important gap in knowledge by characterizing the risk of testing and SARS–CoV‐2 infection patients with IMIDs. We have found that despite increased rates of testing among patients with IMIDs, for most IMID groups, the risk of SARS–CoV‐2 infection was not elevated, although the risk did vary across IMID diagnoses. Similar to the general population, SARS–CoV‐2 infection risk among IMID patients was associated with demographic factors, including urban living (where the underlying prevalence of the infection is highest), multimorbidity, residing in an LTC facility, and lower socioeconomic status (23, 28, 29).
SARS–CoV‐2 infection risk among IMID patients has been evaluated in a meta‐analysis of 62 observational studies mostly from single centers or regional/national patient registries (12). The reported cumulative incidence ranged from 0.002% to 0.01%, which is substantially lower than our crude estimate of 0.9%, which may be explained by more complete reporting in our study and the fact that our study was performed later in the course of the pandemic, allowing accrual of more cases. Importantly, the above study concluded that the odds of contracting SARS–CoV‐2 infection is twice as high in patients with IMIDs compared to patients without IMIDs (pooled OR 2.19), which is in contrast to our estimates that showed similar or lower risk of SARS–CoV‐2 infection risk for most IMID groups compared to non‐IMID patients. The tendency for higher infection risk in previous studies may be related to inherent limitations, such as inaccuracies due to small samples (30, 31, 32), different definition of SARS–CoV‐2 infection (e.g., including both laboratory confirmed and clinically suspected cases), highly selective patient populations (e.g., single academic centers and patients taking biologic medications) (33, 34), and different reporting methods of COVID‐19 in cases and comparators (35, 36, 37).
Additionally, when COVID‐19 risk is evaluated in selected populations (e.g., individuals who tested for SARS–CoV‐2 and hospitalized patients), the results are susceptible to collider bias, a type of selection bias that can occur when an exposure and an outcome independently influence a collider variable. Since the estimated SARS–CoV‐2 infection is influenced by testing, which in turn is influenced by IMID diagnosis, restricting the analysis to tested patients can lead to artifactual associations (13). Confounder adjustment is another important issue to consider in any observational study such as ours. Imbalances in demographic or comorbid factors between cases and controls that are not accounted for in the analysis could lead to biased comparative risk estimates. To address these potential biases, our population‐based study, carefully matched between individuals with IMIDs and those without IMIDs, carefully adjusted for potential confounding factors and used complete information about SARS–CoV‐2 testing. The results of this robust analysis showed that overall SARS–CoV‐2 infection risk was not increased among most IMID groups compared to the general population.
SARS–CoV‐2 testing is an important component of effective COVID‐19 public health response, as it facilitates early detection of cases, self‐isolation, and prevention of onward transmission (38). Studies have found disparities in SARS–CoV‐2 testing among underserved populations, such as marginalized ethnic groups and individuals of low socioeconomic classes (11, 39, 40). Our study results are in line with previous studies that showed increased SARS–CoV‐2 testing among patients with chronic diseases (14, 41). Having any IMID was associated with 20% higher chances of being tested for SARS–CoV‐2 compared to non‐IMID patients, even after adjusting for other confounding factors including comorbidities, and testing was higher across all IMID groups, with ORs ranging from 1.11 to 1.42. Having an IMID or treatment with immunosuppressive medication were some criteria for SARS–CoV‐2 testing in the setting of flu‐like symptoms in Ontario early in the pandemic (25), which could partly explain differences in testing compared to individuals without IMIDs; however, rates of testing remained elevated for patients with IMIDs throughout the course of the study after the IMID‐specific criteria were removed. Higher rates of testing among those with IMIDs may be explained by several factors, including higher awareness and health‐seeking behaviors and willingness to get tested due to perceived risks of COVID‐19 in IMID patients, higher prevalence of disease‐related COVID‐19–mimicking symptoms or other infections, and preexisting patient–physician relationships.
The primary strength of our study is the use of a centralized data resource in the setting of a public health system for the entire population. As with any study, there are potential limitations to consider. First, the IMID case definitions are based on diagnosis codes from physicians and hospital records used for administrative purposes, thus misclassification of IMID diagnoses is possible. It is expected that such potential misclassification will shift the results toward the null; therefore, we cannot exclude the possibility that SARS–CoV‐2 risk in IMID patients is higher than estimated in this study. To mitigate this risk, whenever possible, we used validated case definitions that showed high performance in prior studies, and we used a similar approach requiring multiple IMID diagnosis codes to identify the remaining IMIDs. The population prevalence and demographic characteristics of the different IMIDs provide further face validity to the accuracy of our case definitions.
Second, since we do not have access to medication data prior to the age of 65 years, we could not assess the effect of exposure to glucocorticoids and immune‐modulating agents on SARS–CoV‐2 infection risk. In addition, our estimated SARS–CoV‐2 infection rates may be underestimated, as typically only symptomatic patients and those with known exposures were tested. Due to delays in reporting hospitalizations during the study period, we did not analyze risk of COVID‐19 hospitalizations in the present study. Last, access to testing varies in different countries depending on local recommendations, coverage, and testing capacity, which may affect the generalizability of the results. While our SARS–CoV‐2 testing estimates reflect the situation in Ontario, we believe that the general pattern can be extrapolated to other countries, as a gradual increased testing capacity resulting in wider testing indications has been a global characteristic of the pandemic (42). Furthermore, it is likely that our results pertaining to the relative risk of SARS–CoV‐2 infection in IMIDs are generalizable, as they consider a wide range of patients and carefully matched comparators adjusted for multiple confounding factors.
In conclusion, in this large population‐based study, we found that despite higher rates of SARS–CoV‐2 testing among patients with IMIDs, the risk of SARS–CoV‐2 infection was not elevated for most IMID groups, yet risk varied across disease subgroups, being lower in IBD, MS, and psoriasis and higher in RA and iritis. These results provide baseline estimates of the SARS–CoV‐2 infection rates in patients with IMIDs prior to the rollout of the COVID‐19 vaccination campaign and identify factors that predispose to infection.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Eder had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Eder, Croxford, Drucker, Mendel, Kuriya, Touma, Johnson, Cook, Bernatsky, Haroon, Widdifield.
Acquisition of data
Croxford, Widdifield.
Analysis and interpretation of data
Eder, Croxford, Drucker, Mendel, Kuriya, Touma, Johnson, Cook, Bernatsky, Haroon, Widdifield.
Supporting information
Disclosure Form:
Appendix S1 Supporting Information
Supplementary Table 1 – Characteristics of the study population
Supplementary Table 2 ‐ Description of IMID cohort creation
Supplementary Table 3
Supplementary Table 4
The opinions, results, and conclusions reported herein are those of the authors and are independent of the funding or data sources; no endorsement is intended or should be inferred.
Supported by ICES (formerly known as the Institute for Clinical Evaluative Sciences), which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). Parts of this material are based on data and information compiled and provided by MOHLTC and the Canadian Institute for Health Information. Dr. Eder is Canada Research Chair in Equity in Care of Rheumatic Diseases. Dr. Johnson's work was supported by the Canadian Institutes of Health Research (New Investigator award). Dr. Bernatsky is a James McGill Professor of Medicine. Dr. Widdifield's work was supported by the Arthritis Society (Stars Career Development award STAR‐19‐0610).
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr.24781&file=acr24781‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Somers EC, Thomas SL, Smeeth L, et al. Autoimmune diseases co‐occurring within individuals and within families: a systematic review. Epidemiology 2006;17:202–17. [DOI] [PubMed] [Google Scholar]
- 2. Sardu C, Cocco E, Mereu A, et al. Population based study of 12 autoimmune diseases in Sardinia, Italy: prevalence and comorbidity. PLoS One 2012;7:e32487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eaton WW, Pedersen MG, Atladóttir HO, et al. The prevalence of 30 ICD‐10 autoimmune diseases in Denmark. Immunol Res 2010;47:228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liao TL, Chen YM, Liu HJ, et al. Risk and severity of herpes zoster in patients with rheumatoid arthritis receiving different immunosuppressive medications: a case‐control study in Asia. BMJ Open 2017;7:e014032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li TH, Chen YM, Liu HJ, et al. Risk of severe herpes simplex virus infection in systemic lupus erythematosus: analysis of epidemiology and risk factors analysis in Taiwan. Ann Rheum Dis 2019;78:941–6. [DOI] [PubMed] [Google Scholar]
- 6. Li M, Yang QF, Cao Q, et al. High‐risk human papilloma virus infection and cervical neoplasm in female inflammatory bowel disease patients: a cross‐sectional study. Gastroenterol Rep (Oxf) 2019;7:338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Favalli EG, Ingegnoli F, De Lucia O, et al. COVID‐19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun Rev 2020;19:102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jung JY, Suh CH. Infection in systemic lupus erythematosus, similarities, and differences with lupus flare. Korean J Intern Med 2017;32:429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . Transmission of SARS–CoV‐2: implications for infection prevention precautions. 2020. URL: https://www.who.int/news‐room/commentaries/detail/transmission‐of‐SARS–CoV‐2‐implications‐for‐infection‐prevention‐precautions.
- 10. Stall NM, Wu W, Lapointe‐Shaw L, et al. Sex‐ and age‐specific differences in COVID‐19 testing, cases, and outcomes: a population‐wide study in Ontario, Canada. J Am Geriatr Soc 2020;68:2188–91. [DOI] [PubMed] [Google Scholar]
- 11. Mody A, Pfeifauf K, Bradley C, et al. Understanding drivers of COVID‐19 racial disparities: a population‐level analysis of COVID‐19 testing among Black and White populations. Clin Infect Dis 2020;73:2921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akiyama S, Hamdeh S, Micic D, et al. Prevalence and clinical outcomes of COVID‐19 in patients with autoimmune diseases: a systematic review and meta‐analysis. Ann Rheum Dis 2021;80:384–91. [DOI] [PubMed] [Google Scholar]
- 13. Griffith GJ, Morris T, Tudball M, et al. Collider bias undermines our understanding of COVID‐19 disease risk and severity. Nat Commun 2020;11:5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van der Meer D, Pinzón‐Espinosa J, Lin BD, et al. Associations between psychiatric disorders, COVID‐19 testing probability and COVID‐19 testing results: findings from a population‐based study. BJPsych Open 2020;6:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Government of Ontario . Health care in Ontario: long‐term care in Ontario. 2020. URL: https://www.ontario.ca/page/health-care-ontario.
- 16. World Health Organization . International Classification of Diseases (ICD). 2020. URL: https://www.who.int/classifications/icd/en/. [Google Scholar]
- 17. Eder L, Widdifield J, Rosen CF, et al. Identifying and characterizing psoriasis and psoriatic arthritis patients in Ontario administrative data: a population‐based study from 1991 to 2015. J Rheumatol 2020;47:1644–51. [DOI] [PubMed] [Google Scholar]
- 18. Widdifield J, Bernatsky S, Paterson JM, et al. Accuracy of Canadian health administrative databases in identifying patients with rheumatoid arthritis: a validation study using the medical records of rheumatologists. Arthritis Care Res (Hoboken) 2013;65:1582–91. [DOI] [PubMed] [Google Scholar]
- 19. Barra L, Pope JE, Pequeno P, et al. Incidence and prevalence of giant cell arteritis in Ontario, Canada. Rheumatology (Oxford) 2020;59:3250–8. [DOI] [PubMed] [Google Scholar]
- 20. Benchimol EI, Guttmann A, Mack DR, et al. Validation of international algorithms to identify adults with inflammatory bowel disease in health administrative data from Ontario, Canada. J Clin Epidemiol 2014;67:887–96. [DOI] [PubMed] [Google Scholar]
- 21. Widdifield J, Ivers NM, Young, J , et al. Development and validation of an administrative data algorithm to estimate the disease burden and epidemiology of multiple sclerosis in Ontario, Canada. Mult Scler 2015;21:1045–54. [DOI] [PubMed] [Google Scholar]
- 22. Weiner J, Abrams C. The Johns Hopkins ACG® System Technical Reference Guide, version 10.0. 2011. URL: https://studylib.net/doc/8365333/the‐johns‐hopkins‐acg%C2%AE‐system‐‐technical‐reference‐guide.
- 23. Chung H, Fung K, Ferreira‐Legere LE, et al. COVID‐19 laboratory testing in Ontario: patterns of testing and characteristics of individuals tested, as of April 30, 2020. URL: https://www.ices.on.ca/Publications/Atlases‐and‐Reports/2020/COVID‐19‐Laboratory‐Testing‐in‐Ontario.
- 24. Sundaram ME, Calzavara A, Mishra S, et al. Individual and social determinants of SARS–CoV‐2 testing and positivity in Ontario, Canada: a population‐wide study. CMAJ 2021;193:E723–E34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ontario Ministry of Health . COVID‐19 provincial testing guidance update, April 15, 2020. URL: https://www.rhra.ca/wp‐content/uploads/2020/04/COVID‐19‐Testing‐Update‐CMOH‐2020‐04‐15‐Shared.pdf.
- 26. Ontario Ministry of Health . COVID‐19 provincial testing guidance update, 2020. URL: http://health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/2019_testing_guidance.pdf.
- 27. Mamdani M, Sykora K, Li P, et al. Reader's guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ 2005;330:960–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown KA, Jones A, Daneman N, et al. Association between nursing home crowding and COVID‐19 infection and mortality in Ontario, Canada. JAMA Intern Med 2021;181:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malikov K, Huang Q, Shi S, et al. Temporal Associations between community incidence of COVID‐19 and nursing home outbreaks in Ontario, Canada. J Am Med Dir Assoc 2021;22:260–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Michelena X, Borrell H, López‐Corbeto M, et al. Incidence of COVID‐19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease‐modifying anti‐rheumatic drugs. Semin Arthritis Rheum 2020;50:564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Costantino F, Bahier L, Tarancón LC et al. COVID‐19 in French patients with chronic inflammatory rheumatic diseases: clinical features, risk factors and treatment adherence. Joint Bone Spine 2020;88:105095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhong J, Shen G, Yang H, et al. COVID‐19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol 2020;2:e557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quartuccio L, Valent F, Pasut E, et al. Prevalence of COVID‐19 among patients with chronic inflammatory rheumatic diseases treated with biologic agents or small molecules: a population‐based study in the first two months of COVID‐19 outbreak in Italy. Joint Bone Spine 2020;87:439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khan N, Patel D, Xie D, et al. Impact of anti‐tumor necrosis factor and thiopurine medications on the development of COVID‐19 in patients with inflammatory bowel disease: a Nationwide Veterans Administration Cohort Study. Gastroenterology 2020;159:1545–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Allocca M, Fiorino G, Zallot C, et al. Incidence and patterns of COVID‐19 among inflammatory bowel disease patients from the Nancy and Milan Cohorts. Clin Gastroenterol Hepatol 2020;18:2134–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Favalli EG, Monti S, Ingegnoli F, et al. Incidence of COVID‐19 in patients with rheumatic diseases treated with targeted immunosuppressive drugs: what can we learn from observational data? Arthritis Rheumatol 2020;72:1600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Damiani G, Pacifico A, Bragazzi NL, et al. Biologics increase the risk of SARS–CoV‐2 infection and hospitalization, but not ICU admission and death: real‐life data from a large cohort during red‐zone declaration. Dermatol Ther 2020;33:e13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Centers for Disease Control and Prevention . Overview of testing for SARS–CoV‐2, the virus that causes COVID‐19. 2020. URL: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html.
- 39. Wang L, Ma H, You KCY, et al. Heterogeneity in testing, diagnosis and outcome in SARS–CoV‐2 infection across outbreak settings in the greater Toronto area, Canada: an observational study. CMAJ Open 2020;8:E627–E36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lewis NM, Friedrichs M, Wagstaff S, et al. Disparities in COVID‐19 incidence, hospitalizations, and testing, by area‐level deprivation – Utah, March 3‐July 9, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salerno S, Zhao Z, Prabhu Sankar S, et al. Understanding the patterns of repeated testing for COVID‐19: association with patient characteristics and outcomes [preprint]. medRxiv 2020. 29;2020.07.26.20162453. [Google Scholar]
- 42. Lippi G, Mattiuzzi C, Santos de Oliveira MH, et al. Clinical predictors of SARS–CoV‐2 testing pressure on clinical laboratories: a multinational study analyzing Google trends and over 100 million diagnostic tests. Lab Med 2021;52:311–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form:
Appendix S1 Supporting Information
Supplementary Table 1 – Characteristics of the study population
Supplementary Table 2 ‐ Description of IMID cohort creation
Supplementary Table 3
Supplementary Table 4


