Abstract
Mechanisms discovered to drive increased glucose metabolism in cancer cells are found to be similar to those in viral‐infected cells. In this mini review, we summarize the major pathways by which the sugar analog, 2‐Deoxy‐d‐glucose, has been shown to exploit increased glucose metabolism in cancer and how this information applies to viral‐infected cells. Moreover, we highlight the relevance of these findings to the emergency approval of 2‐Deoxy‐d‐glucose in India to be used against SARS‐CoV‐2, the virus responsible for COVID‐19.
Keywords: 2‐deoxy‐d‐glucose or 2‐DG, cancer, cellular glucose metabolism, ER stress, glycolysis, SARS‐CoV‐2, viruses
Abbreviations
- 2‐DG
2‐deoxy‐d‐glucose
- EIF2 α
eukaryotic initiation factor 2
- ER stress
endopalsmic reticulum stress
- Glut 1
glucose transporter 1
- IP
intraperitoneal
- KSHV
Kaposi's sarcoma herpes virus
- MYC
a transcription factor overexpressed in many cancers
- PET
positron emission tomography
- p53
tumor protein suppressor gene 53
- PERK, PEDV
porcine epidemic diarhea virus, protein kinase RNA‐like endoplasmic reticulum kinase
- RB
retinobalstoma
- RT‐PCR
real time reverse transcription PCR
- SARSCov‐2
severe acute respiratory coronavirus‐2
- UPR
unfolded protein response
1. INTRODUCTION
The mutating nature of viral influenza has necessitated development of a different flu vaccine each year. With the news of more infectious variants of SARS‐CoV‐2 spreading to or originating in different countries, the need for treatments and prophylactics that can overcome viral variation is paramount.
One such treatment appears to be based on the remodeling of glucose metabolism toward a more glycolytic one, which is emerging as a common trait of viral‐infected cells. 1 , 2 , 3 This is similar to the shift that cancer cells undergo from oxidative phosphorylation to glycolysis in order to meet the demands of rapid growth (commonly referred to as the “Warburg effect”).
It has been known for more than 40 years via PET scans that tumors take up more glucose than normal surrounding tissues. An explanation for this phenomenon came from the findings of Chi Dang's laboratory that showed that when fibroblasts were transfected with MYC, an oncogene found in many tumor types, the glucose transporter, Glut 1, as well as several enzymes of the glycolytic pathway were upregulated when compared to the untransfected cells. 4 Similarly, it has been demonstrated that many of the major genes found to drive cancer (oncogenes and or loss of major suppressor genes), are also responsible for driving increased glucose accumulation into tumor cells (Figure 1). Moreover, by mass spectrometry analysis, it has been shown that in addition to being a vital energy source, the glucose molecule provides the building blocks required for a cell to replicate thereby explaining increased glucose metabolism of cancer cells and why the PET scan works. 5
FIGURE 1.
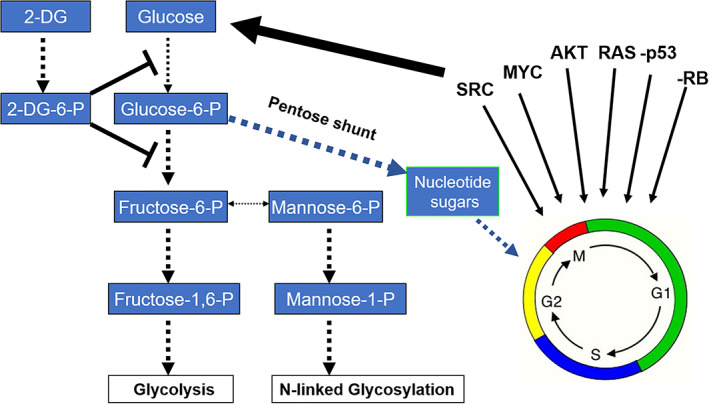
2‐Deoxy‐d‐glucose (2‐DG) exploits cancer‐induced increased glucose uptake and metabolism by blocking glycolysis and interfering with N‐linked glycosylation. The major oncogenes involved in converting a normal cell to a tumor cell have been shown to also upregulate glucose uptake and metabolism. As an analog of glucose, 2‐DG preferentially accumulates in malignant cells and acts to block glycolysis, which supplies the building blocks required for cancer cell replication. Under hypoxia, inhibiting glycolysis leads to cell death, while under normoxia, other energy sources such as fats and amino acids can be used to fuel cells and sustain viability. As an analog of mannose, 2‐DG fraudulently incorporates into the growing oligosaccharide chain required for N‐linked glycosylation leading to unfolded glycoproteins inducing ER stress and activation of PERK (a component of the unfolded protein response [UPR]) which in turn shuts down cap‐dependent translation by phosphorylating EIF2α. We demonstrated that due to 2‐DG's activity as a mannose analog, certain select tumor cell types, even when grown under normoxic conditions, undergo ER stress‐induced UPR‐mediated cell death
Thus, it follows, that upon viral infection, cells undergo a similar increased glucose‐uptake, satisfying their substrate requirements to produce more virus. Although the latter statement was not documented until 2008 when investigators actually measured increases in glycolytic metabolites and enzymes upon adenoviral infection of cells, 6 increased glucose uptake, and metabolism was detected as early as 1957 in cells infected with poliovirus 7 and subsequently reported in a variety of other viral infections. 1 , 8 , 9 , 10 , 11 Therefore, increased glucose uptake is not only recognized as an inherent feature of most cancers but also appears to be a common trait of viral‐infected cells.
2. 2‐DG EXPLOITS CANCER‐INDUCED INCREASED GLUCOSE UPTAKE AND METABOLISM BY BLOCKING GLYCOLYSIS AND INTERFERING WITH N‐LINKED GLYCOSYLATION
To take advantage of this natural trait in cancer, we (T. J. L.) have been developing 2‐deoxy‐d‐glucose (2‐DG), an analog of glucose, as a non‐toxic anti‐cancer agent and have found through an FDA‐approved Phase I clinical trial that it is well‐tolerated and safe in patients. 12 , 13 In the trial, 2‐DG was used for its well‐known property of inhibiting glycolysis, thereby targeting cancer cells that are resistant to chemotherapy or radiation due to their slow growth.
Since most solid tumors contain hypoxic areas that harbor slow growing and or cancer cells in G0, they can be selectively killed by 2‐DG through its inhibitory activity on glycolysis, the pathway cells depend on for survival under the environment of low oxygen. 13 In early experiments, indeed we reported that tumor cells treated with 2‐DG growing in different models of anaerobiosis undergo cell death. 14 Moreover, in a transgenic model of retinoblastoma, the targeting of hypoxic cells in vivo by 2‐DG was demonstrated using the hypoxic sensing agent pimonidazole. 15 In this experiment, not only was it shown that 2‐DG could eliminate the hypoxic population but also that carboplatin used to kill the rapidly replicating cells could not. Thus, shutdown of glycolysis produces energy stress on the cancer cell, which we show in vitro and in vivo is toxic to cells under anerobic growth conditions. Anaerobiosis provides a window of selectivity that can be exploited by 2‐DG, since tumor cells take up more glucose, and therefore more 2‐DG, than normal cells, and even more so when growing under hypoxia.
By mimicking mannose as well as glucose, 2‐DG also produces another kind of stress in the cancer cell when grown under normal oxygen conditions referred to as endoplasmic reticulum (ER) stress, inducing an unfolded protein response (UPR), that in turn shuts down further protein synthesis in order to alleviate this kind of stress. 16 ER stress and the induction of the UPR can be reversed by adding low levels of exogenous mannose but not glucose further supporting a mannose pathway induction of ER stress and UPR activation by 2‐DG under normoxia. 16 Since enveloped viruses use the ER of the cell, they infect to produce massive amounts of viral glycoproteins which would normally lead to UPR‐mediated blockage of protein synthesis, they have had to develop ways to overcome this to allow them to be produced. 17 , 18 , 19
3. 2‐DG EXPLOITS VIRAL‐INDUCED INCREASED GLUCOSE UPTAKE AND METABOLISM BY BLOCKING GLYCOLYSIS AND INTERFERING WITH N‐LINKED GLYCOSYLATION
Based on this understanding, we reasoned that pre‐ or simultaneous treatment of viral‐infected cells with 2‐DG would activate the UPR shutting down protein synthesis and thereby blocking viral replication. This idea was successfully tested in Kaposi's sarcoma Herpes virus, (KSHV) an enveloped oncogenic human virus known to be the etiological agent of Kaposi's sarcoma, the most prevalent AIDS‐associated cancer. 20 Following investigations reported that 2‐DG blocks viral infections in non‐enveloped viruses such as rhinovirus 2 as well as the enveloped virus, Porcine Epidemic Diarhea Virus (PEDV), a porcine alpha coronavirus. 21 Thus, it appears that 2‐DG is unique as an anti‐viral agent, in that, it is able to target both glycolytic reprogramming and UPR during viral infections (Figure 2).
FIGURE 2.
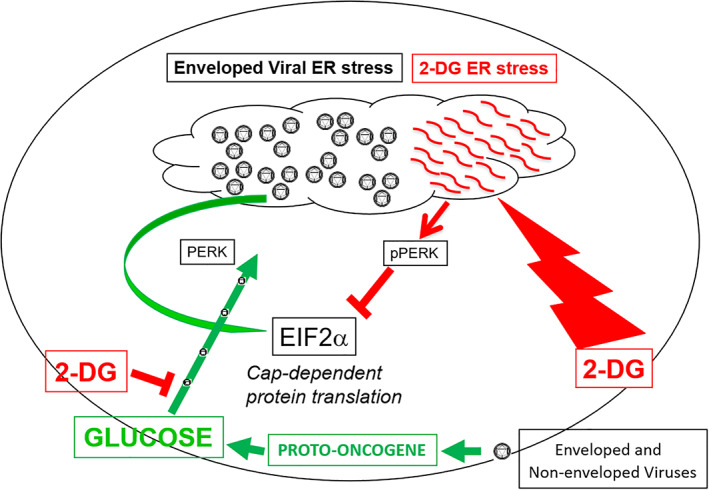
2‐Deoxy‐d‐glucose (2‐DG) exploits viral‐induced increased glucose uptake and metabolism by blocking glycolysis and interfering with N‐linked glycosylation. Viral infection has been shown to increase glucose uptake and metabolism via activation of proto‐oncogenes. 22 As an analog of glucose, 2‐DG preferentially accumulates in viral‐infected cells and acts to block glycolysis, which supplies the building blocks required for non‐enveloped as well as enveloped viral replication and production. As an analog of mannose, 2‐DG fraudulently incorporates into the growing oligosaccharide chain required for N‐linked glycosylation leading to unfolded glycoproteins inducing ER stress and activation of PERK (a component of the unfolded protein response [UPR]) which in turn shuts down cap‐dependent translation by phosphorylating EIF2α resulting in inhibition of enveloped viral maturation and replication. 2‐DG‐induced ER stress has been shown to block viral replication in Kaposi's sarcoma (Herpes) virus and PEDV (a coronavirus) via activating the UPR and blocking EIF2α‐cap dependent protein translation. 20 , 21 Since SARS‐CoV‐2 is also an enveloped virus, this latter 2‐DG effect most likely contribute to its anti‐viral activity
In the report where nasal spray of 2‐DG was used as the delivery system in animal models to block the replication of a rhinovirus (non‐enveloped), which causes the common cold, it was also found that the virus upregulated glycolytic enzymes and glucose metabolism in the cells it infected. Thus, the most likely mechanism by which 2‐DG blocks this type of viral infection is through the inhibition of glycolysis. 2 In the case of the enveloped coronavirus, Porcine Epidemic Diarrhea Virus (PEDV), 2‐DG was shown to be blocking its replication via induction of UPR‐mediated shutdown of protein synthesis. 21
More recently, prompted by proteomic analysis which showed that SARS‐CoV‐2 also led to reprograming of infected cells to a more glycolytic status, 2‐DG was shown to successfully block this metabolic alteration and significantly lower viral titer. 23 Similar increased glycolysis and 2‐DG's inhibitory effects on viral replication were also recently found in monocytes infected with SARS‐CoV‐2. 24 Based on the previous results cited in PEDV, a corona virus as well as for the other enveloped virus, KSHV, where 2‐DG mediated UPR leads to inhibition of viral replication, it is likely that a similar mechanism is contributing to the reported blockage of SARS‐CoV‐2 replication by 2‐DG (Figure 2).
4. RELEVANCE TO USE OF 2‐DG IN INDIA AGAINST SARS‐COV‐2
Thus, it is not very surprising that amid the dire health crisis triggered by the second SARS‐CoV‐2 wave in India, characterized by air‐borne contagion and new variants including vaccine breakthrough cases, as reported in the Indian press, a 2‐DG oral delivery form has now received emergency approval to help control SARS‐CoV‐2 infection. The Drugs Controller General of India cleared the drug after clinical trial results showed that 2‐DG helps in faster recovery of hospitalized patients and reduces supplemental oxygen dependence.
It was found that a higher proportion of patients treated with the drug tested negative for Covid in RT‐PCR tests. In a phase II trial, the dose of 63 mg/kg body patient weight, which was the dose we established in our clinical trial for cancer, 12 has been preliminarily found to be safe in trials in COVID‐19 patients and shown to significantly improve their recovery. The second trial was in 110 patients while the third round of trials was conducted in six hospitals, “dose ranging” in 11 hospitals across India as continuing. In these latter trials, 45 mg/kg 2× per day was found to be safe and effective. It should be noted that the clinical data reported in India to date has not been published. The results shown here, are as reported in the press, as well as recently presented in a virtual meeting held jointly by a laboratory of the Defence Research and Development Organization, in collaboration with Dr. Reddy's Labs who are responsible for the clinical trials.
It is possible that 2‐DG may not only be effective in treating those already infected by coronavirus, but as shown in the rhinoviral report, 2 2‐DG delivered by nasal spray could be used as a barrier to prevent or reduce the chance of viral infection in “at risk” and “highly exposed” individuals. Moreover, the increasing emergence of SARS‐CoV‐2 variants, as well as the occurrence of breakthrough cases, support the need for developing mechanistic‐based drugs such as 2‐DG.
5. PLEIOTROPIC ACTIVITY OF 2‐DG AGAINST DIFFERENT VIRAL TYPES
Dating back to the 1970s, 2‐DG has been demonstrated to block replication of a variety of viral types by a number of different mechanisms which include the following: (a) Incorporating falsely into the structure of the viral capsid (head), leading to attenuation of its ability to become a fully infectious virus 25 ; (b) Interfering with normal glycoprotein folding in the ER leading to ER stress and blocking viral replication by activating the UPR thereby shutting down protein synthesis 20 , 21 ; and (c) By inhibiting glycolysis, 2‐DG shuts off the building blocks required for a virus to replicate. 2
In addition to its anti‐viral effects, 2‐DG has also been shown to have anti‐inflammatory effects that may benefit COVID‐19 patients, 2 especially in the latter phase of their disease, where runaway inflammation appears to play a significant role in the pathogenesis and morbidity of this disease. In support of this possibility, in a recent review 2‐DG's inhibitory effects on subsets of the immune response were summarized and shown to be most pronounced on those commonly referred to as pro‐inflammatory since these immune cells commonly undergo increased glycolysis when activated. 26 Thus, inhibition of glycolysis appears to have selective effects on activated immune cells most closely associated with pro‐inflammatory activities.
Using what is known as “in silico molecular modeling” (a non‐cellular method which predicts how a drug will bind to its target and therefore inhibit its function), a new mechanism of how 2‐DG may be effective against SARS has recently been preliminarily (not yet peer‐reviewed) reported. 27 It was found that the structure of 2‐DG fits into several of the binding sites that allow SARS‐CoV‐2 to attach as well as to replicate once it gets into a cell.
This is the first report that examines the SARS‐CoV‐2 sites that 2‐DG directly could have significant anti‐viral activity on. It predicts that 2‐DG will interfere with viral pathogenesis by binding what is known as its protease as well as its endoribonuclease. Each of these sites is required for proper viral replication and infectivity. Moreover, the authors show that the docking of 2‐DG with the main protease 3CLpro and NSP15 endoribonuclease of COVID‐19 is significantly better than that of the standard anti‐viral drugs lopinavir and favipiravir.
This recent finding together with (a) the aforementioned reports of anti‐replicative activity of 2‐DG against SARS‐CoV‐2 in vitro; (b) the anti‐viral activity of 2‐DG previously reported in a number of different virus types; and (c) the number of mechanisms by which it inhibits viral replication, adds further support for 2‐DG (nasal spray) to be tested against COVID‐19 in animals and if found to be beneficial, in humans.
6. CAUTION REQUIRED IN DETERMINING BEST WAY TO TREAT PATIENTS WITH 2‐DG
It should be noted that although the above studies provide overwhelming support for 2‐DG to be used in the fight against COVID‐19, caution must be taken to determine how humans undergoing infection with this particular virus will respond to 2‐DG. Although the human trials done in India to date indicate that it is safe, it remains to be seen whether this continues as the number of patients treated with 2‐DG expand.
The caution is based on a report that at high doses of 2‐DG delivered by injection systemically (2.5 mg/ml twice per day) 2‐DG, or fasting, are detrimental in an animal model of influenza, increasing the death rate. 28 In a herpes simplex eye infection animal model, 29 similar detrimental results were obtained when 2‐DG was applied at high dose by IP injection at early stages, but beneficial effects were observed when applied in the latter stages of the viral infection.
In the former report, differential alterations in glucose metabolism in the different phases of diseases appeared to account for opposite effects when either fasting or 2‐DG treatment was used, with the anti‐inflammatory effects of 2‐DG most likely accounting for the beneficial effects observed in the latter phase of viral‐induced pathology. 28 There appear to be a complicated relationship between a beneficial immune response leading to protection against foreign insults and a consequential inflammatory response, both of which may be beneficial or detrimental.
Fortunately, in the human trials completed in India (to date) apparently detrimental effects were not observed (at least in the number of patients studied [>400 < 500]). Moreover, the work published in the rhinovirus animal model 2 also did not describe this complication and in fact showed a dramatic drop in both viral load as well as inflammation leading to a prevention and/or rapid resolution of a deadly form of pneumonia. These two observations bring up the possibility that either the delivery system of 2‐DG (nasal spray 2 as opposed to IP injection, 28 , 29 ) plasma concentration of 2‐DG, or viral type contribute to the differences reported in these three different viral animal models as well as in human trials.
Noteworthy, is that the inflammatory response which appears to contribute significantly to the unusually deadly pathogenesis of COVID‐19 may clearly benefit from the known anti‐inflammatory activity of 2‐DG mentioned above. 2 , 26
7. METRONOMIC DELIVERY OF 2‐DG IN STAGE 4 CANCER PATIENTS
To add to the safety of 2‐DG treatment that has been established in previous human trials, recently 2‐DG has been preliminarily observed to be safe and well tolerated in a small number of end‐stage diseased cancer patients when treated metronomically (slow‐drip 24–48 hr slow infusion) with low doses for periods up to 2 years (unpublished results D. Stanciu and T. J. Lampidis). Metronomic delivery of 2‐DG was developed to circumvent the insulin surge we observed when cancer patients in our phase I trial were treated with a daily bolus oral dose of 2‐DG. 23 It, therefore, remains to be seen whether COVID‐19 patients treated orally (as in the trials in India) experience increase in insulin, although it proved to be transitory and cleared up in cancer patients shortly after each treatment.
This information together with a preclinical study in a human xenograft cancer model 30 suggests that metronomic delivery of 2‐DG may be the best and safest way to proceed with those patients who are in the later stages of COVID‐19 disease. If these patients' viral titers have already reached their peak and they are suffering from a runaway inflammatory reaction, then indeed 2‐DG with its anti‐inflammatory as well as its anti‐viral activity may significantly benefit this group and save lives. However, if the disease has progressed to a stage where the lungs or other organs have been permanently damaged, 2‐DG unfortunately will yield little benefit. From the human trial information in India, so far made available to the public, this seems to be the case.
A thorough understanding of when and how 2‐DG can be given in the course of COVID‐19 infection is key to being able to apply it safely and effectively. Although there may be other analogs of glucose in addition to 2‐DG that may prove to be as or more effective in either of its pleiotropic mechanisms of action, at this point based on all we know about its mechanisms, safety record in humans, and availability, 2‐DG seems best suited for further testing and rapid development for clinical use.
8. VIRUS ACTIVATION OF ONCOGENES IN UP‐REGULATING GLUCOSE METABOLISM
Interestingly, a pathway by which virus infection increases glucose metabolism was shown to be through activation of MYC, one of the earliest oncogenes reported to drive glucose metabolism in cancer cells. 31 This finding not only highlights the relationship between cancer and viral‐infected cells in their shared trait of increased glucose uptake, but also raises the question of whether certain viral infections could perhaps contribute to the onset of oncogenesis by transitory oncogene activation?
In fact, in a recent review, 32 it was suggested that the presence of NSP15 endonuclease in SARS‐CoV‐2 which was shown in SARS‐CoV‐1 to downregulate RB thereby promoting cell proliferation, 33 could do the same in SARS‐CoV‐2 infections. In another report, SARS coronavirus was shown to interact with p53 further suggesting a relationship between SARS‐C0V‐2 infections and cancer related genes. 34 For a more comprehensive account of the pathways by which oncogenes are involved in increased glucose metabolism by different viral types, please see the review by Mayer et al. 22
9. SUMMARY
In summary, viruses, as well as cancer cells, use glucose not only as a vital energy source but also as the building blocks for further production, which has been demonstrated with carbon 13 tracer experiments and mass spectrometry. Thus, taking advantage of increased glucose metabolism, 2‐DG will preferentially accumulate in cancer as well viral‐infected cells, which separates it from other anti‐cancer and or anti‐viral agents that enter normal or viral‐infected and non‐infected cells equally. The selectivity of 2‐DG in addition to it being easily applied, nontoxic, and a rapid inexpensive means of treating viral outbreaks, further supports its use in India and strongly encourages further development and expansion which hopefully will prove to be practical and effective for treating SARS‐CoV‐2 as well other emerging coronavirus variants worldwide.
DISCLOSURE OF INTERESTS
Theodore J. Lampidis is an unpaid member of the scientific advisory board of G.ST Anti‐virals, a startup company in Vienna Austria that is developing delivery of 2‐DG via nasal spray for rhinoviral infections.
The University of Miami, where Dr. Lampidis is employed, has signed a collaborative contract with G.ST Anti‐virals.
Enrique Mesri reports no conflicts of interest.
ACKNOWLEDGMENTS
We would like to acknowledge the helpful discussions with Dr. Daniel Stanciu at MCS Foundation for Life, on the mechanisms and clinical applications of 2‐DG as well as his assistance in developing and implementing the metronomic delivery of 2‐DG protocol for stage 4 cancer patients who are currently under treatment in several different countries. Additionally, we recognize, Dr. Metin Kurtoglu, Dr. John Maher, and Dr. Huaping Liu who were instrumental for generating much of the basic information on how 2‐DG preferentially accumulates and the mechanisms by which it exploits oncogene‐driven increased glucose metabolism in cancer.
E. A. M. is supported by PHS grant CA136387.
T. J. L. as co‐investigator is partially supported by National Institutes of Health grant GM138557.
Mesri EA, Lampidis TJ. 2‐Deoxy‐d‐glucose exploits increased glucose metabolism in cancer and viral‐infected cells: Relevance to its use in India against SARS‐CoV‐2. IUBMB Life. 2021;73:1198–1204. 10.1002/iub.2546
We dedicate this review to distinguished fellow of The Royal Society, Professor William J. Whelan, who as an eminent biochemist discovered how glucose is converted to glycogen by identifying and characterizing the enzyme known as glycogenin. Professor Whelan has made everlasting contributions to science and to its worldwide dissemination, which humanity will continue to benefit from.
[Correction added after first online publication on September 27 2021. MCS Cancer Treatments Foundation has been replaced with MCS Foundation for Life in Acknowledgment section]
Funding information PHS, Grant/Award Number: CA136387; National Institutes of Health, Grant/Award Number: GM138557
REFERENCES
- 1. Delgado T, Carroll PA, Punjabi AS, et al. Induction of the Warburg effect by Kaposi's sarcoma herpesvirus is required for the maintenance of latently infected endothelial cells. Proc Natl Acad Sci USA. 2010;107:10696–10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gualdoni GA, Mayer KA, Kapsch AM, et al. Rhinovirus induces an anabolic reprogramming in host cell metabolism essential for viral replication. Proc Natl Acad Sci USA. 2018;115(30):E7158–E7165. 10.1073/pnas.1800525115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thaker SK, Ch'ng J, Christofk HR. Viral hijacking of cellular metabolism. BMC Biol. 2019;17:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osthus RC, Shim H, Kim S, et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c‐Myc. J Biol Chem. 2000;275(29):21797–21800. [DOI] [PubMed] [Google Scholar]
- 5. Vander Heiden MG, Locasale JW, Swanson KD, et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Munger J, Bennett BD, Parikh A, et al. Systems‐level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat Biotechnol. 2008;26(10):1179–1186. 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levy HB, Baron S. The effect of animal viruses on host cell metabolism II. Effect of poliomyelitis virus on glycolysis and uptake of glycine by monkey kidney tissue cultures. J Infect Dis. 1957;100:109–118. 10.1093/infdis/100.2.109. [DOI] [PubMed] [Google Scholar]
- 8. Bardell D. Glucose uptake and lactic acid production of adenovirus type 5‐infected HEp‐2 cells cultured under exponential growth and stationary phase conditions. Microbios. 1977;20:139–144. [PubMed] [Google Scholar]
- 9. Landini MP. Early enhanced glucose uptake in human cytomegalovirus‐infected cells. J General Virol. 1984;65(Pt 7):1229–1232. [DOI] [PubMed] [Google Scholar]
- 10. Ritter JB, Wahl AS, Freund S, Genzel Y, Reichl U. Metabolic effects of influenza virus infection in cultured animal cells: Intra‐ and extracellular metabolite profiling. BMC Syst Biol. 2010;4:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fontaine KA, Sanchez EL, Camarda R, Lagunoff M. Dengue virus induces and requires glycolysis for optimal replication. J Virol. 2015;89:2358–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raez LE, Papadopoulos K, Ricart AD, et al. A phase I dose‐escalation trial of 2‐deoxy‐D‐glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013;71(2):523–530. 10.1007/s00280-012-2045-1. [DOI] [PubMed] [Google Scholar]
- 13. Xi H, Kurtoglu M, Lampidis TJ. The wonders of 2‐deoxy‐D‐glucose. IUBMB Life. 2014;66(2):110–121. 10.1002/iub.1251. [DOI] [PubMed] [Google Scholar]
- 14. Maher JC, Krishan A, Lampidis TJ. Greater cell cycle inhibition and cytotoxicity induced by 2‐deoxy‐D‐glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer Chemother Pharmacol. 2004;53:116–122. [DOI] [PubMed] [Google Scholar]
- 15. Boutrid H, Jockovich ME, Murray T, et al. Targeting hypoxia, a novel treatment for advanced retinoblastoma. Invest Ophthalmol Vis Sci. 2008;49:2799–2805. [DOI] [PubMed] [Google Scholar]
- 16. Kurtoglou M, Gao N, Shang J. Under normoxia, 2‐deoxy‐D‐glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N‐linked glycosylation. Mol Cancer Ther. 2007;6(11):3049–3058. [DOI] [PubMed] [Google Scholar]
- 17. Child SJ, Hakki M, De Niro KL, Geballe AP. Evasion of cellular antiviral responses by human cytomegalovirus TRS1 and IRS1. J Virol. 2004;78:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double‐stranded RNA‐ activated protein kinase. Proc Natl Acad Sci U S A. 1997;94:843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mulvey M, Arias C, Mohr I. Maintenance of endoplasmic reticulum (ER) homeostasis in herpes simplex virus type 1‐infected cells through the association of a viral glycoprotein with PERK, a cellular ER stress sensor. J Virol. 2007;81:3377–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leung HJ, Duran EM, Kurtoglu M, Andreansky S, Lampidis TJ, Mesri EA. Activation of the unfolded protein response by 2‐deoxy‐D‐glucose inhibits Kaposi's sarcoma‐associated herpesvirus replication and gene expression. Antimicrob Agents Chemother. 2012;56(11):5794–5803. 10.1128/AAC.01126-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Li JR, Sun MX, et al. Triggering unfolded protein response by 2‐deoxy‐D‐glucose inhibits porcine epidemic diarrhea virus propagation. Antiviral. Res. 2014;106:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mayer KA, Stöckl J, Zlabinger GJ, Gualdoni GA. Hijacking the supplies: Metabolism as a novel facet of virus‐host interaction. Front Immunol. 2019;10:1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bojkova D, Klann K, Koch B, et al. SARS‐CoV‐2 infected host cell proteomics reveal potential therapy targets. Nature. 2020;583:469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Codo AC, Davanzo GG, de Brito Monteiro L, et al. Elevated glucose levels favor SARS‐CoV‐2 infection and monocyte response through a HIF‐1α/glycolysis‐dependent Axis. Cell Metab. 2020;32(3):437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Datema R, Schwarz RT. 2‐DG incorporates fraudulently into oligosaccharide chain mannose disrupting influenza viral glycoprotein capsid formation. Eur J Biochem. 1979;184:113–123. [Google Scholar]
- 26. Kornberg MD. The immunologic Warburg effect: Evidence and therapeutic opportunities in autoimmunity. Wiley Interdiscip Rev Syst Biol Med. 2020;12(5):e1486. 10.1002/wsbm.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Balkrishna A, Thakur P, Singh S, et al. Glucose antimetabolite 2‐Deoxy‐D‐Glucose and its derivative as promising candidates for tackling COVID‐19: Insights derived from in silico docking and molecular simulations. Authorea. 2021. 10.22541/au.158567174.40895611/v2. [DOI] [Google Scholar]
- 28. Wang A, Huen SC, Luan HH, Gallezot J‐D, Booth CJ, Medzhitov R. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. J Immunol. 2017;199(5):1748–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Varanasi SK, Donohoe D, Jaggi U, Rouse BT. Manipulating glucose metabolism during different stages of viral pathogenesis can have either detrimental or beneficial effects. J Immunol. 2017;199(5):1748–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu H, Kurtoglu M, León‐Annicchiarico CL, et al. Combining 2‐deoxy‐D‐glucose with fenofibrate leads to tumor cell death mediated by simultaneous induction of energy and ER stress. Oncotarget. 2016;7(24):36461–36473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thai M, Graham NA, Braas D, et al. Adenovirus E4ORF1‐induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metab. 2014;19:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alpalhão M, Ferreir AJ, Filipea P. Persistent SARS‐CoV‐2 infection and the risk for cancer. Med Hypotheses. 2020;143:109882. 10.1016/j.mehy.2020.109882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bhardwaj K, Liu P, Leibowitz JL, Kao CC. The coronavirus endoribonuclease Nsp15 interacts with retinoblastoma tumor suppressor protein. J Virol. 2012;86:4294–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ma‐Lauer Y, Carbajo‐Lozoya J, Hein MY, et al. P53 downregulates SARS coronavirus replication and is targeted by the SARS‐unique domain and PLpro via E3 ubiquitin ligase RCHY1. Proc Natl Acad Sci USA. 2016;113:E5192–E5201. [DOI] [PMC free article] [PubMed] [Google Scholar]


