To the Editor:
Patients with rheumatic and musculoskeletal diseases (RMDs) who are taking immunosuppressants have been considered to be at increased risk of developing SARS–CoV‐2 infection during the COVID‐19 pandemic, and vaccination is the mainstay for the prevention of this infection (1). To date, recommendations and data for COVID‐19 vaccination in adolescent patients with RMDs are lacking (2). Reports from international societies and post‐authorization safety studies of the novel messenger RNA (mRNA) COVID‐19 vaccines are generally reassuring; however, in adolescent RMD patients treated with immunomodulators, the safely profile of mRNA COVID‐19 vaccines is unknown because adolescents with RMD were excluded from the vaccine trials (3, 4, 5). Furthermore, there is a theoretical risk of RMD flare related to the mRNA COVID‐19 vaccines (1, 2). Nevertheless, the estimated risks and benefits clearly favor vaccination (1, 2). In a population of adult RMD patients receiving non–B cell–depleting therapy, it was demonstrated that after 2 doses of a COVID‐19 mRNA vaccine, the vast majority of patients developed a positive antibody response (though data on relative amount of antibody responses are still lacking) and experienced only minor side effects with no apparent disease exacerbation/flare (6).
Recently we performed a study that aimed to evaluate the safety and tolerability of the BNT162b2 COVID‐19 vaccine (BioNTech; Pfizer) in adolescents with juvenile idiopathic arthritis (JIA) treated with tumor necrosis factor (TNF) inhibitors. This single‐center study included adolescent patients (ages 16–21 years) with stable JIA who had been receiving treatment with TNF inhibitors for at least 1 year following the diagnosis. Written informed consent was obtained at enrollment. The patients received 2 doses of the COVID‐19 mRNA vaccine intramuscularly, with the initial dose and follow‐up dose administered between April 15 and May 15, 2021 (designated 0 weeks and 3 weeks, respectively). Follow‐up visits were planned for 1, 2, and 3 months after vaccination. All participants were observed for 30 minutes after the injection and were given a diary card to record the occurrence of local or systemic symptoms for the following 14 days. Adverse reactions were defined as any reaction that lasted for >7 days after vaccination, and serious adverse reactions were defined as any reaction requiring medical attention or hospitalization. Disease activity was evaluated using the Juvenile Arthritis Disease Activity Score in 27 joints (JADAS‐27) (7). Data were analyzed using SPSS version 18.0 software. P values less than 0.05 were considered significant.
A total of 21 adolescent patients were enrolled in our study. Demographic and clinical characteristics are shown in Table 1. Both doses of the vaccine were well tolerated by all of the participants. Local reactions were frequent in the majority of participants (74%) (Table 1). No difference in reaction was noted between the patients taking etanercept versus those taking adalimumab (71% versus 75%, respectively; P = 0.09) or in patients with different JIA types. In addition, systemic reactions were relatively infrequent (19%) (Table 1). There were no differences in the rates of systemic reactions according to the type of JIA or the medication received. Most localized and systemic reactions were noted after the second dose of the vaccine (P = 0.02). One patient developed hives after the second dose, which was alleviated with antihistamines. JIA was in clinical remission in all patients at the time of vaccination. No exacerbation of underlying disease was noted, based on evaluation of the JADAS‐27 at 1 month before the vaccination, as well as at 1 and 3 months after the second dose of vaccination (Figure 1). There were no significant changes in the JADAS‐27 (P = 0.417) or in laboratory test results (C‐reactive protein, erythrocyte sedimentation rate, and white blood cell count) at follow‐up over a period of 3 months (P = 0.1, P = 0.09, and P = 0.4, respectively) (data not shown). None of the participants discontinued treatment with TNF inhibitors at the time of vaccine administration or during the follow‐up period.
Table 1.
Baseline characteristics, treatments, and frequency of AEs occurring after immunization with the COVID‐19 messenger RNA vaccine among adolescent patients with JIA treated with TNF inhibitors*
| Demographic and clinical characteristics (n = 21) | |
| Age, median (IQR) years | 17 (16–21) |
| Sex | |
| Male | 5 (24) |
| Female | 16 (76) |
| Polyarticular JIA | 8 (38) |
| Psoriatic JIA | 7 (33) |
| ERA | 6 (29) |
| Treatment (n = 21) | |
| TNF inhibitors | |
| Adalimumab | 10 (48) |
| Etanercept | 11 (52) |
| Other concurrent treatment, methotrexate | 15 (71) |
| Postvaccination AEs (n = 21 patients, n = 42 vaccine doses) | |
| Local | 31 (74) |
| Erythema | 21 (50) |
| Pain | 32 (76) |
| Swelling | 12 (29) |
| Systemic | 8 (19) |
| Headache | 7 (17) |
| Myalgias | 5 (12) |
| Fatigue | 6 (14) |
| Transient arthralgia | 5 (12) |
| Allergic reaction | 1 (2) |
| Exacerbation of JIA | 0 (0) |
| Serious AEs | 0 (0) |
Except where indicated otherwise, values are the number (%). AEs = adverse events; JIA = juvenile idiopathic arthritis; TNF = tumor necrosis factor; IQR = interquartile range; ERA = enthesitis‐related arthritis.
Figure 1.
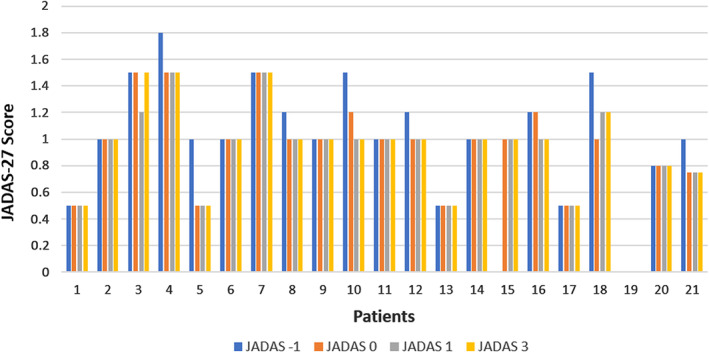
Disease Activity Score in 27 joints (JADAS‐27) after vaccination with the BNT162b2 COVID‐19 messenger RNA vaccine (BioNTech; Pfizer) in 21 adolescent patients with juvenile idiopathic arthritis treated with tumor necrosis factor inhibitors. No significant changes in the JADAS‐27 were noted at 1 month prior to the vaccination (JADAS −1), at the time of vaccination (JADAS 0), 1 month after vaccination (JADAS 1), or 3 months after vaccination (JADAS 3) (P = 0.417 by Kruskal‐Wallis H test).
This is the first study demonstrating that mRNA vaccines appear to be safe and well tolerated in adolescents with JIA receiving treatment with TNF inhibitors. Although our sample size was small and a limited number of patients were included within each JIA type and treatment group, it may be concluded that the vaccine has an adequate safety and tolerability profile and does not provoke disease flare. As there are no studies examining the safety and effectiveness of COVID‐19 vaccines in this population, further studies are needed to evaluate the immune response, analyze the immunogenicity of the 2‐dose schedule, and determine the real duration of immune protection.
Supporting information
Disclosure Form
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Fart.41977&file=art41977-sup-0001-Disclosureform.pdf.
References
- 1. Schulze‐Koops H, Specker C, Skapenko A. Vaccination of patients with inflammatory rheumatic diseases against SARS‐CoV‐2: considerations before widespread availability of the vaccines. RMD Open 2021;7:e001553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Furer V, Rondaan C, Agmon‐Levin N, van Assen S, Bijl M, Kapetanovic MC, et al. Point of view on the vaccination against COVID‐19 in patients with autoimmune inflammatory rheumatic diseases. RMD Open 2021;7:e001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, et al. American College of Rheumatology guidance for COVID‐19 vaccination in patients with rheumatic and musculoskeletal diseases: version 1. Arthritis Rheumatol 2021;73:1093–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bijlsma JW. EULAR December 2020 view‐points on SARS‐CoV‐2 vaccination in patients with RMDs. Ann Rheum Dis 2021;80:411–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paediatric Rheumatology European Association (PRES) . Guidelines and recommendations. PRES update regarding COVID‐19 vaccines in pediatric rheumatic patients. December 2020. URL: https://www.pres.eu/clinical-affairs/guidelines.html.
- 6. Braun‐Moscovici Y, Kaplan M, Braun M, Markovits D, Giryes S, Toledano K, et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS‐CoV2. Ann Rheum Dis 2021;80:1317–21. [DOI] [PubMed] [Google Scholar]
- 7. Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni‐Manzoni S, Filocamo G, et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum 2009;61:658–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form


