Abstract
Kidney transplant recipients (KTRs) are extremely vulnerable to SARS-CoV-2 infection and show an impaired immune response to SARS-CoV-2 vaccination. We analyzed factors related to vaccination efficiency in KTRs. In a multicenter prospective observational study (NCT04743947), IgG antibodies levels against SARS-CoV-2 spike S1 subunit and their neutralization capacity after SARS-CoV-2 vaccination were analyzed in 225 KTRs and compared to 176 controls. After the vaccination, 56 (24.9%) KTRs became seropositive of whom 68% had neutralizing antibodies. This immune response was significantly lower compared to controls (239 [78–519] BAU/ml versus 1826 [560–3180] BAU/ml for KTRs and controls, p < .0001). The strongest predictor for an impaired response was mycophenolate mofetil (MMF) treatment. Multivariate regression analysis revealed that MMF-free regimen was highly associated with seroconversion (OR 13.25, 95% CI 3.22–54.6; p < .001). In contrast, other immunosuppressive drugs had no significant influence. 187 out of 225 KTRs were treated with MMF of whom 26 (13.9%) developed antibodies. 23 of these seropositive KTRs had a daily MMF dose ≤1 g. Furthermore, higher trough MMF concentrations correlated with lower antibody titers (R −0.354, p < .001) supporting a dose-dependent unfavorable effect of MMF. Our data indicate that MMF dose modification could lead to an improved immune response.
KEYWORDS: clinical research/practice, immunosuppression/immune modulation, immunosuppressive regimens, infection and infectious agents - viral, kidney transplantation/nephrology, vaccine
Abbreviations: BAU, binding antibody units; CPE, cytopathic effect; KTRs, kidney transplant recipients; MMF, mycophenolate mofetil; NT, neutralization efficacy; SARS-CoV-2, severe, acute respiratory syndrome coronavirus type 2
1. BACKGROUND
In comparison to the general population, kidney transplant recipients (KTRs) have a significantly higher risk of severe, life-threatening acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection.1 Currently, vaccination against SARS-CoV-2 appears to be the best prophylaxis against the severe course of COVID-19 infection. Unfortunately, recent observational studies suggest, that the majority of KTRs do not develop sufficient antibody levels after SARS-CoV-2 vaccination.2, 3, 4, 5 Moreover, the occurrence of COVID-19 infection among vaccinated KTRs is almost always related to seronegative status.6 , 7 Based on these disappointing data, it is essential to identify factors influencing the immune response in KTRs. The overall goal is to develop new SARS-CoV-2 vaccination strategies which increase the probability for a positive immune response.
2. METHODS
During this prospective multicenter observational study, the humoral immune response to SARS-CoV-2 vaccination (either BNT162b2; Biontech/Pfizer or mRNA-1273; Moderna) was measured in 225 KTRs (NCT04743947) and compared to 176 volunteers (controls). Shortly, the previously described control group was composed of volunteers from a nursing home, who had no history of kidney failure.8 Twenty-eight of the 225 KTRs were included in a previously published study.9 All participants had to be older than 18 years, with no history of previous COVID-19 and able to give informed consent to participate in the study. All KTRs were on stable immunosuppressive medication. None of the KTRs had an acute graft rejection. Eight KTRs were treated for a rejection in the last 12 months. Mentioned vaccines were administered as advised by the manufacturer. The study was approved by the ethics committee of the Medical Faculty at the Heinrich-Heine University, Düsseldorf, Germany (study numbers 2020–1237 and 2021–1287, respectively) and in line with the Declaration of Helsinki, as revised in 2013.
Immune response to SARS-CoV-2 vaccination was measured at mean 14 ± 2 days and 17 days post vaccination in KTRs and control group respectively. All samples were tested for IgG antibodies against SARS-CoV-2 spike S1 subunit using Anti-SARS-CoV-2-QuantiVac-ELISA (Euroimmun AG) as well as for SARS-CoV-2 neutralization efficacy (NT) at the Institute of Virology, University Hospital Düsseldorf, Germany. According to the manufacturer’s instruction results <25.6 BAU/ml were considered as negative, ≥25.6 BAU/ml and ≤35.2 BAU/ml as indeterminate, and >35.2 BAU/ml as positive (BAU, Binding Antibody Units). The upper detection limit for undiluted samples was >384 BAU/ml, the lower detection limit was <3.2 BAU/ml. For samples above the detection limit, 1:10 or 1:100 dilutions were performed in IgG sample buffer according to the manufacturer’s instructions.
To detect the neutralizing capacity of the Anti-SARS-CoV-2 antibodies after the second vaccination, an endpoint dilution neutralization test with the infectious SARS-CoV-2 B.1 isolate (EPI_ISL_425126) at a TCID 50 of 100 was performed in a BSL-3 facility as described previously.10 At the time of this study, no other variants were established in the lab for the neutralization test. The neutralization titer was determined as the highest serum dilution without virus-induced cytopathic effect (CPE).
Statistical analysis was performed using SPSS version 23 (SPSS Inc.) and Graph Prism 5.3 (GraphPad Software). Data distribution was evaluated using Shapiro–Wilk normality test and expressed as mean ± standard deviation (SD) or median (interquartile ranges expressed as two numbers, Q1–Q3, respectively). Categorical variables are expressed as number (percentage). The difference among immune response groups was evaluated using the Chi-square test or Mann–Whitney test where appropriate. Multivariate logistic regression was used for indicating variables associated with a positive immune response after SARS-CoV-2 vaccination. p values less than 0.05 were considered statistically significant.
3. RESULTS
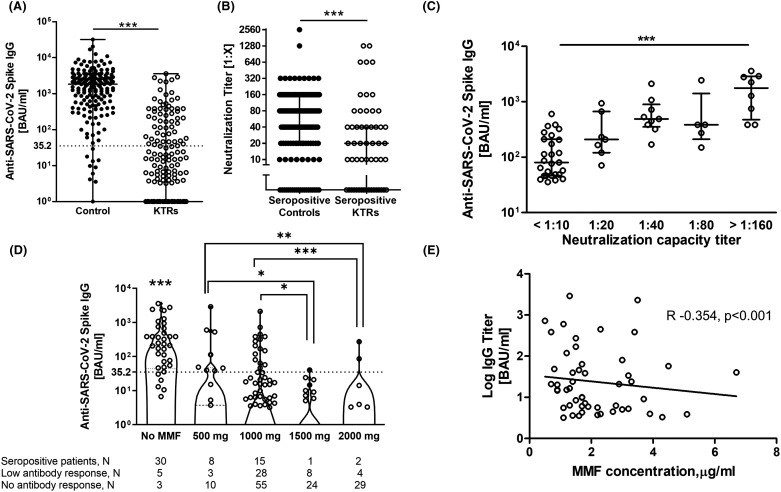
Characteristics of the KTR population are presented in Table 1. The median age of controls (60 years [54–69]) did not differ to that of KTRs (62 years [54–70]). 64.8% of KTRs and 37% of controls were male. Two weeks after the second vaccination, 56 (24.9%) of 225 KTRs and 165 (93.8%) out of 176 controls became seropositive. Median IgG levels were significantly lower in KTRs compared to controls (239 [78–519] BAU/ml vs. 1826 [560–3180] BAU/ml for KTRs and controls respectively, p < .0001) ( Figure 1A). The neutralizing capacity of these antibodies was significantly lower in KTRs. 38 (68%) out of 56 seropositive KTRs had neutralizing antibodies whereas in the control group 144 seropositive patients (87%) developed neutralizing antibodies (Figure 1B). Similarly, the median values of neutralizing antibodies were significantly higher in the control group (median 1:20 [0–1:40] for KTR’s and with 1:80 [1:20–1:160] for the control group respectively, p < .05). Although KTRs with previous COVID-19 infection were excluded from this study, we have observed that 6 KTRs with prior COVID-19 infection developed considerably good immune response after full vaccination with median anti-SARS-CoV-2 spike IgGs of 765,1 BAU/ml (384–4300,5) and median neutralizing antibodies of 1:1280 (1:800–1:2240).
TABLE 1.
Patient characteristics at study entry
| Parameter | All (N = 225) | Seropositive (N = 56) | Seronegative (N = 169) |
|---|---|---|---|
| Age, y | 62 (54–70) | 61 (57–68) | 62 (53–70) |
| M:F | 1:0.5 | 1:0.6 | 1:0.5 |
| Time after transplantation, mo | 81 (31–148) | 150 (94–227) | 56 (26–124)** |
| Creatinine, mg/dl | 1.6 (1.2–2.0) | 1.6 (1.2–1.9) | 1.5 (1.2–2.1) |
| eGFR, ml/min/1.73 m2 | 45 (31–58) | 45 (31–61) | 45 (31–58) |
| Immunosuppression | |||
| CNI | 217 (96.4%) | 54 (96.4%) | 163 (96.4%) |
| Steroids | 212 (94.2%) | 51 (91.1%) | 161 (95.3%) |
| mTOR inhibitor | 7 (3.1%) | 4 (7.1%) | 3 (1.8%) |
| Azathioprine | 4 (1.8%) | 2 (3.6%) | 2 (1.2%) |
| Belatacept | 5 (2.2%) | 0 (0%) | 5 (3%) |
| MMF | 187 (83.1%) | 26 (46.4%) | 161 (95.3%)** |
| ≤1 g/d | 119 (63.6%) | 23 (88.5%) | 96 (59.6%)** |
| ≥1 g/d | 68 (36.4%) | 3 (11.5%) | 65 (40.4%)** |
| Dual therapy | 39 (17.3%) | 26 (46.4%) | 13 (7.7%)** |
| With MMF | 12 (30.8%) | 3 (11.5%) | 9 (69.2%)** |
| No MMF | 27 (69.2%) | 23 (88.5%) | 4 (30.8%)** |
| Triple therapy | 184 (81.8%) | 28 (50%) | 156 (92.3%)** |
| With MMF | 175 (95.1%) | 23 (82.1%) | 152 (97.4%)** |
| No MMF | 9 (4.9%) | 5 (17.9%) | 4 (2.6%)* |
| Monotherapy | 2 (0.9%) | 2 (3.6%) | 0 (0%) |
Note: Seropositivity was defined as IgG antibody against SARS-CoV-2 spike S1 subunit titer above 35.2 BAU/ml measured 2 weeks after the second vaccine dose. Dichotomous data are presented as percentages whereas continuous data as means ± SD or median (Q1–Q3).
Abbreviations: CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; MMF, mycophenolate mofetil.
Represent significant difference between the groups with p < .001.
p < .01.
p < .05 using Chi-square test or Mann–Whitney test.
FIGURE 1.
Immune response to SARS-CoV-2 vaccination in kidney transplant recipients. (A) Comparison of antibody titers against Sars-CoV-2 spike S1 subunit between controls and KTRs. Dashed line was set at 35.2 BAU/ml to outline seropositive patients. (B) Comparison of neutralizing antibody capacity between seropositive controls and KTRs. (C) Association between anti-SARS-CoV-2 antibodies and neutralizing antibody titers in seropositive KTRs. (D) Development of antibody titer between different MMF regime groups. Patients who developed measurable antibody levels were only included. Dashed line was set to outline seropositive patients. (E) Correlation between MMF concentration in the blood and development of IgG antibodies (R −0.354, p < .001). Differences were assessed using Mann-Whitney test or Kruskal-Wallis test were applicable. ****Represent p value < .0001, ***p < .001, **p < .01, *p < .05.
KTRs in the seropositive group defined as anti-SARS-CoV-2 spike S1 antibody levels >35.2 BAU/ml compared to seronegative patients, had lower rates of triple immunosuppressive therapy and less use of MMF accompanied by significantly longer time after transplantation (Table 1). Other immunosuppressive agents as well as differences in age, gender and graft function had no influence on the immune response (Table 1). Seropositive patients with neutralizing antibodies (n = 38) compared to seropositive patients without neutralizing antibodies (n = 18) did not differ with respect to immunosuppression, graft function or time after transplantation ( Table 2). KTRs with higher IgG antibody levels were more likely to develop neutralizing antibodies with higher titers (Figure 1C; Table 2).
TABLE 2.
Characteristics of seropositive patients with regard to development of neutralizing antibody capacity
| Parameter | NTs (N = 38) | No NTs (N = 18) |
|---|---|---|
| Age, y | 59 (56–63) | 66 (60–72) |
| M:F | 1:0.6 | 1:0.6 |
| Time after transplantation, mo | 150 (94–229) | 149 (108–214) |
| Creatinine, mg/dl | 1.4 (1.0–1.9) | 1.7 (1.3–2.0) |
| eGFR, ml/min/1.73 m2 | 49 (35–75) | 36 (29–50) |
| IgG antibody titer, BAU/ml | 384 (169–749) | 56 (46–220)** |
| Immunosuppression | ||
| CNI | 37 (97.4%) | 17 (94.4%) |
| Steroids | 33 (86.8%) | 18 (100%) |
| mTOR inhibitor | 2 (5.3%) | 2 (11.1%) |
| Azathioprine | 2 (5.3%) | 0 (0%) |
| MMF | 18 (47.4%) | 8 (44.4%) |
| ≤1 g/d | 16 (88.9%) | 7 (87.5%) |
| ≥1 g/d | 2 (11.1%) | 1 (12.5%) |
| Dual therapy | 17 (44.7%) | 9 (50%) |
| With MMF | 3 (17.6%) | 0 (0%) |
| No MMF | 14 (82.4%) | 9 (100%) |
| Triple therapy | 19 (50%) | 9 (50%) |
| With MMF | 15 (78.9%) | 8 (88.9%) |
| No MMF | 4 (21.1%) | 1 (11.1%) |
| Monotherapy | 2 (5.3%) | 0 (0%) |
Note: Dichotomo
Abbreviations: CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; MMF, mycophenolate mofetil; NT, neutralizing antibody capacity.
Represent significant difference between the groups (p < .001) using Mann Whitney test.
To evaluate independent factors which modify the immune response in KTRs, logistic multivariate regression analysis was performed ( Table 3). By adjusting to age, gender, graft function and immunosuppressive agents, a MMF-free regimen (OR 13.25, 95% CI 3.22–54.6; p < .001) and degree of graft function (OR 1.03, 95% CI 1.00–1.05; p = .019) were the most significant factors influencing antibody production. Of note, univariate analysis revealed that within the dual immunosuppressive regimen, a positive immune response is only associated with those who were treated with a MMF-free dual regime (OR 18.75, 95% CI 3.5–100.6, p = .001). Of note, in seropositive patients MMF is not associated with the development of neutralizing antibodies. However, while analyzing the whole study population of 225 KTRs, MMF free regime increases the possibility of NT production by 10 times (OR 10.432, 95% CI 4.683–23.241, p < .001).
TABLE 3.
Logistic regression model representing factors associated with the development of antibodies against SARS-CoV-2 spike S1 subunit after the second vaccination
| Variables | β | OR | CI 95% | p value |
|---|---|---|---|---|
| Gender (male) | 0.389 | 1.48 | 0.64–3.41 | .363 |
| Age | −0.012 | 0.99 | 0.96–1.02 | .432 |
| eGFR | 0.024 | 1.03 | 1.00–1.05 | .019 |
| Time after transplantation | 0.000 | 1.00 | 0.99–1.00 | .809 |
| No use of MMF | 2.584 | 13.25 | 3.22–54.60 | <0.001 |
| No use of CNI | −0.822 | 0.44 | 0.04–5.26 | .516 |
| No use of steroids | −0.264 | 0.77 | 0.09–6.14 | .768 |
| Dual therapy | 1.527 | 4.60 | 0.85–24.95 | .077 |
Abbreviations: CI, confidence interval; CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; MMF, mycophenolate mofetil; OR, odds ratio.
p values <.05 represent statistical significance.
Only 26 out of 187 KTRs under the treatment with MMF developed antibodies. Interestingly, a higher MMF dose was related with an impaired development of antibodies (Figure 1D). Eight of the 21 (38%) KTRs treated with the lowest daily MMF dose showed a positive humoral immune response to vaccination (Figure 1D). In contrast, KTRs treated with a daily MMF dose above 1 g almost failed to develop antibodies after SARS-CoV-2 vaccination (Figure 1D). KTRs taking ≤1 g MMF per day (OR 5.19, 95% CI 1.49–18.00, p = .009) had a higher possibility to develop antibodies than KTRs treated with higher MMF doses according to logistic regression. There was a positive correlation between MMF dose and MMF trough concentration (R 0.279, p = .011). Moreover, MMF trough concentrations showed a negative correlation with antibody titers (R −0.354, p < .001, Figure 1E).
4. DISCUSSION
In this study, a very low humoral immune response to SARS-CoV-2 vaccine in KTRs compared to controls with no history of kidney failure was demonstrated. This is in accordance with other recent studies.2, 3, 4, 5 Importantly, KTRs have significantly lower neutralizing capacity against SARS-CoV-2 as compared to controls suggesting a severely impaired sero-protection. Only 68% of seropositive patients showed neutralizing antibodies. Smaller cohorts representing KTRs failed to demonstrate any neutralizing antibody capacity in these patients.11 , 12 In fact, the presence and in particular the titer levels of the neutralizing antibodies correlate with efficient protection against COVID-19 infection and more importantly against novel virus variants such as B.1.617.2 and B.1.351.13 , 14 Thus, significantly lower neutralizing antibody titers in KTRs as shown in the present study may confer lower protection against COVID-19 infection especially those caused by SARS-CoV-2 variants.
KTRs are extremely vulnerable to severe COVID-19 infection despite vaccination.6 , 7 Thus, there is a medical need to develop strategies for a better immune response to SARS-CoV-2 vaccination. Therefore, we analyzed whether immunosuppressive drug regimens, renal function or demographic factors are associated with seroconversion after SARS-CoV-2 vaccination. Especially, we aimed to find factors which could be modified to improve vaccination success. Here, we demonstrate that MMF had a highly significant effect on the development of SARS-CoV-2 antibodies after vaccination. KTRs treated with a MMF-free immunosuppressive regimen are 13-times more likely to develop antibodies against SARS-CoV-2 than KTRs treated with MMF. Among those KTRs who were treated with MMF, a daily dose equal or less than 1 g was associated with an up to 5 times improved humoral immune response to vaccination in comparison to KTRs treated with the higher MMF dose. In line with these observations, Rozen-Zvi et al. showed a relationship between MMF dose and immune response. Every 360 mg reduction in MMF was associated with 2.3 times increased possibility to develop antibodies.15 In addition to that, we were the first to demonstrate a negative correlation between trough MMF concentrations in the blood and antibody titers. This observation supports the dose-dependent unfavorable effect of MMF on humoral immune response. The detrimental effects of a MMF containing immunosuppressive treatment on the vaccination-induced humoral immune response are not only evident after vaccination against SARS-CoV-2. Previous studies observed also a significantly attenuated immune response of MMF treatment in KTRs after vaccination against Influenza virus and Pneumococcus pneumoniae.16 , 17
Aside MMF treatment, graft function also influenced the immune response in our cohort significantly. Thus, every increase in eGFR of 1 ml/min/1.73 m2 increased the probability of developing IgGs by 3%. This observation is in line with a previous study15 and might be explained by the fact that renal failure is associated with an impaired immune response especially with dysfunctional B cells.18 , 19 In addition, other studies have indicated that calcineurin inhibitors, mTor inhibitors and age are additional factors influencing the immune response after SARS-CoV-2 vaccination in KTRs.4 , 15 These associations could not be found in our study as the patient number taking these immunosuppression drugs was too low.
Recent observational case series report, that a third vaccination against SARS-CoV-2 improves the immune response especially in those KTRs with detectable but low antibody titers. However, approximately half of the KTRs remained seronegative even after the third vaccination.20 , 21
A limitation of this study is that we did not measure T cell response after vaccination. Recent studies have shown that the specific T cell immune response is also impaired in KTRs compared to controls or dialysis patients.4 , 12 Interestingly, an anti-spike-specific T cell response after SARS-CoV-2 vaccination was detected in half of the KTRs and even in those with no detectable humoral immune response.12 Thus, one can assume that at least some of the patients with no significant humoral immune response may have a sufficient T cell response to prevent severe COVID-19 infection. However, this needs to be investigated in large outcome studies. Of note, recently published study on a general population has showed that higher anti-SARS-CoV-2 IgG levels and higher neutralization titers are closely correlated with lower numbers of symptomatic SARS-CoV-2 infections.22 This study underlines the importance of the humoral immune response and measuring this response as a surrogate parameter for a sufficient immune response.
Based on these results, our observation suggests that MMF dose modification could be an option for an improved humoral immune response. Furthermore, trough MMF levels may guide dose reduction strategies in individual patients. However, we do not know whether or to which extent a reduction of MMF will allow a sufficient immune response after vaccination. Therefore, the risk of rejection due to modification of immunosuppression in the context of vaccination needs to be minimized and should be decided individually. In this context, further prospective studies investigating the impact of the modification of the immunosuppressive therapy are necessary and need to be conducted.
ACKNOWLEDGMENTS
The authors thank Yvonne Dickschen for their technical assistance, Liliane Janssen and Natascha Rapp for their support for organizing the visits. This work was supported by Forschungskommission of the Medical Faculty, Heinrich-Heine-Universität Düsseldorf, the Ministry of Culture and Science of North Rhine-Westphalia (VIRus Alliance NRW) and BMBF (COVIM 01KX2021).
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
AUTHOR CONTRIBUTIONS
TK, KI, HS, LCR, JT, and JS contributed to conceptualization. MK, SF, LM, and OA contributed to formal analysis. SF, TK, KI, CS, MA, and HMO contributed to investigation. MK contributed to writing—original draft preparation. TK, TL, HMO, LCR, JT, and JS contributed to review and editing. JT and JS contributed to supervision.
DATA AVAILABILITY STATEMENT
Raw data were generated at University Hospital Düsseldorf, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany. The data that support the findings of this study are available on request from the corresponding author, JS. The data are not publicly available due to privacy restrictions.
Funding information Heinrich-Heine-Universität Düsseldorf; Ministerium für Kultur und Wissenschaft des Landes Nordrhein-Westfalen; Bundesministerium für Bildung und Forschung, Grant/Award Number: COVIM 01KX2021
REFERENCES
- 1.Elias M, Pievani D, Randoux C, et al. COVID-19 infection in kidney transplant recipients: disease incidence and clinical outcomes. J Am Soc Nephrol. 2020;31(10):2413–2423. doi: 10.1681/ASN.2020050639. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204. doi: 10.1001/jama.2021.7489. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benotmane I, Gautier-Vargas G, Cognard N, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99(6):1498–1500. doi: 10.1016/j.kint.2021.04.005. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32(9):2147–2152. doi: 10.1681/ASN.2021040480. ASN.2021040480. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korth J, Jahn M, Dorsch O, et al. Impaired humoral response in renal transplant recipients to sars-cov-2 vaccination with bnt162b2 (Pfizer-biontech) Viruses. 2021;13(5):756. doi: 10.3390/v13050756. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caillard S, Chavarot N, Bertrand D, et al. Occurrence of severe COVID-19 in vaccinated transplant patients. Kidney Int. 2021;100(2):477–479. doi: 10.1016/j.kint.2021.05.011. S0085-2538-3. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsapepas D, Paget K, Mohan S, Cohen DJ, Husain SA. Clinically significant COVID-19 following SARS-CoV-2 vaccination in kidney transplant recipients. Am J Kidney Dis. 2021;78(2):314–317. doi: 10.1053/j.ajkd.2021.05.004. S0272-6386-1. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller L, Andrée M, Moskorz W, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis. 2021. doi: 10.1093/cid/ciab381 [DOI] [PMC free article] [PubMed]
- 9.Kolb T, Fischer S, Müller L, et al. Impaired immune response to SARS-CoV-2 vaccination in dialysis patients and in kidney transplant recipients. Kidney360. 2021. doi: 10.34067/KID.0003512021 [DOI] [PMC free article] [PubMed]
- 10.Müller L, Ostermann PN, Walker A, et al. Sensitivity of anti-SARS-CoV-2 serological assays in a high-prevalence setting. Eur J Clin Microbiol Infect Dis. 2021;40(5):1063–1071. doi: 10.1007/s10096-021-04169-7. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rincon-Arevalo H, Choi M, Stefanski A-L, et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6(60):eabj1031. doi: 10.1126/sciimmunol.abj1031. doi: [DOI] [PubMed] [Google Scholar]
- 12.Sattler A, Schrezenmeier E, Weber U, et al. Impaired humoral and cellular immunity after SARS-CoV2 BNT162b2 (Tozinameran) prime-boost vaccination in kidney transplant recipients. medRxiv. 2021:2021.04.06.21254963. doi: 10.1101/2021.04.06.21254963 [DOI] [PMC free article] [PubMed]
- 13.Garcia-Beltran WF, Lam EC, St. Denis K, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184(9) doi: 10.1016/j.cell.2021.03.013. 2372-2383.e9. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wall EC, Wu M, Harvey R, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet (London, England). 2021;397(10292):2331–2333. doi: 10.1016/S0140-6736(21)01290-3. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rozen-Zvi B, Yahav D, Agur T, et al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect. 2021;27(8) doi: 10.1016/j.cmi.2021.04.028. 1173.e1-1173.e4. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar D, Rotstein C, Miyata G, Arlen D, Humar A. Randomized, double-blind, controlled trial of pneumococcal vaccination in renal transplant recipients. J Infect Dis. 2003;187(10):1639–1645. doi: 10.1086/374784. doi: [DOI] [PubMed] [Google Scholar]
- 17.Smith KGC, Isbel NM, Catton MG, Leydon JA, Becker GJ, Walker RG. Suppression of the humoral immune response by mycophenolate mofetil. Nephrol Dial Transplant. 1998;13(1):160–164. doi: 10.1093/ndt/13.1.160. doi: [DOI] [PubMed] [Google Scholar]
- 18.Krishnamurthy G, Kher V, Naik S. Low response to HBsAg vaccine in chronic renal failure patients is not due to intrinsic defect of B cells. Scand J Urol Nephrol. 2002;36(5):377–382. doi: 10.1080/003655902320783908. doi: [DOI] [PubMed] [Google Scholar]
- 19.Betjes MGH. Uremia-associated immunological aging and severity of COVID-19 infection. Front Med. 2021;8 doi: 10.3389/fmed.2021.675573. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and Immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174(9):1330–1332. doi: 10.7326/l21-0282. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. medRxiv. 2021:2021.06.21.21258528. doi: 10.1101/2021.06.21.21258528 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data were generated at University Hospital Düsseldorf, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany. The data that support the findings of this study are available on request from the corresponding author, JS. The data are not publicly available due to privacy restrictions.