CONFLICT OF INTEREST
The authors have nothing to declare.
ETHICAL APPROVAL
No ethical approval or waiver was obtained from the METC. Patients did not have to adhere to any study protocol.
PATIENT CONSENT STATEMENT
Patient consent was not needed. Data are decoded and there is a direct treatment relationship with the patients.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
We did not reproduce material from other sources.
CLINICAL TRIAL REGISTRATION
No clinical trial registration was performed.
Dear Editors,
Sickle cell disease (SCD) is frequently complicated by painful vaso‐occlusive crises (VOCs) often resulting in healthcare utilization including hospital admission. A vaso‐occlusive crisis (VOC) can be induced by multiple factors including infection, exposure to cold, physical exercise, dehydration, and stress. Given the current SARS‐CoV‐2 pandemic, we hypothesized that SARS‐CoV‐2 (even without symptoms of upper airway infection) might play a major causal role in VOC. In order to test this hypothesis, consecutive SCD patients presenting in our center with symptoms compatible with VOC were tested for SARS‐CoV‐2 by real‐time polymerase chain reaction (RT‐PCR) of nasopharyngeal swabs. Presentations of SCD patients with a proven prior SARS‐CoV‐2 infection were excluded. Given the limited sensitivity of the RT‐PCR in SARS‐CoV‐2 high incidence groups, low‐dose noncontrast chest CT‐scans were initially performed in addition to the RT‐PCR. 1
In total, 122 VOCs in 70 adult SCD patients, presenting to the emergency department between March 16th 2020 and March 16th 2021, were evaluated (Figure 1). Five presentations in two individual patients were excluded due to a prior RT‐PCR proven SARS‐CoV‐2 infection. Furthermore, 13 presentations in six patients were excluded due to nonprotocol adherence (not obtaining a RT‐PCR at presentation). In total, 104 episodes of VOC in 62 patients with SCD were prospectively analyzed on SARS‐CoV‐2 by RT‐PCR irrespective of respiratory symptoms. Physical examination, including temperature and oxygen saturation, and laboratory analysis including blood cell count, lactate dehydrogenase (LDH), and C‐reactive protein (CRP), were completed in most of the patients. Patients were treated with intravenous pain medication according to a standard protocol or an individual pain plan. The baseline characteristics are presented in Table 1. In 104 consecutive presentations, five presentations tested positive on SARS‐CoV‐2 (4.8%). Only one of these five patients presented with respiratory symptoms in addition to the symptoms of a VOC and one patient was diagnosed with acute chest syndrome (ACS). Five of the 99 presentations of patients with a negative SARS‐CoV‐2 PCR presented with respiratory symptoms. In this group, seven patients developed an ACS. This difference was not statistically significant (Fisher's exact test, P = .335). The laboratory results at presentation were comparable for both groups (Table 2).
FIGURE 1.
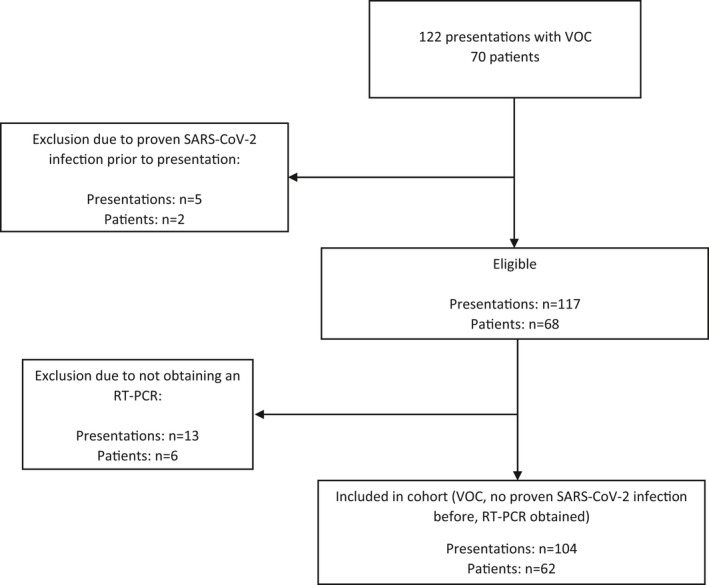
Flowchart
TABLE 1.
Baseline characteristics
|
Patients n = 62 |
Presentations n = 104 |
|
|---|---|---|
| Female/Male (n) | 30/32 | ‐ |
| Age, y (IQR) | 25 (21‐33) | ‐ |
| Concomitant hydroxyurea use (n, percentage) | 22 (35.5%) | ‐ |
| Genotype (n, percentage) | ‐ | |
| Homozygous hemoglobin S | 33 (53.2%) | |
| Hemoglobin SB0‐thalassemia | 7 (11.3%) | |
| Hemoglobin SB+‐thalassemia | 5 (8.1%) | |
| Hemoglobin SC | 15 (24.2%) | |
| Unknown | 2 (3.2%) | |
| Hemoglobin F, % (IQR) | 3.7 (1.5‐6.4) | ‐ |
| Hemoglobin, g/dL (SD) | ‐ | 9.2 (1.77) |
| White blood cell count, 109/L (IQR) | ‐ | 12.3 (10.1‐15.4) |
| Lymphocytes, 109/L (IQR) | ‐ | 2.9 (2.0‐4.6) |
| CRP, mg/L (IQR) | ‐ | 4.8 (1.9‐10.4) |
| LDH, U/L (SD) | ‐ | 675.0 (299.6) |
| Temperature, °C (SD) | ‐ | 36.4 (0.6) |
| Oxygen saturation, % (IQR) | ‐ | 98 (97‐99) |
| Hospitalization (n, percentage) | ‐ | 90 (86.5%) |
| Length of stay, d (IQR) | ‐ | 3.5 (1‐7) |
Parametric data were described by mean and standard deviation (SD). Nonparametric data were described by median and interquartile range (IQR).
TABLE 2.
Lab values sorted by RT‐PCR outcome
|
Negative RT‐PCR n = 99 |
Positive RT‐PCR n = 5 |
|
|---|---|---|
| Hemoglobin, g/dL (SD) | 9.3 (1.7) | 8.1 (1.2) |
| White blood cell count, 109/L (IQR) | 12.3 (10.2‐15.5) | 9.9 (5.3‐15.0) |
| Lymphocytes, 109/L (IQR) | 2.8 (1.9‐4.1) | 2.4 (2.0‐4.1) |
| CRP, mg/L (IQR) | 4.8 (1.9‐9.9) | 2.4 (1.3‐27.3) |
| LDH, U/L (SD) | 437.8 (147.6) | 429 (94.0) |
Fisher's exact test, P = .05, with a confidence interval of 95% was used to determine statistical significance.
From March 16th till May 31st 2020, routine CT‐scans were performed in addition to the RT‐PCR, in order to increase the diagnostic accuracy. A CT‐scan was performed in 23 out of 27 VOCs presenting to the emergency department in this period. In 19 of these presentations, that were tested negative for SARS‐COV‐2, no CT‐abnormalities suggestive for COVID‐19 were found. One of four CT‐scans performed in patients with a positive RT‐PCR showed abnormalities not specific for COVID‐19.
This is, to the best of our knowledge, the first study that prospectively analyzed the incidence of SARS‐COV‐2 in consecutive SCD patients presenting with a painful crisis in a 12‐month period during the SARS‐COV‐2 pandemic. We show that only a limited number of patients, primarily presenting with a VOC during the current pandemic, appeared to be SARS‐CoV‐2 positive, suggesting that a SARS‐CoV‐2 infection is not a frequent provoking factor for VOC in patients with SCD. So far, a number of case‐series of SCD patients with COVID‐19 presenting with pain have been published, 2 , 3 including a report from our institution in which two patients presented with pain and appeared to have a positive PCR on SARS‐COV‐2. 2 These observations are in contrast to our current analysis and could be explained by the limited number of patients assessed in these studies as compared to the consecutive analyses in our cohort and a possible bias due to the absence of a consecutive registry.
It is not possible to determine in our study whether the VOCs of the patients with a positive SARS‐CoV‐2 PCR were related to the SARS‐COV‐2 infections or whether the SARS‐COV‐2 infections should be considered as co‐incidental asymptomatic infections, in particular in patients without any respiratory symptoms. In a retrospective cohort study, comprising of asymptomatic patients consecutively screened before surgery with a RT‐PCR of nasopharyngeal swabs combined with a pulmonary CT scan, an overall SARS‐COV‐2 incidence of 1.5% was found. 4 Despite the lower incidence rate in that screening study as compared to our cohort, multiple other factors may play a role including differences in ethnicity and age.
Other studies reported the clinical characteristics of SCD patients hospitalized with COVID‐19. First, a registry study showed that 41 out of 81 patients hospitalized with COVID‐19 concurrently presented with symptoms of a VOC, indicating that pain indeed can be observed in SCD patients admitted with COVID‐19. 5 However, this study did not systematically assess SCD patients diagnosed with COVID‐19 based on hospital records, but was based on the reporting of admitted patients from individual clinicians of different centers, possibly resulting in missed cases and recall bias. Second, in a case series, eight out of 10 patients with COVID‐19 presented with a VOC, although these patients also presented with pulmonary symptoms or fever. 6 A possible explanation of these contrasting findings could be that mild/severe SARS‐COV‐2 infections may lead to VOCs, as any viral infection, whereas asymptomatic SARS‐COV‐2 infections without hypoxia and/or an inflammatory response may not frequently cause VOCs. Indeed, no statistically significant difference was found in the occurrence of ACS between patients with a positive SARS‐CoV‐2 and a negative SARS‐CoV‐2 RT‐PCR, although this comparison might be hampered by lack of power.
In our cohort, a negative SARS‐CoV‐2 diagnosis was based on one negative RT‐PCR in most patients. This is likely sufficient for exclusion if there is a low pre‐test likelihood of a positive SARS‐CoV‐2 RT‐PCR in a SCD patient with a VOC, hence sufficient for estimating the true incidence of SARS‐CoV‐2 in this cohort. The low rate of abnormalities in the routinely performed pulmonary CT‐scans obtained in the subset of patients suggests this as well. Indeed, studies that focused on the value of a pulmonary CT‐scan in adjunction to the RT‐PCR were mostly performed in populations with a high SARS‐CoV‐2 incidence in which most patients presented with respiratory symptoms or fever. 1 In conclusion, we found a low incidence of SARS‐CoV‐2 infections in our cohort of consecutive SCD patients presenting with VOCs which suggests that COVID‐19 is not a frequent provoking factor for clinical VOCs during the pandemic and may even be considered as a coincidental finding, given the low incidence and the fact that only one of the five patients with a positive RT‐PCR presented with pulmonary symptoms.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Waller JV, Kaur P, Tucker A, et al. Diagnostic Tools for Coronavirus Disease (COVID‐19): comparing CT and RT‐PCR viral nucleic acid testing. Am J Roentgenol. 2020;215(4):834‐838. [DOI] [PubMed] [Google Scholar]
- 2. Nur E, Gaartman AE, Van Tuijn CFJ, Tang MW, Biemond BJ. Vaso‐occlusive crisis and acute chest syndrome in sickle cell disease due to 2019 novel coronavirus disease (COVID‐19). Am J Hematol. 2020;95(6):725‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hussain FH, Njoku FU, Saraf SL, Molokie RE, Gordeuk VR, Han J. SARS‐CoV‐2 infection in patients with sickle cell disease. Br J Haematol. 2020;189(5):851‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Puylaert CAJ, Scheijmans JCG, Borgstein ABJ, et al. SCOUT study group. Yield of screening for COVID‐19 in asymptomatic patients before elective or emergency surgery using chest CT and RT‐PCR (SCOUT): multicenter study. Ann Surg. 2020;272(6):919‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arlet JB, De Luna G, Khimoud D, et al. Prognosis of patients with sickle cell disease and COVID‐19: a French experience. Lancet Haematol. 2020;7(9):e632‐e634. doi: 10.1016/S2352-3026(20)30204-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCloskey KA, Meenan J, Hall R, Tsitsikas DA. SARS‐CoV‐2 infection and sickle cell disease: a UK centre experience. Br J Haematol. 2020;190(2):e57‐e58. doi: 10.1111/bjh.16779 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


