Key Messages.
COVID 19 crisis reduced the number of patients attended in allergy departments
Aeroallergen immunotherapy prescriptions decreased during the COVID‐19 pandemic
Among SCIT prescribers, an increase in SLIT prescriptions has been recorded
To the Editor,
The COVID‐19 pandemic represents a global health crisis and a challenge at all societal, economic and health levels. With the first cases having been reported in most of the European countries in January 2020, 1 it was not until March that the pandemic hit the territory and lead to an unprecedent hard lock‐down in most countries.
In response to the crisis, the scientific society has put tremendous efforts in producing numerous clinical practice guidelines for non‐COVID patients. In the field of allergy, 2 , 3 and more specifically in allergen immunotherapy (AIT), 4 position papers have given recommendations on several aspects of AIT practice for the safety of patients and health care professionals. After 1 year since the beginning of the pandemic, few real‐life data have been reported on how AIT has been managed during and post the lockdown periods.
To assess how COVID‐19 crisis affected aeroallergen AIT prescription patterns during the lockdown period and the following post peak period, we collected real world data from doctors in Spain and France. We set this survey in the framework of the ongoing academic “CHOICE” (Criteria Used by Health Professionals on the Selection of Allergen Immunotherapy in Real Clinical Practice: an international e‐survey) project. The study has been approved by the Ethical Committee in both countries, in Spain at the Hospital Universitario Reina Sofia, Internal code FCO‐CHO‐19, and in France by the Institutional Review Board from the CHU Montpellier, internal code 2019_IRB‐MTP_09‐02. Written consent was not required.
A transversal, web‐based questionnaire was conducted from October 4 to November 4, 2020. Doctor inclusion criteria was aeroallergen AIT prescribers in clinical care routine in France and Spain. At the time of creating this COVID‐19 branch of the CHOICE project, the number of enrolled doctors was 221 (137 France and 83 Spain).
The questionnaire gathered information on how doctors handled AIT prescriptions, such as the number of respiratory patients (allergic rhinitis and/or allergic asthma) attending doctors’ offices, the number of new AIT prescriptions, the changes in clinical practice other than merely reducing the number of prescriptions, and the modifications in AIT administration in patients undergoing the maintenance phase. The same information was collected for the first lockdown and for the subsequent period (Full questionnaire, online Data S1).
Descriptive information was analysed and compared with Fisher's exact test or Student's t test. Direct comparisons for the quantitative variables of the two reported periods was done using Wilcoxon for paired samples. Statistical significance was set for p values below .05. A multivariate analysis was performed using logistic regression with stepwise selection. All statistical analyses were performed with SPSS software (version 25, IBM Corporation, Armonk, NY, USA).
The reply ratio of completed questionnaires was 71.1% (59/83) for Spain and 26.3% (36/137) for France. Both cohorts have similar gender and age distribution (48 ± SD10.2 years old), with an overall majority of female (72.7%) physicians. Most Spanish participants (89%) worked in public hospitals, whereas French doctors mainly worked (71.9%) in private practice (p < .001). Spanish doctors, compared with French ones, showed slightly longer experience in AIT (20.6 ± SD8.7 years vs. 15.3 ± SD11.1 years, p = .012).
In both countries, doctors perceived a strong reduction in the number of respiratory patients assessed in their clinics, compared with the same period of the previous year. Furthermore, most of them (96% in Spain and 82% in France; p = .053) declared prescribing fewer new AIT courses compared with the same period during the previous year (Table 1). Interestingly, the estimated percentage of decrease in prescriptions (median 75% [0–100]) was beyond the decrease in patients (median 50% [0–100], p < .001), suggesting other reasons than the mere reduction in attended patients. Logistic regression analysis identified that the risk of prescribing less AIT was increased among Spanish doctors compared with their French colleagues (OR 13.7; 95% CI 1.4–135.9, p = .025). As for new AIT prescriptions, 27% of Spanish doctors, the only SCIT prescribers in the cohort, reported an increase of use of SLIT. No relevant changes were reported by French doctors, who generally do not have access to SCIT for aeroallergens. 5
TABLE 1.
Characteristics of doctors and the effect of COVID‐19 in AIT practice during and after the sanitary lockdown in France and Spain
| Allergen immunotherapy and COVID−19 | |||||
|---|---|---|---|---|---|
| France n = 36 | Spain n = 59 | Total n = 95 | p‐value | ||
| Doctors’ characteristics | |||||
| Participants treating COVID−19 patients, n (%) | 10 (27.8%) | 32 (54.2%) | 42 (44.2%) | .019 | |
| Participants suffering COVID−19, n (%) | 1 (2.8%) | 9 (15.3%) | 10 (10.5%) | .142 | |
| Duration of hard lockdown (weeks) | 8 weeks | 6 weeks | NA | NA | |
| Patients assessed during lockdown, n (%) | 34 (94.4%) | 52 (88.1%) | 86 (90.5%) | .475 | |
| Lockdown, doctors attending allergy clinic (n = 86) | |||||
| Fewer respiratory patients attended compared with same period of the previous year, n (%) | 20 (58.8%) | 41 (78.8%) | 61 (70.9%) | .55 | |
| Decrease in the number of respiratory patients assessed, median % [range] | 50 [0–100] | 50 [0–100] | 50 [0–100] | .383 | |
| Doctors declaring prescribing fewer new AIT courses compared with same period of the previous year, n (%) | 28 (82.4%) | 50 (96.2%) | 78 (90.7%) | .053 | |
| Decrease in the number of new AIT courses compared with same period of previous year, median % [range] | 75 [0–100] | 75 [0–100] | 75 [0–100] | .420 | |
| Post‐lockdown, doctors attending allergy clinic (n = 95) | |||||
| Patient´s lockdown influenced the clinical data available for doctors to prescribe AIT, n (%) | 12 (33.3%) | 42 (71.2%) | 54 (56.8%) | .001 | |
| Patient´s attitude towards AIT, n (%) | No change | 30 (83.3%) | 31 (52.5%) | 61 (64.2%) | .014 |
| Reluctancy to AIT | 2 (5.6%) | 15 (25.4%) | 17 (17.9%) | ||
| Receptive to AIT | 2 (5.6%) | 7 (11.9%) | 9 (9.5%) | ||
| Unclear | 2 (5.6%) | 6 (10.2%) | 8 (8.4%) | ||
| Fewer respiratory patients attended compared with same period of the previous year, n (%) | 7 (19.4%) | 27 (45.8%) | 34 (35.8%) | .015 | |
| Decrease in the number of respiratory patients attended, median % [range] | 0 [25–100] | 25 [0–100] | 25 [0–100] | .001 | |
| Doctors declaring prescribing fewer new AIT courses than to same period of the previous year, n (%) | 11 (30.6%) | 39 (66.1%) | 50 (52.6%) | .001 | |
| Decrease in the number of new AIT courses compared with same period of previous year, median % [range] | 0 [0–100] | 50 [0–100] | 50 [0–100] | <.001 | |
More than one option could be selected.
Regarding patients who were on the maintenance phase during the lock‐down, most (98.7%) physicians did not modify the treatment of their patients on SLIT, whereas 57.7% of doctors with patients on SCIT considered changes of some sort in some or all their patients: 44.2% paused the treatment, 17.3% changed the place of administration and 1.9% switched to SLIT (Figure 1).
FIGURE 1.
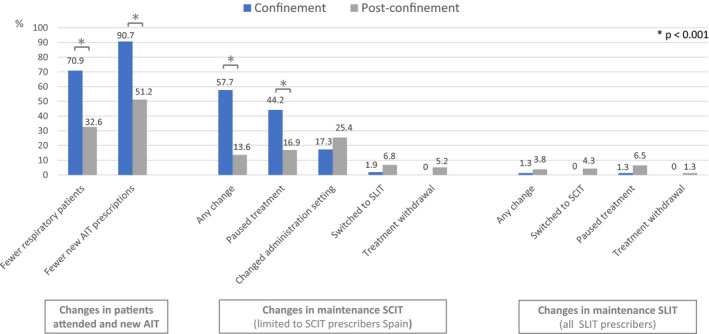
AIT Management Strategies during COVID‐19. Legend: For new AIT comparisons, the 9 doctors not attending allergy clinic during the confinement were not included. For maintenance dosing strategies, more than one strategy could be selected. AIT, allergen immunotherapy; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy
The second section of the questionnaire was dedicated to understanding AIT management after the end of the lockdown. A moderate increase in the estimated number of respiratory patients who attended the allergy clinics was detected in both countries (Figure 1). Interestingly, the decrease in the number of new prescriptions was still greater than the decrease in the number of respiratory patients, but only in Spain. Among SCIT prescribers, 27% still prescribed more SLIT than previously. It was the perception of 56.8% of participants that the mobility restrictions during the lockdown influenced the clinical data available to prescribe AIT afterwards, with more impact on the Spanish doctors compared with their French peers (71.2% vs 33.3%, p = .001) (Table 1). For 27.4% of all doctors, patient´s attitude to AIT changed either in feeling more reluctant to receive AIT (17.9%) or more open to it (9.5%), a trend significantly more marked among Spanish participants. Indeed, a higher risk of prescribing less AIT was recorded among doctors reporting their patients having a different attitude towards AIT (OR 6.140 [95% IC 1.714–24.995], p = .005). Maintenance SCIT administration was more affected than SLIT (Figure 1), and pausing the treatment and changing the administration setting were the two most frequent applied strategies.
Our research provides real‐life data showing a significant reduction in the number of respiratory patients referred to allergy specialists, the number of new AIT administered and also changes in qualitative prescription patterns during the first COVID‐19 pandemic peak, that only partially recovered after the lockdown.
AIT initiation during the pandemic peak (high community prevalence) has been generally discouraged in guidelines whilst already started treatments were advised to be maintained on the treatment. 2 , 6 , 7 This trend has been recently supported with real life data from a worldwide survey. 8 For the post pandemic (“controlled”) phase, returning to regular prescription patterns for new AIT was allowed. 2 , 4 , 6 , 7 Our data mirror these recommendations for the two periods, with a dramatic median decrease (75%) in new AIT initiations, and a significant but not full recover during the post lockdown period (50%). For this period, determinant factors behind this reduction were patient´s convenience and patient´s attitude towards AIT, which is a direct sign of the relevance of patient´s involvement in the AIT selection process, as part of precision medicine. 9
For AIT initiation, 27% of SCIT prescribers used SLIT more frequently, a trend that remained stable during the post peak phase. Several authors 10 , 11 suggested that SLIT might be more appropriate during the pandemic because of the possibility of home‐based administration and of a better safety profile, thus minimizing visits to healthcare facilities.
Although SLIT maintenance treatments experienced nearly no changes, 55.5% of SCIT prescribers applied treatment modifications during lockdown. It has been very recently published 12 that among 183 SCIT prescribers, up to 79%, made changes in their SCIT maintenance patients, and 31% discontinued the treatment if they were in the up‐dosing phase. Our cohort reported very few treatment withdrawals, but frequently paused treatments (41.8%), which is comparable with the 72% of participants having increased dosing intervals in the referenced manuscript 12 .
COVID‐19 confinement has induced environmental‐exposition changes 13 acting both as exacerbating factors (higher indoor allergens exposure, decreased medical visits, stress and anxiety) and as ameliorating factors (decreased exposition to outdoor allergens and pollution). These changes are probably behind the 56.8% of participants reporting that hard lockdown impacted the clinical available data used as the basis for AIT prescription.
Although COVID‐19 hit Spain and France in a similar way, both among the 10 worldwide countries most severely affected by the disease 14 , in terms of AIT management, some country‐disparities were noted, especially for the post‐lockdown period. Spanish practitioners compared with their French colleagues reported higher impact of lockdown in available clinical data, larger changes in patient attitude towards AIT and poorer recovery of regular clinic routine in terms of number of respiratory patients and prescriptions. Probably some, but not all these differences, are due to a different profile of populations and medical settings, since French physicians worked more frequently in private medicine and with SLIT products.
The retrospective nature of the information and the poor response ratio from French doctors may represent a weakness; however, we consider that the data provided in this brief communication are highly relevant to understand how aeroallergen AIT has been managed in real‐life settings; provide insights on the impact of the crisis; and may help implementing new strategies to ensure a quality and timely healthcare for those who missed their consultations and for new patients.
CONFLICT OF INTEREST
Authors declare no conflicts of interest.
AUTHORS CONTRIBUTION
All authors have made substantial contributions for the conception and design of the study, the acquisition of data, the analysis and interpretation of data and drafted and/or revised it critically providing their approval to the final version.
ETHICAL STATEMENT
The data contained is only related to overall practice of physicians, who voluntarily provided all information. There was no need for ethical committee evaluation.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to all physicians for their participation in the survey and generously devoting time and effort to provide their practice data.
Rodriguez del Rio P, Caimmi D, Rico P, et al. Real‐life report of allergen immunotherapy management during the COVID‐19 outbreak in France and Spain. Clin Exp Allergy. 2022;52:167–170. 10.1111/cea.14043
Funding information
The project is partially supported by a non‐restricted grant provided by ALK and Stallergenes Greer.
DATA AVAILABILITY STATEMENT
Data can be available on reasonable justified request to the corresponding author.
REFERENCES
- 1. Lescure F‐X, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID‐19 in Europe: a case series. Lancet Infect Dis. 2020;20(6):697‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shaker MS, Oppenheimer J, Grayson M, et al. COVID‐19: pandemic contingency planning for the allergy and immunology clinic. J Allergy Clin Immunol Pract. 2020;8(5):1477‐1488.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pfaar O, Klimek L, Jutel M, et al. COVID‐19 pandemic: practical considerations on the organization of an allergy clinic‐An EAACI/ARIA position paper. Allergy. 2021;76(3):648‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klimek L, Jutel M, Akdis C, et al. Handling of allergen immunotherapy in the COVID‐19 pandemic: an ARIA‐EAACI statement. Allergy. 2020;75(7):1546‐1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caimmi Davide E, Demoly P. Allergen immunotherapy using NPP: Perspectives for the treatment and prevention of respiratory allergies; the case of pollinosis. REVUE FRANÇAISE D’ALLERGOLOGIE. 2019;59(8):617‐623. [Google Scholar]
- 6. Cianferoni A, Votto M. COVID‐19 and allergy: how to take care of allergic patients during a pandemic? Pediatr Allergy Immunol. 2020;31(Suppl 26):96‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Searing DA, Dutmer CM, Fleischer DM, et al. A phased approach to resuming suspended allergy/immunology clinical services. J Allergy Clin Immunol Pract. 2020;8(7):2125‐2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pfaar O, Agache I, Bonini M, et al. COVID‐19 pandemic and allergen immunotherapy ‐ an EAACI survey. Allergy. 2021;76(11):3504‐3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hellings PW, Fokkens WJ, Bachert C, et al. Positioning the principles of precision medicine in care pathways for allergic rhinitis and chronic rhinosinusitis ‐ A EUFOREA‐ARIA‐EPOS‐AIRWAYS ICP statement. Allergy. 2017;72(9):1297‐1305. [DOI] [PubMed] [Google Scholar]
- 10. Compalati E, Erlewyn‐Lajeunesse M, Runa Ali F, et al. Allergen immunotherapy in the era of SARS‐CoV‐2. J Investig Allergol Clin Immunol. 2020;30(6):459‐461. [DOI] [PubMed] [Google Scholar]
- 11. Malipiero G, Paoletti G, Puggioni F, et al. An academic allergy unit during COVID‐19 pandemic in Italy. J Allergy Clin Immunol. 2020;146(1):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ozturk AB, Baççıoğlu A, Soyer O, Civelek E, Şekerel BE, Bavbek S. Change in allergy practice during the COVID‐19 pandemic. Int Arch Allergy Immunol. 2021;182(1):49‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oreskovic NM, Kinane TB, Aryee E, Kuhlthau KA, Perrin JM. The unexpected risks of COVID‐19 on asthma control in children. J Allergy Clin Immunol Pract. 2020;8(8):2489‐2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. COVID‐19 Map [Internet] . Johns Hopkins Coronavirus Resource Center. [consulted 27th May 2021]. https://coronavirus.jhu.edu/map.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data can be available on reasonable justified request to the corresponding author.


