Abstract
Studies are urgently needed to characterize immunogenicity, efficacy, and safety of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccines in kidney transplant (KT) recipients, excluded from major clinical trials. Complex ELISPOT and other cellular response techniques have been applied, but simpler tools are needed. An easy-to-use real-world monitoring of SARS-CoV-2 IgG antibodies against the Spike protein and QuantiFERON® SARS-CoV-2 IFNγ release assay (IGRA) were performed at baseline and 28 days after the second dose in KT recipients and controls (dialysis patients and healthy ones). All healthy controls and >95% dialysis controls became positive for anti-S IgG antibodies, while only 63.3% of KT patients seroconverted with a very low antibody level. A positive IGRA was documented in 96.9% of controls, 89.3% peritoneal dialysis, 77.6% hemodialysis, 61.3% of KT patients transplanted more than 1 year ago and only 36% of those transplanted within the previous 12 months. Overall, 100% of healthy controls, 95.4% of dialysis patients and 78.8% KT recipients developed any immune response (humoral and/or cellular) against SARS-CoV-2. KT patients showed low rates of immune responses to mRNA Coronavirus infectious disease 2019 vaccines, especially those with recent transplantations. Simple humoral and cellular monitoring is advisable, so that repeated doses may be scheduled according to the results.
KEYWORDS: antibody biology, clinical research/practice, dialysis, immunobiology, COVID-19, infectious disease, kidney transplantation/nephrology, T cell biology, vaccine
Abbreviations: AE, adverse events; AUC, area under the curve; CI, confidence interval; CKD, chronic kidney disease; COVID-19, coronavirus infectious disease 2019; eGFR, estimated glomerular filtration rate; HD, hemodialysis; IFNγ, interferon gamma; IGRA, interferon gamma release assay; IQR, interquartile range; KT, kidney transplant; PD, peritoneal dialysis; ROC, receiver operating characteristic; S, Spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; Th1, T helper 1
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for coronavirus infectious disease 2019 (COVID-19), which has caused the worst pandemic in the last decades. COVID-19 cases can be asymptomatic or mild in around 80% of individuals, especially in young adults and children. However, patients over 60 years old and with comorbidities are in major risk, requiring intensive respiratory support and presenting more frequently complications such as multiorganic failure or death.1 , 2 Several studies indicate that chronic kidney disease (CKD) is the most common comorbidity in severe COVID-19.3, 4, 5, 6 Furthermore, patients on renal replacement therapy, on dialysis or with a kidney transplant (KT), have shown the highest morbidity and mortality.3 , 7, 8, 9
A functional immune system is essential to overcome SARS-CoV-2 infection. In the acute phase, activation of CD4 and CD8-T cells is observed in most infected patients. CD4-T cells conduct T helper 1 (Th1) responses, expressing cytokines like interferon gamma (IFNγ) that contribute to viral clearance. CD8-T cells directly destroy infected cells through cytotoxicity.10 , 11 Regarding humoral response, SARS-CoV-2 antigen-specific antibodies are detected in the first weeks since symptoms onset, reaching peak levels in the third week. Neutralizing antibodies, which bind to the Spike (S) protein and prevent interaction with the cellular receptor ACE2 are also generated, granting immune protection against SARS-CoV-2 by disabling viral entry.11 Antibody durability has not yet been uniformly established, although some studies suggest that levels decrease just after reaching a peak.12 , 13 Despite this, as antibody levels that offer protection have not been determined, this decrease may not imply loss of immunity against reinfections.10 Two studies have shown persistence of antibody and cellular responses 6 months after COVID-19 in over 100 adult hemodialysis (HD) patients.14 , 15 Transplant recipients can also show robust although delayed humoral and cellular responses after infection.16 , 17
Since the beginning of the pandemic, governments of states affected by COVID-19 implemented sanitary measures to limit virus propagation and reduce high morbidity and mortality rates. The most suitable way to achieve these objectives is generating herd immunity with vaccines.18 Vaccines must induce antibody production and T cell activation to prevent infection and spread to others.19 Cellular immunity stimulated by mRNA-1273 is characterized by activation of S-specific CD4-T cells with Th1 profile, while BNT162b2 additionally induces a considerable CD8-T cell response,20 , 21 and both induce antibodies against the S protein.
Due to the increased risk of severe and fatal COVID-19 in KT patients, they have been prioritized for COVID-19 vaccination. Because the response to several vaccines is recognized to be poorer in these patients,22 , 23 it is imperative to assess the proportion of responders, the quality of the response as well as the best time for vaccination across the lifetime of the CKD patient with simple and reliable immune response tools. In 104 heart and liver transplant recipients, 64% developed antibodies and 79% T cell responses measured with ELISPOT against the S protein 1 month after completing mRNA-1273 vaccination.24 Although ELISPOT responses have been documented in transplant recipients,24, 25, 26 the technique is time-consuming and cumbersome. Thus, we designed a cohort study to assess and compare antibody and cellular responses in KT patients, using simple tools, 28 days after the administration of two doses of either Moderna mRNA-1273 or Pfizer BNT162b2 SARS-CoV-2 mRNA vaccines.
2. MATERIALS AND METHODS
2.1. Population, endpoints, and vaccines
An observational prospective cohort study was conducted in the Nephrology department of Hospital del Mar and two HD centers in Barcelona, Spain, including 251 individuals without known COVID-19 who were going to receive mRNA vaccines. Exclusion criteria were history of severe adverse reaction associated with a vaccine, previous known COVID-19 infection, active malignancy, inherited immune deficiency, pregnancy and a condition that would contraindicate intramuscular injection. At the hospital, all individuals, including all KT recipients received two 100 µg doses of the mRNA-1273 vaccine (Moderna) separated by 28 days, and in the HD centers, controls and dialysis patients received two doses of 30 µg BNT162b2, Comirnaty® (Pfizer/BioNTech) 21 days apart. The primary endpoint was the antibody-based immune response on day 28 after the second vaccination, to classify individuals as responders or non-responders defined with thresholds prior to data analyses. T cell COVID-19 specific response and safety were secondary endpoints.
The study was approved by the Internal Review Board at Hospital del Mar (2021/9726/I) and adheres to the Declaration of Istanbul. Before receiving the first dose, all participants signed informed consent.
2.2. SARS-CoV-2 Spike serological assay
SARS-CoV-2 IgG antibodies against Spike were assessed using LIAISON® SARS-CoV-2 TrimericS IgG kit (Diasoin Inc.). Serum samples obtained before vaccination with positive serology served as exclusion criterion. Following 28 days (±3 days) from the last vaccine dose, humoral response was assessed again. Antibody levels are expressed in arbitrary units per milliliter (AU/ml) and considered positive if values reached ≥13.0 AU/ml, in accordance with the manufacturer’s guidelines. Serum samples that exceeded assay measuring range (>800 AU/ml) were diluted with LIAISON® TrimericS IgG Diluent Accessory (Diasoin Inc.) by a 1:20 factor and tested again.
2.3. IFNγ release assay
Cellular immune response in vaccinated patients was evaluated using QuantiFERON® SARS-CoV-2 IFNγ release assay (IGRA). At baseline and 28 days (±3 days) after the second dose, whole blood samples were collected into the four tubes of QuantiFERON® SARS-CoV-2 Blood Collection Tubes (QIAGEN). Two (Antigens tubes) were coated with a combination of SARS-CoV-2 Spike antigens to stimulate T lymphocytes: tube 1 contains CD4+ epitopes derived from the S1 subunit (Receptor Binding Domain) of the S protein while tube 2 contains CD4+ and CD8+ epitopes from the S1 and S2 subunits of the S protein. The other two tubes, Nil and Mitogen, were used to adjust the background and as a positive control, respectively. Samples were incubated for 16–24 h at 37 ± 1℃, followed by centrifugation at 2000–3000 g for 15 min. Finally, IFNγ levels in plasma were quantified using QuantiFERON® ELISA (QIAGEN), according to the manufacturer’s instructions. Samples are considered positive for T cell response when exceeding the cut-off value in one or both Antigen tubes.
2.4. Safety assessment
Patients were called by telephone and answered a standardized questionnaire 7 days after receiving both the first and second dose of the mRNA vaccine. This questionnaire, created from previous data,27 , 28 included solicited local (pain, redness and swelling at injection site) and systemic (fever, fatigue, headache, chills, vomits, diarrhea, myalgia, and arthralgia) adverse events (AE) and their severity. Patients were also followed through electronic chart records 28 days after the second dose to detect unsolicited adverse reactions, serious AE or medically attended side effects.
2.5. Statistical analysis
Categorical variables were analyzed with chi-squared or Fisher’s exact tests and expressed as counts and percentages. Continuous variables were first tested for normal distribution using Kolmogorov–Smirnov test. If normally distributed, continuous data were analyzed using t-test or ANOVA and expressed as mean values ± standard deviation; if not, Mann–Whitney or Kruskal–Wallis test were used and values were expressed as the median and interquartile range (IQR). Univariate and multivariate logistic regression analyses was performed to identify factors associated with seroconversion and low or absent humoral response. Spearman test was used to explore correlations between continuous variables. A receiver operating characteristic (ROC) analysis was employed to obtain cut-off values for IGRA. p < .05 were considered significant. Statistical analysis was performed using IBM SPSS Statistics 25 (SPSS Inc.).
3. RESULTS
3.1. Population
A total of 251 individuals were recruited, and 209 were finally included. Excluded cases were: 25 positives for SARS-CoV-2 anti-S IgG or IGRA at baseline, 6 did not receive the second dose and 11 could not be contacted or rejected second sample extraction. The definitive group included 90 KT recipients, 87 dialysis controls, and 32 healthy controls. Two doses of Moderna were administered to 90 KT recipients, 48 dialysis controls and 11 healthy ones vaccinated at Hospital del Mar, and Pfizer vaccine was administered to 39 HD and 21 controls vaccinated at HD centers. Baseline characteristics are shown in Table 1. Characteristics of KT recipients transplanted within the previous year or later are depicted in Table 2.
TABLE 1.
Baseline characteristics of patients and controls
| Healthy controls (n = 32) | Hemodialysis controls (n = 58) | Peritoneal dialysis controls (n = 29) | Kidney transplantation (n = 90) | p-value | |
|---|---|---|---|---|---|
| Sociodemographics | |||||
| Age (years, mean ± SD) | 52.7 ± 10.7 | 67.0 ± 13.9 | 67.0 ± 13.9 | 59.7 ± 12.5 | .001 |
| Sex (female, n [%]) | 27 (84.4) | 19 (32.8) | 7 (24.1) | 35 (38.9) | .329 |
| Body mass index (kg/m2, mean ± SD) | 25.0 ± 4.3 | 27.5 ± 6.1 | 29.2 ± 5.5 | 27.7 ± 5.9 | .396 |
| Time on RRT, months (median [IQR]) | — | 27 (10–49) | 12 (2–24) | 42 (9–99) | .001 |
| Administered vaccine, n (%) | |||||
| mRNA−1273 (Moderna) | 11 (34.4) | 19 (32.8) | 29 (100) | 90 (100) | <.001 |
| BNT162b2 (Pfizer) | 21 (65.6) | 39 (67.2) | — | — | |
| Other vaccines in the last 12 months | |||||
| No, n (%) | 19 (59.4) | 23 (39.7) | 13 (44.8) | 59 (65.6) | .012 |
| Influenza vaccine, n (%) | 13 (40.6) | 31 (53.4) | 16 (55.2) | 28 (31.1) | |
| Others, n (%) | — | 4 (6.9) | — | 3 (3.3) | |
| Time since last vaccine, months (median [IQR]) | 4 (3–4) | 3 (3–4) | 3 (2.3–3) | 3 (3–4) | .319 |
| Darbepoetin treatment, n (%) | — | 43 (74.1) | 20 (69.0) | 10 (11.1) | <.001 |
| Vitamin D supplementation, n (%) | — | 37 (63.8) | 21 (72.4) | 18 (20.0) | <.001 |
| Comorbidities | |||||
| Arterial hypertension, n (%) | 6 (18.8) | 56 (96.6) | 29 (100) | 88 (97.8) | .594 |
| Diabetes mellitus, n (%) | 1 (3.1) | 36 (62.1) | 17 (58.6) | 37 (41.1) | .030 |
| Cardiovascular disease, n (%) | 1 (3.1) | 24 (41.4) | 15 (51.7) | 32 (35.6) | .295 |
| Pulmonary disease, n (%) | 0 (0) | 24 (41.4) | 12 (41.4) | 18 (20.0) | .008 |
| Underlying diabetic kidney disease, n (%) | — | 22 (37.9) | 4 (13.8) | 10 (11.1) | .002 |
| Blood tests | |||||
| White blood cells, ×103 U/µl (mean ± SD) | 7.82 ± 2.28 | 6.91 ± 1.90 | 8.24 ± 2.71 | 8.20 ± 2.57 | .005 |
| T lymphocytes, ×103 U/µl (mean ± SD) | 2.29 ± 0.81 | 1.45 ± 0.57 | 1.29 ± 0.65 | 2.17 ± 1.06 | <.001 |
| Creatinine, mg/dl (mean ± SD) | 0.70 ± 0.15 | — | — | 1.63 ± 0.75 | <.001 |
| eGFR, ml/min per 1.73 m2 (mean ± SD) | 98.1 ± 14.6 | — | — | 49.9 ± 22.9 | <.001 |
| C-reactive protein, mg/dl (mean ± SD) | 0.31 ± 0.53 | 2.21 ± 3.30 | 1.35 ± 2.96 | 0.61 ± 1.06 | .001 |
| Albumin, g/dl (mean ± SD) | 4.56 ± 0.25 | 3.83 ± 0.36 | 3.47 ± 0.43 | 4.29 ± 0.50 | <.001 |
| Maintenance immunosuppression in kidney transplant recipients | |||||
| Prednisone, n (%) | — | — | — | 82 (91.1) | — |
| Mycophenolic acid derivatives, n (%) | — | — | — | 49 (54.4) | — |
| Dose, mg/kg/day (mean ± SD) | — | — | — | 8.06 ± 2.90 | — |
| Tacrolimus, n (%) | — | — | — | 82 (91.1) | — |
| Dose, mg/kg/day (mean ± SD) | 0.048 ± 0.034 | ||||
| Blood levels, ng/ml (mean ± SD) | 6.12 ± 2.06 | — | |||
| Cyclosporin A, n (%) | — | — | — | 3 (3.3) | — |
| Dose, mg/kg/day (mean ± SD) | — | — | — | 1.19 ± 0.32 | — |
| Blood levels, ng/ml (mean ± SD) | — | — | — | 217.3 ± 139.3 | — |
| Everolimus, n (%) | — | — | — | 22 (24.4) | — |
| Dose, mg/kg/day (mean ± SD) | — | — | — | 0.037 ± 0.02 | — |
| Blood levels, ng/ml (mean ± SD) | — | — | — | 4.36 ± 1.25 | — |
Bold values indicate statistically significant p values (p < .05).
Abbreviations: eGFR, estimated glomerular filtration rate; IQR, interquartile range; RRT, renal replacement therapy; SD, standard deviation.
TABLE 2.
Baseline characteristics of KT recipients comparing those performed within the previous year and those performed earlier
| KT <1 year (n = 26) | KT >1 year (n = 64) | p-value | |
|---|---|---|---|
| Sociodemographics | |||
| Age (years, mean ± SD) | 56.5 ± 12.7 | 60.9 ± 12.3 | .132 |
| Sex (female, n [%]) | 12 (46.2) | 23 (35.9) | .368 |
| Body mass index (kg/m2, mean ± SD) | 28.7 ± 7.45 | 27.3 ± 5.15 | .309 |
| Darbepoetin treatment, n (%) | 4 (15.4) | 6 (9.4) | .411 |
| Vitamin D supplementation, n (%) | 4 (15.4) | 14 (21.9) | .485 |
| Blood tests | |||
| White blood cells, ×103 U/µl (mean ± SD) | 7.23 ± 2.33 | 8.60 ± 2.58 | .021 |
| T lymphocytes, ×103 U/µl (mean ± SD) | 1.95 ± 0.85 | 2.26 ± 1.12 | .202 |
| Creatinine, mg/dl (mean ± SD) | 1.75 ± 0.71 | 1.58 ± 0.76 | .338 |
| eGFR, ml/min per 1.73 m2 (mean ± SD) | 45.1 ± 19.5 | 51.9 ± 24.0 | .202 |
| C-reactive protein, mg/dl (mean ± SD) | 0.72 ± 1.40 | 0.56 ± 0.88 | .524 |
| Albumin, g/dl (mean ± SD) | 4.22 ± 0.36 | 4.32 ± 0.55 | .374 |
| Comorbidities | |||
| Arterial hypertension, n (%) | 26 (100) | 62 (96.9) | .362 |
| Diabetes mellitus, n (%) | 10 (38.5) | 27 (42.2) | .745 |
| Cardiovascular disease, n (%) | 9 (34.6) | 23 (35.9) | .905 |
| Pulmonary disease, n (%) | 5 (19.2) | 13 (20.3) | .907 |
| Underlying diabetic kidney disease, n (%) | 4 (15.4) | 6 (9.4) | .728 |
| Induction therapy | |||
| No, n (%) | 0 (0) | 1 (1.6) | .64 |
| Anti-IL2R, n (%) | 23 (88.5) | 52 (81.3) | |
| Antithymocyte globulin, n (%) | 3 (11.5) | 11 (17.2) | |
| Maintenance immunosuppression | |||
| Prednisone, n (%) | 26 (100) | 56 (87.5) | .059 |
| Mycophenolic acid derivatives, n (%) | 14 (53.8) | 35 (54.7) | .942 |
| Dose, mg/kg/day (mean ± SD) | 9.07 ± 3.65 | 7.65 ± 2.49 | .122 |
| Tacrolimus, n (%) | 25 (96.2) | 57 (89.1) | .469 |
| Dose, mg/kg/day (mean ± SD) | 0.063 ± 0.041 | 0.041 ± 0.028 | .018 |
| Blood levels, ng/ml (mean ± SD) | 6.55 ± 2.58 | 5.93 ± 1.77 | .214 |
| Cyclosporin A, n (%) | 0 (0) | 3 (4.7) | — |
| Dose, mg/kg/day (mean ± SD) | — | 1.19 ± 0.32 | — |
| Blood levels, ng/ml (mean ± SD) | — | 217.3 ± 139.3 | — |
| mTOR inhibitors (everolimus, n [%]) | 7 (36.9) | 15 (23.4) | .727 |
| Dose, mg/kg/day (mean ± SD) | 0.048 ± 0.020 | 0.03 ± 0.019 | .098 |
| Blood levels, ng/ml (mean ± SD) | 4.49 ± 1.35 | 4.30 ± 1.24 | .757 |
| Biopsy proven acute rejectiona | |||
| Cellular acute rejection, n (%) Treated with methylprednisolone bolus therapy 250 mg x 3 |
1 (3.8) | — | — |
| SARS-CoV−2 immune response | |||
| Seropositivity, n (%) | 13 (50) | 44 (68.8) | .094 |
| Antibody levels, AU/ml (median [IQR]) | 12.0 (1.85–30.2) | 80.4 (5.09–316.8) | .003 |
| T cell response, n (%) | 9 (36.0) | 38 (61.3) | .032 |
Bold values indicate statistically significant p values (p < .05).
Abbreviations: eGFR, estimated glomerular filtration rate; IQR, interquartile range; KT, kidney transplant; SD, standard deviation.
Twelve months before the vaccine.
3.2. Humoral response and factors associated with response
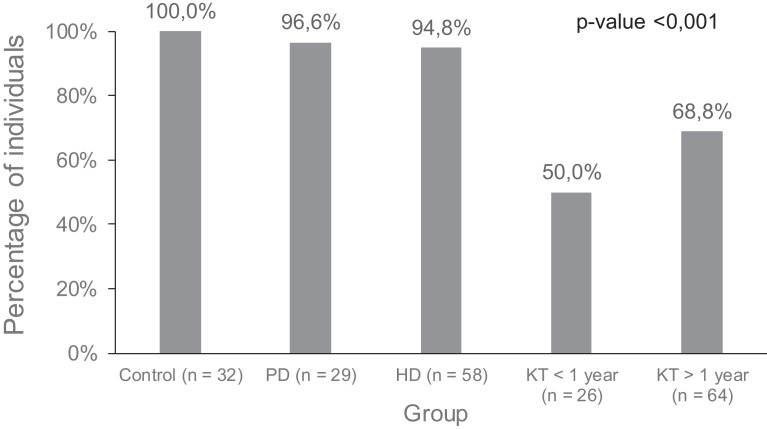
Four weeks after completing vaccination, all healthy controls elicited detectable humoral responses, as did almost all peritoneal dialysis (PD) (96.6%) and HD controls (94.8%) ( Figure 1). Only 57 out of 90 KT (63.3%) seroconverted.
FIGURE 1.
Seropositivity percentages after vaccination in the different groups of patients and controls
Factors associated with lack of seroconversion in KT recipients in univariate analysis were darbepoetin need for anemia management, a KT performed during the previous 6 months, high serum creatinine or low estimated glomerular filtration rate (eGFR). At multivariate analysis, only KT <6 months showed marginal association with a negative antibody response to vaccination according to the manufacturer cut-off point (p = .05) ( Table 3).
TABLE 3.
Factors associated with a negative antibody response after vaccination in KT recipients
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Sociodemographics | ||||||
| Age (years) | 1.035 | 0.99–1.07 | .06 | 1.03 | 0.98–1.074 | .16 |
| Sex (ref: female) | 0.53 | 0.22–1.27 | .15 | |||
| Body mass index (kg/m2) | 1.034 | 0.96–1.11 | .36 | |||
| Seasonal vaccine (ref: no) | 0.85 | 0.33–2.22 | .75 | |||
| Darbepoetin treatment (ref: no) | 8.80 | 1.74–44.46 | .009 | 6.02 | 0.89–34.35 | .17 |
| Vitamin D supplementation (ref: no) | 1.50 | 0.52–4.92 | .44 | |||
| Comorbidities | ||||||
| Hypertensiona | — | — | — | |||
| Diabetes mellitus | 1.08 | 0.45–2.59 | .84 | |||
| Cardiovascular disease | 1.05 | 0.43–2.58 | .90 | |||
| Pulmonary disease | 1.50 | 0.52–4.29 | .44 | |||
| Underlying diabetic nephropathy | 2.53 | 0.50–12.70 | .25 | |||
| Blood tests | ||||||
| White blood cells (×103 U/µl) | 0.83 | 0.69–1.013 | .12 | |||
| T lymphocytes (×103 U/µl) | 0.68 | 0.42–1.10 | .11 | |||
| C-reactive protein (mg/dl) | 1.19 | 0.77–1.82 | .41 | |||
| Albumin (g/dl) | 0.77 | 0.30–2.00 | .60 | |||
| Transplantation characteristics | ||||||
| Time after transplantation (months) | 0.99 | 0.98–1.01 | .10 | |||
| Transplantation >1 year | 0.45 | 0.17–1.15 | .09 | 0.42 | 0.14–1.12 | .11 |
| Transplantation >6 months | 0.37 | 0.12–1.12 | .07 | 0.29 | 0.08–1.01 | .05 |
| Serum creatinine (mg/dl)b | 2.17 | 1.12–4.22 | .02 | 1.60 | 0.75–3.40 | .22 |
| eGFR (ml/min per 1.73 m2)b | 0.97 | 0.95–0.99 | .02 | 0.98 | 0.96–1.01 | .30 |
| Immunosuppression treatment | ||||||
| Prednisonea | — | — | — | |||
| Mycophenolic acid | 1.48 | 0.62–3.54 | .37 | |||
| Tacrolimus or cyclosporin A | 2.56 | 0.27–23.94 | .41 | |||
| Tacrolimus dose (mg/kg/day) | 0.013 | 0.01–22.28 | .38 | |||
| Tacrolimus blood levels (ng/ml) | 1.97 | 0.92–1.03 | .45 | |||
| Everolimus | 1.98 | 0.36–2.66 | .97 | |||
| Everolimus dose (mg/kg/day) | 0.11 | 0.01–3.99 | .45 | |||
| Everolimus blood levels (ng/ml) | 1.65 | 0.74–3.66 | .21 | |||
Bold values indicate statistically significant p values (p < .05).
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; KT, kidney transplant; OD, odds ratio.
Impossible calculation, as patients without prednisone and a negative response were = 0.
Different models, using either serum creatinine or eGFR.
Establishing good response in the 25th percentile of antibody production, risk factors for not reaching this cut-off in the univariate analysis were darbepoetin treatment, recent KT, and low eGFR. In the multivariate analysis, a recent KT was the only significant factor associated with a lack of substantial production of antibodies ( Table 4). Every month of posttransplant period increased by 1% the possibilities of achieving the 25th percentile of antibody production.
TABLE 4.
Factors associated with insufficient humoral response to vaccine in KT recipients, defined as not reaching the 25th percentile of antibody levels
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Sociodemographics | ||||||
| Age (years) | 1.030 | 0.99–1.06 | .08 | 1.01 | 0.97–1.05 | .38 |
| Sex (ref: female) | 0.49 | 0.20–1.17 | .10 | |||
| Body mass index (kg/m2) | 1.078 | 1.00–1.16 | .05 | 1.06 | 0.97–1.16 | .15 |
| Seasonal vaccine (ref: no) | 0.63 | 0.25–1.56 | .32 | |||
| Darbepoetin treatment (ref: no) | 9.94 | 1.20–82.21 | .03 | 5.96 | 0.59–54.41 | .13 |
| Vitamin D supplementation (ref: no) | 2.90 | 0.93–8.99 | .07 | 2.90 | 0.81–10.43 | .10 |
| Comorbidities | ||||||
| Hypertension | 1.09 | 0.06–18.06 | .94 | |||
| Diabetes mellitus | 1.13 | 0.48–2.62 | .77 | |||
| Cardiovascular disease | 1.87 | 0.36–2.06 | .75 | |||
| Pulmonary disease | 1.57 | 0.57–4.51 | .40 | |||
| Underlying diabetic nephropathy | 1.57 | 0.15–2.18 | .41 | |||
| Blood tests | ||||||
| White blood cells (×103 U/µl) | 0.87 | 0.73–1.034 | .11 | |||
| T lymphocytes (×103 U/µl) | 0.74 | 0.48–1.12 | .16 | |||
| C-reactive protein (mg/dl) | 1.012 | 0.66–1.54 | .95 | |||
| Albumin (g/dl) | 0.75 | 0.31–1.81 | .53 | |||
| Transplantation characteristics | ||||||
| Time after transplantation (months)a | 0.99 | 0.983–0.998 | .009 | 0.99 | 0.98–0.99 | .012 |
| Transplantation >1 yeara | 0.21 | 0.07–0.61 | .004 | 0.16 | 0.50–0.57 | .004 |
| Transplantation >6 monthsa | 0.11 | 0.24–0.54 | .006 | 0.60 | 0.01–0.33 | .001 |
| Serum creatinine (mg/dl)b | 1.94 | 0.98–3.85 | .05 | 1.45 | 0.64–3.28 | .37 |
| eGFR (ml/min per 1.73 m2)b | 0.97 | 0.95–0.99 | .01 | 0.98 | 0.95–1.005 | .12 |
| Immunosuppression treatment | ||||||
| Prednisonec | — | — | — | |||
| Mycophenolic acid | 1.07 | 0.46–2.47 | .86 | |||
| Tacrolimus or cyclosporin A | 5.11 | 0.54–47.73 | .15 | |||
| Tacrolimus dose (mg/kg/day) | 0.037 | 0.001–8.35 | .24 | |||
| Tacrolimus blood levels (ng/ml) | 1.96 | 0.93–1.02 | .32 | |||
| Everolimus | 1.13 | 0.43–2.96 | .80 | |||
| Everolimus dose (mg/kg/day) | 0.47 | 0.01–3.32 | .97 | |||
| Everolimus blood levels (ng/ml) | 1.44 | 0.68–3.04 | .33 | |||
Bold values indicate statistically significant p values (p < .05).
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; KT, kidney transplant; OR, odds ratio.
Different multivariate models maintain the three different variables within the model, all significant: months after transplant (continuous variable), KT of more than a year or KT of more than 6 months.
Not significant either including serum creatinine or eGFR.
Impossible to calculate, as patients negative without prednisone were = 0.
Including only seropositive patients, median (IQR) antibody levels were 412 (165–704) AU/ml ( Table 5). As expected, healthy controls generated the highest amount of antibodies (734 [532–1149] AU/ml), while PD and HD controls achieved 559 (216–908) AU/ml and 378 (195–664) AU/ml, respectively. KT elicited significantly lower median antibody levels: 139 (43–440) AU/ml. In this case, time since transplantation was again an important factor as patients with a KT <1 year barely generated antibodies in contrast to KT >1 year (p = .017).
TABLE 5.
Humoral and cellular responses between Moderna and Pfizer vaccines
| Group | Humoral response |
Cellular response |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (N Pfizer) | Seropositivity n (%) | Antibody levels (median [IQR]) |
p-value* | N (N Pfizer) | Positive IGRA n (%) |
|||||
| All | Moderna | Pfizer | All | Moderna | Pfizer | |||||
| All | 209 (60) | 172 (82.3) | 412 (165–704) | — | — | — | 205 (60) | 148 (72.2) | 97 (66.9) | 51 (85.0) |
| KT | 90 | 57 (63.3) | — | 139 (43.4–440) | — | — | 87 | 47 (54.0) | — | — |
| KT <1 year | 26 | 13 (50.0) | — | 25.7 (23–167) | — | — | 25 | 9 (36.0) | — | — |
| KT >1 year | 64 | 44 (68.8) | — | 221 (66–494) | — | — | 62 | 38 (61.3) | — | — |
| Healthy controls | 32 (21) | 32 (100) | 734 (532–1149) | 1570 (793–1742) | 678 (489–753) | .003 | 32 (21) | 31 (96.9) | 11 (100) | 20 (95.2) |
| Dialysis controls | 87 (39) | 83 (95.4) | 445 (203–702) | — | — | — | 86 (39) | 70 (81.3) | 39 (83.0) | 31 (79.5) |
| HD controls | 58 (39) | 55 (94.8) | 378 (195–664) | 470 (218–744) | 377 (194–626) | .547 | 58 (39) | 45 (77.6) | 14 (73.7) | 31 (79.5) |
| PD controls | 29 | 28 (96.6) | — | 559 (216–908) | — | — | 28 | 25 (89.3) | — | — |
Note: Median antibody levels in AU/ml of responders in each group with two different vaccines. Differences between controls and patients were significant (controls vs. HD p < .001, controls vs. PD p = .033, controls vs. KT p < .001). Differences between HD and PD were not significant. Differences between HD and KT, and between PD and KT were significant, either considering KT > or <1 year (p < .005–.001 in each case). Differences between KT >1 year and KT <1 year were also significant (p = .017). Differences were similar when considering only individuals vaccinated with Moderna.
Bold values indicate statistically significant p values (p < .05).
Abbreviations: HD, hemodialysis; IGRA, interferon gamma release assay; IQR, interquartile range; KT, kidney transplant; PD, peritoneal dialysis.
Comparison between Moderna and Pfizer.
Regarding the type of vaccine, individuals of the healthy control group immunized with Moderna elicited higher antibody levels than with Pfizer. Conversely, no significant differences were observed in HD controls (Table 5).
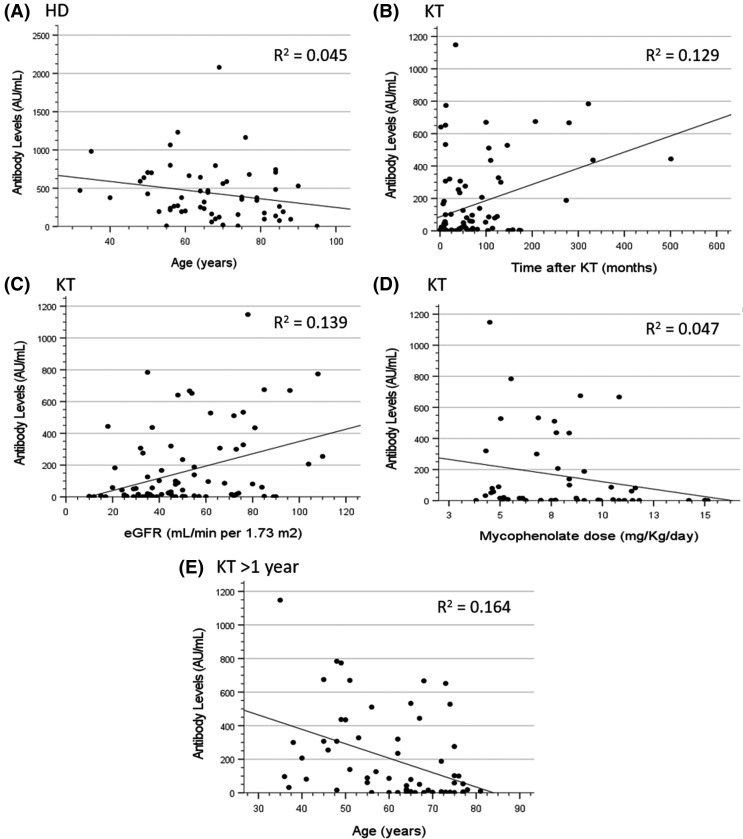
Antibody levels negatively correlated with increasing age in HD controls (Spearman ρ = −0.28 [95% confidence interval, CI −0.504 to −0.021], p = .035) and KT recipients >1 year (Spearman ρ = −0.404 [−0.592 to −0.174], p = .001). Additionally, eGFR and time after KT positively correlated with the amount of antibodies (Spearman ρ = 0.355 [0.157–0.525], p = .001 and Spearman ρ = 0.34 [0.141–0.513], p = .001), while a higher dose of mycophenolate correlated with lower antibody response (Spearman ρ = −0.295 [−0.475 to −0.091], p = .042) ( Figure 2).
FIGURE 2.
Correlations of serum antibody levels and several variables: (A) age in HD patients (Spearman ρ = –0.28 [95% CI –0.504 to –0.021], p = .035), (B) time after KT in KT recipients (Spearman ρ = 0.34 [95% CI 0.141–0.513], p = .001), (C) eGFR in KT recipients (Spearman ρ = 0.355 [95% CI 0.157–0.525], p = .001), (D) mycophenolate dose in KT recipients (Spearman ρ = –0.295 [95% CI –0.475 to –0.091], p = .042), and (E) age in KT recipients with more than 1 year of a functioning graft (Spearman ρ = –0.404 [95% CI –0.592 to –0.174], p = .001). CI, confidence interval; HD, hemodialysis; KT, kidney transplant
3.3. Cellular response and concordance with humoral response
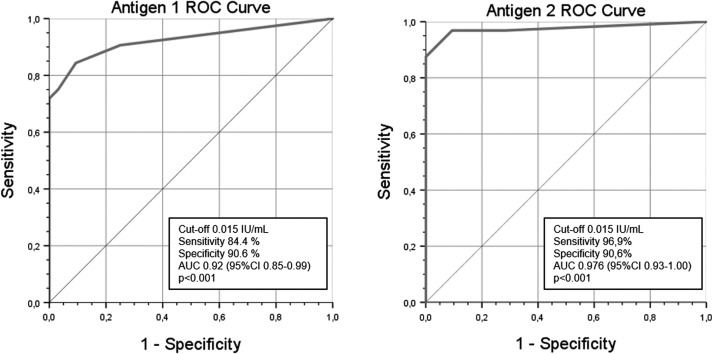
A ROC analysis was used to obtain cut-off values for IFNγ-response detection with IGRA. Data from the healthy control group were used, considering pre-vaccination results as negative and post-vaccination as positive. The resulting cut-off was 0.015 IU/ml for each antigen tube (area under the curve [AUC] 0.92 [95% CI 0.85–0.99], p < .001 and AUC 0.976 [95% CI 0.93–1.00], p < .001), with a sensitivity and specificity of 84.4% and 90.6% for antigen tube 1 and 96.9% and 90.6% for antigen tube 2, respectively ( Figure 3).
FIGURE 3.
ROC analysis to identify the cut-off for positive IGRA using results of healthy controls before (considered negative) and after vaccination (considered positive). IGRA, interferon gamma release assay; ROC, receiver operating characteristic
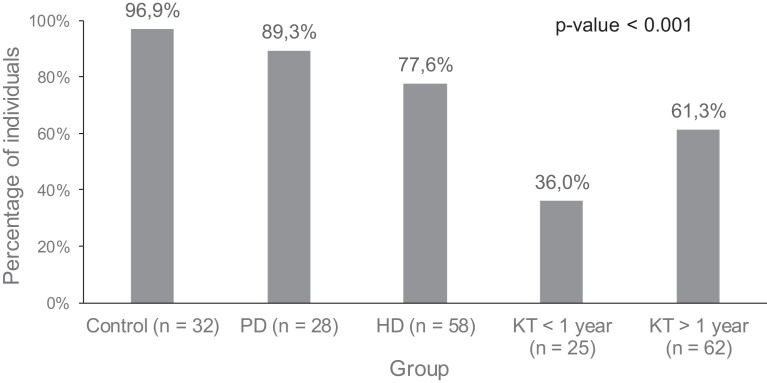
Like seropositive rates, 96.9% of healthy controls showed a positive IGRA test. With this cut-off value, 89.3% PD and 77.6% HD controls generated reactive T cells 28 days after vaccination, respectively. Within the KT group, only 36% of KT <1-year patients developed cellular responses and 61.3% of KT >1 year did ( Figure 4). No significant differences in cellular response in dialysis and healthy controls were observed between vaccines; no comparison could be established in KT as they were all vaccinated with Moderna (Table 5). Factors associated with IGRA response are described in Table 6.
FIGURE 4.
Percentages of T cell responses after vaccination in the different groups of patients and controls
TABLE 6.
Factors associated with a negative cell IGRA response after vaccination in KT recipients
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Sociodemographics | ||||||
| Age (years) | 0.98 | 0.95–1.023 | .49 | |||
| Sex (ref: female) | 0.63 | 0.26–1.50 | .10 | |||
| Body mass index (kg/m2) | 0.99 | 0.92–1.06 | .83 | |||
| Seasonal vaccine (ref: no) | 1.02 | 0.40–2.57 | .96 | |||
| Darbepoetin treatment (ref: no) | 0.46 | 0.11–1.921 | .29 | |||
| Vitamin D supplementation (ref: no) | 0.92 | 0.32–2.62 | .88 | |||
| Comorbidities | ||||||
| Hypertension | 0.84 | 0.05–14.04 | .90 | |||
| Diabetes mellitus | 0.98 | 0.41–2.32 | .96 | |||
| Cardiovascular disease | 0.55 | 0.22–1.38 | .20 | |||
| Pulmonary disease | 2.16 | 0.75–6.26 | .15 | |||
| Underlying diabetic nephropathy | 3.11 | 0.74–12.94 | .11 | |||
| Blood tests | ||||||
| White blood cells (×103 U/µl) | 0.91 | 0.77–1.088 | .32 | |||
| T lymphocytes (×103 U/µl) | 0.56 | 0.34–0.93 | .02 | .58 | 0.35–0.97 | .04 |
| C-reactive protein (mg/dl) | 1.29 | 0.80–2.09 | .29 | |||
| Albumin (g/dl) | 0.56 | 0.20–1.54 | .26 | |||
| Transplantation characteristics | ||||||
| Time after transplantation (months)a | 0.99 | 0.99–1.002 | .20 | |||
| Transplantation >1 yeara | 0.35 | 0.13–0.93 | .035 | .58 | 0.14–1.05 | .06 |
| Transplantation >6 monthsa | 0.50 | 0.16–1.56 | .23 | |||
| Serum creatinine (mg/dl)b | 1.02 | 0.56–1.84 | .93 | |||
| eGFR (ml/min per 1.73 m2)b | 0.99 | 0.97–1.01 | .54 | |||
| Immunosuppression treatment | ||||||
| Prednisone | 2.78 | 0.52–14.62 | .22 | |||
| Mycophenolic acid | 1.61 | 0.26–1.43 | .26 | |||
| Tacrolimus or cyclosporin Ac | — | — | — | |||
| Everolimus | 1.40 | 0.52–3.75 | .50 | |||
| Everolimus dose (mg/kg/day) | 0.01 | 0.01–6.34 | .83 | |||
| Everolimus blood levels (ng/ml) | 1.71 | 0.32–1.55 | .39 | |||
Bold values indicate statistically significant p values (p < .05).
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; OD, odds ratio; RRT, renal replacement therapy.
Different multivariate models maintain the three different variables within the model, marginal significance with KT of more than a year.
Not significant either including serum creatinine or eGFR.
Impossible to calculate, as patients negative without tacrolimus or cyclosporine were = 0.
Roughly two thirds (65.9%) of IGRA(+) individuals were also positive for anti-S IgG, while only 11.2% were negative for assays. However, 47 patients (22.9%) had discordant results: 34 were IgG(+)/IGRA(–) and 13 IgG(–)/IGRA(+). All healthy controls and most dialysis controls (96.6% PD, 94.8% HD) developed any response (humoral and/or cellular) against SARS-CoV-2 after vaccination. This percentage was only 77.8% in KT patients.
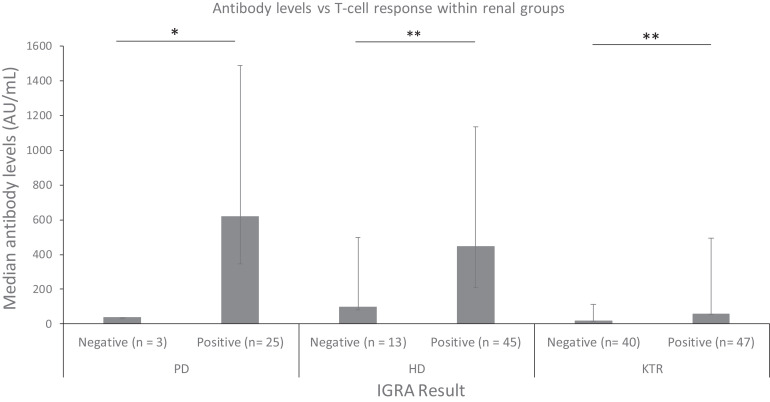
Antibody levels of IGRA(+) individuals (n = 148) were significantly higher than IGRA(–) (n = 57) (451 [158–725] vs. 24 [1.8–180], p < .001). This tendency prevailed within the different groups of patients, especially in HD (p = .001) and KT (p = .007) ( Figure 5).
FIGURE 5.
Median antibody levels in positive and negative individuals for T cell responses in each group of PD controls, HD controls, and KT recipients (*p < .001, **p < .01). HD, hemodialysis; KT, kidney transplant; PD, peritoneal dialysis
3.4. Safety
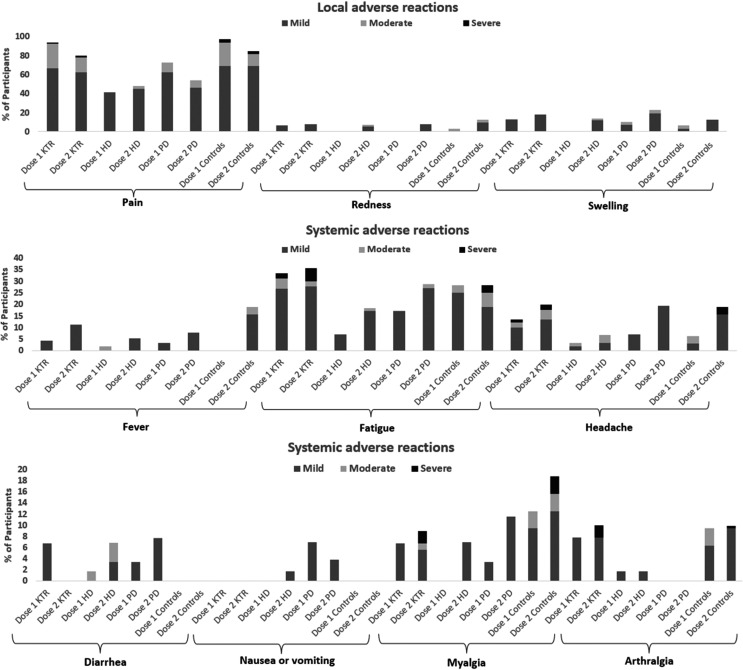
COVID-19 mRNA vaccines were safe and well tolerated in renal patients, showing mild AE without serious reactions ( Figure 6). Pain (76.6%) and swelling (8.1%) were the most prevalent local AE after the first dose, and fatigue (23%) and headache (8.8%) were the most frequent systemic reactions.
FIGURE 6.
Safety assessment in vaccinated individuals using a standardized questionnaire
No cases of COVID-19 infection, acute rejection, Guillain-Barré syndrome, anaphylactic reactions, or enhanced respiratory disease were observed. No participant had any serious AE requiring emergency hospitalization.
A similar pattern was observed after the second dose, with pain (67.8%) and swelling (16.8%) being the main local solicited AE and fatigue (29.3%) and headache (15.9%) the systemic reactions. The second dose was significantly worse tolerated in terms of swelling (p = .021), fever (p = .002), headache (p = .007), and myalgia (p = .024), whereas the incidence of pain was lower (p = .006). Again, no serious AE was detected.
Compared to Pfizer, those that received Moderna vaccine presented more pain after both doses (first: 84.6% vs. 56.6%, p < .001; second: 74.7% vs. 53.3%, p = .012) and arthralgia (first: 6.7% vs. 1.7%, p = .036; second: 11.7% vs. 0%, p = .022), whereas swelling (11.4% vs 0%, p = .024), fatigue (10.7% vs 3.3%, p = .035) and myalgia (6.7% vs. 1.7%, p = .036) were more prevalent only after the first dose.
We performed subgroup analyses comparing patients and controls. Data are shown in Figure 6.
4. DISCUSSION
Clinical trials of both mRNA vaccines disregarded the immune response that CKD or immunosuppressed patients could generate,21 , 27 , 29 , 30 therefore we attempted to shed light in this topic. To the best of our knowledge, this is one of the first studies that evaluates cellular immunogenicity of mRNA COVID-19 vaccines with an IGRA in a KT cohort. Additionally, we also provided data on humoral response and safety in KT and compared them with healthy controls and dialysis controls. Our analysis evidences a robust and timely humoral and cellular response to mRNA COVID-19 vaccines in healthy controls and CKD patients on HD or PD 28 days after full vaccination. Contrarily, the response of KT recipients is less frequent and significantly less intense, especially in those transplanted within the previous year.
Almost all HD and PD controls generated antibodies against SARS-CoV-2 S protein, but antibody levels were significantly lower than in our healthy controls. Seroconversion rates in this study resemble those described in other ones.31, 32, 33, 34
KT recipients had discouraging antibody levels after vaccination, indicating a poor response to mRNA vaccines. They are in a more concerning situation as their antibody levels slightly exceeded the cut-off to be considered positive. Two different studies reported seropositive rates of 22%35 and 37.5%36 after BNT162b2 vaccination, lower than in the present study using kits from the same manufacturer (DiaSorin) with similar cut-off values. However, their post-vaccination analysis was conducted earlier, 10–14 days after the second dose, so there may not have been enough time to develop a detectable response in some patients. At 28 days after booster dose, Benotmane et al. observed with an Abbott kit that 48% of 205 KT recipients vaccinated with mRNA-1273 elicited antibody levels >50 AU/ml.37 Surprisingly, the Berlin group detected lower anti-BNT162b2 responses in naive dialysis and KT recipients (70.5% and 0%) 3 to 4 weeks after vaccination with ELISA and cut-offs based on optical density ratios.38 Technical design, sensitivity and established cut-off values of the different kits may explain the disparity of seropositive rates seen in KT recipients. However, studies involving patients on dialysis report similar rates even when using different kits.31, 32, 33, 34
KT with worse kidney graft function were less likely to seroconvert in univariate analysis, as reported previously.36 , 37 However, multivariate analyses showed that the association was not significant. Older age has been associated with lower antibody levels in dialysis patients31 , 39 and this correlation was seen in our HD and KT patients, but again the multivariate adjustment diluted the effect. A short time since transplantation became the only detectable risk factor for a negative response to vaccination, and a lower production of antibodies, as seen in transplant recipients.24 , 34 This finding suggests that the capacity to produce antibodies is impaired early after KT, probably related to the amount of immunosuppression administered, independently of recipient age.22 , 23 It is worth noting, however, that immunosuppressive drug class, dose and levels were not associated to a negative response.
T cell responses showed trends like humoral responses across all groups. Lower T cell responses were observed in the KT patients than in healthy and dialysis controls, especially in those who had received the kidney graft <1 year before vaccination. A previous smaller study reported that 57.8% of KT recipients elicited SARS-CoV-2 cell-mediated immunity in contrast to all their HD patients using an ELISPOT assay.25 We employed a new and simple kit that assesses T cell immunity through IGRA, looking for a simpler tool for monitoring. Using this assay, Stumpf et al. described similar positive rates in their KT and dialysis patients (30% and 78%).34 In contrast to ELISPOT or intracellular cytokine staining, QuantiFERON® SARS-CoV-2 allows the processing of a larger number of samples without requiring much effort.40 Moreover, concordance between detection of IFNγ-expressing cells by intracellular cytokine staining and quantification of soluble IFNγ through ELISA was high in SARS-CoV-2 convalescent patients41 , 42 and vaccinated renal patients.34 This format has been used in tuberculosis detection for over a decade. Several societies recommend their use, and the latest generation of QuantiFERON®-TB has proved to increase sensitivity even in immunocompromised patients.43 In addition, tuberculosis and cytomegalovirus QuantiFERON® assays have shown a good correlation with ELISPOT in renal patients.44 , 45 Interestingly, the most relevant factor associated with a good IGRA response was the number of peripheral blood T lymphocytes. Cut-off values for IFNγ detection with this ELISA kit have not yet been stablished, however we obtained one through ROC analysis similarly to other studies involving COVID-19 convalescent patients.40 , 46 Once standardization is achieved, QuantiFERON® SARS-CoV-2 IGRA will serve as a simple but effective tool for cell-mediated immunity evaluation after COVID-19 vaccination, as reflected by recent studies that only incorporate this assay in their cellular analysis.47 , 48
Regarding safety, most of the observed AE were like those reported in randomized trials and observational studies conducted in KT recipients.26 , 27 , 30 , 49 mRNA vaccines were well tolerated without significant safety issues in renal patients. As expected, the second dose was worse tolerated than the first one,27 , 28 and some AE were more frequent with the mRNA-1273 vaccine.
Strengths in our study were the inclusion of a direct comparison of a KT cohort with healthy controls and control patients with advanced CKD on PD or HD programs, who underwent simple and reliable monitoring of humoral and cellular response to mRNA vaccines. The main limitation is the reduced number of KT recipients during their first year of postransplantation follow-up, which precludes detailed analyses of some factors potentially associated with lack of response. As technical limitations, we recognize the lack of results regarding neutralizing activity and durability of immune responses. However, the kit used to quantify anti-S IgG has a good correlation with a microneutralization test,50 therefore data on antibody levels presented in this study could highly relate with serum neutralizing capacity. As opposed to other studies that used the same kit,35 , 39 we diluted samples that exceeded assay measuring range to obtain a more accurate value of antibody levels. Concerning durability, this project will keep ongoing and evaluate cellular and humoral immunity at 6 and 12 months. However, Boyarsky et al. have demonstrated that antibody levels increase or remain stable between months 1 and 3 post-vaccination in 64% of solid organ transplant recipients.51 The incorporation of IGRA, as an easier way to assess cellular immunity, may be key for the complete follow-up of many patients at risk of COVID-19.
Vaccination is the best way to prevent SARS-CoV-2 infection. Nevertheless, KT patients develop weaker responses than healthy individuals and dialysis patients, probably related to the immunosuppression associated with anti-rejection treatment.52 Precautions still need to be taken in order to protect this vulnerable population, as COVID-19 cases in vaccinated KT recipients have already been described.53 , 54 Our findings have direct clinical implications. It is mandatory to find a suitable strategy to improve their immunological responses. In France, authorities have approved the administration of a third mRNA vaccine dose in immunocompromised individuals, which includes transplant recipients and patients on dialysis.55 Following this recommendation, a study with 396 solid organ transplant recipients observed an increase of the seropositive rate from 41.4% to 67.9% 4 weeks after receiving a third BNT162b2 dose.56 Another alternative, known as the cocoon strategy, would be prioritizing vaccination of patients’ relatives to create a safe environment with a lower risk of SARS-CoV-2 infection.26 This is of particular interest in recent transplant recipients, who unfrequently develop immune responses after vaccination. In this regard, it is of utmost importance to find out if the response to the vaccine obtained on the waiting list persists despite the intense initial immunosuppression at transplantation.
In summary, a small percentage of KT recipients developed humoral responses 28 days after vaccination with mRNA vaccines, and the median antibody level was considerably low in responders, with a shorter time since transplantation associated to impaired response. Regarding cell-mediated immunity, a similar trend was observed, in which patients with higher antibody levels were more likely to mount T cell responses. KT patients, especially within the first year after transplantation, must be followed to prevent possible infections as we cannot ensure that they are fully protected against SARS-CoV-2. In addition, the immunological memory endurance in renal patients must be evaluated so that other approaches can be carried out if the vaccine effects wane.
ACKNOWLEDGMENTS
The authors thank Laura Claramunt, Rosa Causadias, and Anna Bach for their assistance with patients. The authors also thank the staff of the Hospital del Mar and the Laboratori de Referència de Catalunya for technical assistance. AB-J did this study as part of his Master degree at the Department of Experimental and Health Sciences from the Universitat Pompeu Fabra of Barcelona (UPF). MJPS has been granted by the Spanish Society of Transplant.
All of the Mariscovid research group members participated in the study: Marta Crespo, Julio Pascual, M. J. Pérez-Sáez, D. Redondo-Pachón, C. Arias, A. Buxeda, C. Burballa, A. Bach, G. Pedreira, M. D. Arenas, F. Barbosa, H. Cao, S. Collado, Marisol Fernández, E. Barbero, E. Rodríguez, L. Sans, E. Márquez, S. Vázquez, A. Oliveras, B. Galcerán, E. Solà-Porta, S. Pascual, S. Nuñez, A. Ribas, M. Iriarte, J. Farrera, A. Faura, O. Savall, M. Folgueiras, and Rosa Causadias (Nephrology Department); S. Hurtado and L. Ribera (tertiary dialysis centers); Antoni Barrilado-Jackson, Eduardo Padilla, José Muñoz, Jorge Eguía, Eduardo Villegas, and Mireia Canal (Departments of Microbiology and Immunology); Daniel Echeverria-Esnal, Laura Río-No, and Santiago Grau (Department of Pharmacy); Milagro Montero and Judit Villar (Department of Infectious Diseases); and Pilar Días (Department of Occupational Health).
This study was performed with partial funding from grants FIS-FEDER PI19/00037 and PI20/00090. The manuscript was produced as part of the Master degree of AB-J.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding information Instituto de Salud Carlos III, Grant/Award Number: FIS- FEDER PI19/00037 and FIS-FEDER PI20/00090
Footnotes
Marta Crespo and Antoni Barrilado-Jackson share first authorship.
Dolores Redondo-Pachón and Julio Pascual share senior authorship.
REFERENCES
- 1.Hu B, Guo H, Zhou P, Shi Z-L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang MY, Zhao R, Gao LJ, Gao XF, Wang DP, Cao JM. SARS-CoV-2: structure, biology, and structure-based therapeutics development. Front Cell Infect Microbiol. 2020;10 doi: 10.3389/fcimb.2020.587269. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coca A, Burballa C, Centellas-Pérez FJ, et al. Outcomes of COVID-19 among hospitalized patients with non-dialysis CKD. Front Med. 2020;7 doi: 10.3389/fmed.2020.615312. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortiz A, Cozzolino M, Fliser D, et al. Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant. 2021;36(1):87–94. doi: 10.1093/ndt/gfaa314. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Posso M, Comas M, Román M, et al. Comorbidities and mortality in patients with COVID-19 aged 60 years and older in a University Hospital in Spain. Arch Bronconeumol. 2020;56(11):756–758. doi: 10.1016/j.arbres.2020.06.012. doi: [DOI] [PubMed] [Google Scholar]
- 7.Hilbrands LB, Duivenvoorden R, Vart P, et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35(11):1973–1983. doi: 10.1093/ndt/gfaa261. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crespo M, Mazuecos A, Rodrigo E, et al. Respiratory and gastrointestinal COVID-19 phenotypes in kidney transplant recipients. Transplantation. 2020;104(11):2225–2233. doi: 10.1097/TP.0000000000003413. doi: [DOI] [PubMed] [Google Scholar]
- 9.Villanego F, Mazuecos A, Pérez-Flores IM, et al. Predictors of severe COVID-19 in kidney transplant recipients in the different epidemic waves: analysis of the Spanish Registry. Am J Transplant. 2021;21:2573–2582. doi: 10.1111/ajt.16579. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grigoryan L, Pulendran B. The immunology of SARS-CoV-2 infections and vaccines. Semin Immunol. 2020;50:101422. doi: 10.1016/j.smim.2020.101422. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim K-D, Hwang I, Ku KB, Lee S, Kim S-J, Kim C. Progress and challenges in the development of COVID-19 vaccines and current understanding of SARS-CoV-2- specific immune responses. J Microbiol Biotechnol. 2020;30(8):1109–1115. doi: 10.4014/jmb.2006.06006. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. doi: [DOI] [PubMed] [Google Scholar]
- 13.Ma H, Zhao D, Zeng W, et al. Decline of SARS-CoV-2-specific IgG, IgM and IgA in convalescent COVID-19 patients within 100 days after hospital discharge. Sci China Life Sci. 2021;64(3):482–485. doi: 10.1007/s11427-020-1805-0. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forbes S, Davari M, Gnanasampanthan S, et al. Persistence of antibody response to SARS-CoV-2 in a cohort of haemodialysis patients with COVID-19. Nephrol Dial Transplant. 2021;36:1292–1297. doi: 10.1093/ndt/gfab066. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke CL, Prendecki M, Dhutia A, et al. Longevity of SARS-CoV-2 immune responses in hemodialysis patients and protection against reinfection. Kidney Int. 2021;99(6):1470–1477. doi: 10.1016/j.kint.2021.03.009. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Favà A, Donadeu L, Sabé N, et al. SARS-CoV-2-specific serological and functional T cell immune responses during acute and early COVID-19 convalescence in solid organ transplant patients. Am J Transplant. 2021;21(8):2749–2761. doi: 10.1111/ajt.16570. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartzell S, Bin S, Benedetti C, et al. Evidence of potent humoral immune activity in COVID-19-infected kidney transplant recipients. Am J Transplant. 2020;20(11):3149–3161. doi: 10.1111/ajt.16261. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauer K, Harris T. An effective COVID-19 vaccine needs to engage T cells. Front Immunol. 2020;11:581807. doi: 10.3389/fimmu.2020.581807. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 — preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahin U, Muik A, Vogler I, et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. medRxiv. 2020;18(6) doi: 10.1101/2020.12.09.20245175. doi: [DOI] [Google Scholar]
- 22.Crespo M, Collado S, Mir M, et al. Efficacy of influenza A H1N1/2009 vaccine in hemodialysis and kidney transplant patients. Clin J Am Soc Nephrol. 2011;6(9):2208–2214. doi: 10.2215/CJN.02160311. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danziger-Isakov L, Kumar D. Vaccination of solid organ transplant candidates and recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9) doi: 10.1111/ctr.13563. doi: [DOI] [PubMed] [Google Scholar]
- 24.Herrera S, Colmenero J, Pascal M, et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am J Transplant. 2021. doi: 10.1111/ajt.16768 [DOI] [PMC free article] [PubMed]
- 25.Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32:2147–2152. doi: 10.1681/ASN.2021040480. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21:2727–2739. doi: 10.1111/ajt.16701. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaccines and Related Biological Products Advisory Committee Meeting FDA Briefing Document Pfizer-BioNTech COVID-19 Vaccine Sponsor. http://www.fda.gov/ohrms/dockets/ac/08/minutes/2008-4384M.htm. Published 2020. Accessed June 17, 2021.
- 29.Chu L, McPhee R, Huang W, et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine. 2021;39(20):2791–2799. doi: 10.1016/j.vaccine.2021.02.007. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grupper A, Sharon N, Finn T, et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021;16(6) doi: 10.2215/CJN.03500321. CJN.03500321. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanay NB, Freiman S, Shapira M, et al. Experience with SARS-CoV-2 BNT162b2 mRNA vaccine in dialysis patients. Kidney Int. 2021;99(6):1496–1498. doi: 10.1016/j.kint.2021.04.006. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lacson E, Argyropoulos CP, Manley HJ, et al. Immunogenicity of SARS-CoV-2 vaccine in dialysis. medRxiv. April 2021. doi: 10.1101/2021.04.08.21254779 [DOI] [PMC free article] [PubMed]
- 34.Stumpf J, Siepmann T, Lindner T, et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021:100178. doi: 10.1016/j.lanepe.2021.100178. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korth J, Jahn M, Dorsch O, et al. Impaired humoral response in renal transplant recipients to SARS-CoV-2 vaccination with BNT162b2 (Pfizer-BioNTech) Viruses. 2021;13(5):756. doi: 10.3390/v13050756. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21:2719–2726. doi: 10.1111/ajt.16615. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benotmane I, Gautier-Vargas G, Cognard N, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99(6):1498–1500. doi: 10.1016/j.kint.2021.04.005. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rincon-Arevalo H, Choi M, Stefanski A-L, et al. Impaired antigen-specific memory B cell and plasma cell responses including lack of specific IgG upon SARS-CoV-2 BNT162b2 vaccination among Kidney Transplant and Dialysis patients. medRxiv. April 2021:2021.04.15.21255550. doi: 10.1101/2021.04.15.21255550 [DOI]
- 39.Jahn M, Korth J, Dorsch O, et al. Humoral response to SARS-CoV-2-vaccination with BNT162b2 (Pfizer-BioNTech) in patients on hemodialysis. Vaccines. 2021;9(4):360. doi: 10.3390/vaccines9040360. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murugesan K, Jagannathan P, Pham TD, et al. Interferon-γ release assay for accurate detection of severe acute respiratory syndrome coronavirus 2 T-cell response. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa1537 [DOI] [PMC free article] [PubMed]
- 41.Vallejo A, Vizcarra P, Quereda C, Moreno A, Casado JL. IFN-γ+ cell response and IFN-γ release concordance after in vitro SARS-CoV-2 stimulation. Eur J Clin Invest. 2021. doi: 10.1111/eci.13636 [DOI] [PMC free article] [PubMed]
- 42.Mouton W, Compagnon C, Saker K, et al. Specific detection of memory T-cells in COVID-19 patients using standardized whole-blood interferon gamma release assay. Eur J Immunol. 2021:eji.202149296. doi: 10.1002/eji.202149296 [DOI] [PMC free article] [PubMed]
- 43.Fernández-Blázquez A, Argüelles Menéndez P, Sabater-Cabrera C, García-García J-M, Asensi Álvarez V, Palacios Gutiérrez JJ. Diagnóstico de la infección tuberculosa en pacientes inmunodeprimidos y/o candidatos a terapias biológicas mediante el uso combinado de dos pruebas IGRA: T-SPOT.TB/QuantiFERON TB Gold In-Tube vs. T-SPOT.TB/QuantiFERON TB Gold Plus. Arch Bronconeumol. 2020;1(1):1–6. doi: 10.1016/j.arbres.2020.04.011. doi: [DOI] [PubMed] [Google Scholar]
- 44.Winthrop KL, Nyendak M, Calvet H, et al. Interferon-γ release assays for diagnosing mycobacterium tuberculosis infection in renal dialysis patients. Clin J Am Soc Nephrol. 2008;3(5):1357–1363. doi: 10.2215/CJN.01010208. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abate D, Saldan A, Mengoli C, et al. Comparison of cytomegalovirus (CMV) enzyme-linked immunosorbent spot and CMV quantiferon gamma interferon-releasing assays in assessing risk of CMV infection in kidney transplant recipients. J Clin Microbiol. 2013;51(8):2501–2507. doi: 10.1128/JCM.00563-13. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aiello A, Najafi Fard S, Petruccioli E, et al. Spike is the most recognized antigen in the whole-blood platform in both acute and convalescent COVID-19 patients. Int J Infect Dis. 2021;106:338–347. doi: 10.1016/J.IJID.2021.04.034. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moor MB, Suter-Riniker F, Horn MP, et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021. doi: 10.1016/S2665-9913(21)00251-4 [DOI] [PMC free article] [PubMed]
- 48.Echeverría G, Guevara Á, Coloma J, et al. Pre-existing T-cell immunity to SARS-CoV-2 in unexposed healthy controls in Ecuador, as detected with a COVID-19 interferon-gamma release assay. Int J Infect Dis. 2021;105:21–25. doi: 10.1016/j.ijid.2021.02.034. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyarsky BJ, Ou MT, Greenberg RS, et al. Safety of the first dose of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021;105:e56–e57. doi: 10.1097/tp.0000000000003654. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diasorin. DiaSorin’s LIAISON® SARS-CoV-2 Diagnostic Solutions. https://www.diasorin.com/en/node/11792/. Published 2020. Accessed June 13, 2021.
- 51.Boyarsky BJ, Chiang TP-Y, Teles AT, et al. Antibody kinetics and durability in SARS-CoV-2 mRNA vaccinated solid organ transplant recipients. Transplantation. 2021. doi: 10.1097/TP.0000000000003863 [DOI] [PMC free article] [PubMed]
- 52.Kato S, Chmielewski M, Honda H, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3(5):1526–1533. doi: 10.2215/CJN.00950208. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsalouchos A, Rossolini GM, Maggi L, Mazzoni A, Annunziato F, Dattolo PC. COVID-19 in a kidney transplant recipient after mRNA-based SARS-CoV-2 vaccination. Transpl Infect Dis. 2021;23 doi: 10.1111/tid.13649. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wadei HM, Gonwa TA, Leoni JC, Shah SZ, Aslam N, Speicher LL. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am J Transplant. 2021:ajt.16618. doi: 10.1111/ajt.16618 [DOI] [PMC free article] [PubMed]
- 55.CORRUSS-Centre opérationnel de régulation et de réponse aux urgences sanitaires et sociales. DGS-URGENT N°2021_43 Vaccins contre la COVID-19: Modalites d’administration des rappels. https://solidarites-sante.gouv.fr/IMG/pdf/dgs_urgent_n43_vaccination_modalites_d_administration_des_rappels.pdf. Published 2020. Accessed June 24, 2021.
- 56.Del Bello A, Abravanel F, Marion O, et al. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am J Transplant. 2021:ajt.16775. doi: 10.1111/ajt.16775 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.