Abstract
Pyroptosis is a specialized form of inflammatory cell death which aids the defensive response against invading pathogens. Its normally tight regulation is lost during infection by the severe acute respiratory coronavirus 2 (SARS‐CoV‐2), and thus, uncontrolled pyroptosis disrupts the immune system and the integrity of organs defining the critical conditions in patients with high viral load. Molecular pathways engaged downstream of the formation and stabilization of the inflammasome, which are necessary to execute the process, have been uncovered and drugs are available for their regulation. However, the pharmacology of the upstream events, which are critical to sense and interpret the initial damage by the pathogen, is far from being elucidated. This limits our capacity to identify early markers and targets to ameliorate SARS‐CoV‐2 linked pyroptosis. Here, we focus attention on the mitochondria and pathways leading to their dysfunction, in order to elucidate the early steps of inflammasome formation and devise tools to predict and counter pathological states induced by SARS‐CoV‐2.
LINKED ARTICLES
This article is part of a themed issue on The second wave: are we any closer to efficacious pharmacotherapy for COVID 19? (BJP 75th Anniversary). To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v179.10/issuetoc
Keywords: mitochondria and pharmacology, pyroptosis, SARS‐CoV‐2
The 2019 coronavirus disease (COVID‐19) has changed our lifestyles causing an unimaginable and unprecedented health crisis with serious effects at many levels. The causative aetiological agent behind what is the fastest spreading disease of the 21st century is the severe acute respiratory coronavirus 2 (SARS‐CoV‐2). This virus is an enveloped, positive‐sense, single‐stranded RNA virus that enters the host cell by binding to the ACE2 receptor through the interaction with the trimeric S spike glycoprotein (Hoffmann et al., 2020; Kim et al., 2020).
Like other coronaviruses (i.e., the highly pathogenic severe acute respiratory syndrome coronavirus [SARS‐CoV] and the Middle East respiratory syndrome coronavirus [MERS‐CoV]), SARS‐CoV‐2 infection is associated with overbearing and uncontrolled inflammatory response (He et al., 2006; Lau et al., 2013; Zhou et al., 2014).
Although many patients infected by SARS‐CoV‐2 remain asymptomatic or develop very mild symptoms, others experience a severe and acute respiratory syndrome leading to hospitalization and critical care treatment. Notably, patients with severe COVID‐19 present elevated level of pro‐inflammatory mediators (TNF‐α and IL‐6) in their peripheral blood which epitomizes poor prognosis, linked with mortality (Hojyo et al., 2020; Santa Cruz et al., 2021).
The uncontrolled increase of cytokines delivers distress at a systemic level, irreparably damaging organs essential for life such as the heart and kidneys (Long et al., 2020).
Since the very beginning of the outbreak, it was clear that, in SARS‐CoV‐2 patients, the so‐called “cytokine storm” played a crucial role in the pathogenesis of the disease and its most severe manifestations: immune dysregulation, systemic inflammation and multi‐organ dysfunction are all due to the cytokine storm. This is present in the severe COVID‐19 cases in which exacerbation of inflammation is a consequence of the unrestrained pathogen‐associated molecular patterns. Clinical manifestations of COVID‐19 include acute respiratory distress syndrome (ARDS), systemic inflammatory response syndrome (SIRS) and cardiac failure (Patel et al., 2021). Specifically, in COVID‐19 cases, the uncontrolled inflammatory response results in leukopenia reflecting a high degree of cell lysis which follows the sustained pyroptosis (Ferreira et al., 2021). In addition, poor prognosis is associated with coagulopathy (Klok et al., 2020) which is also linked with the inflammasome‐mediated pyroptosis in macrophages, leading to the release of tissue factor, an essential mediator of blood coagulation cascades (Wu et al., 2019).
Pyroptosis was first described in myeloid cells infected by pathogens (Cookson & Brennan, 2001) and originates etymologically from the Greek words pyro (fire) and ptosis (falling). It is a programmed execution of the cell which follows the stabilization of the supramolecular protein complex called the inflammasome. Pyroptosis (schematically summarized in Figure 1) is characterized by cellular swelling and rupture (lysis) which aids the release of pro‐inflammatory mediators such as IL‐1β and IL‐18 (Figure 1.1), whose maturation follows activation of caspase‐1 (Yang et al., 2019). Pyroptosis is thus a caspase‐dependent process in which gasdermin D is proteolytically cleaved enabling interactions with phosphatidylinositol phosphates and phosphatidylserine on the inner cell membrane to form pores (Shi et al., 2015) (Figure 1.2).
FIGURE 1.
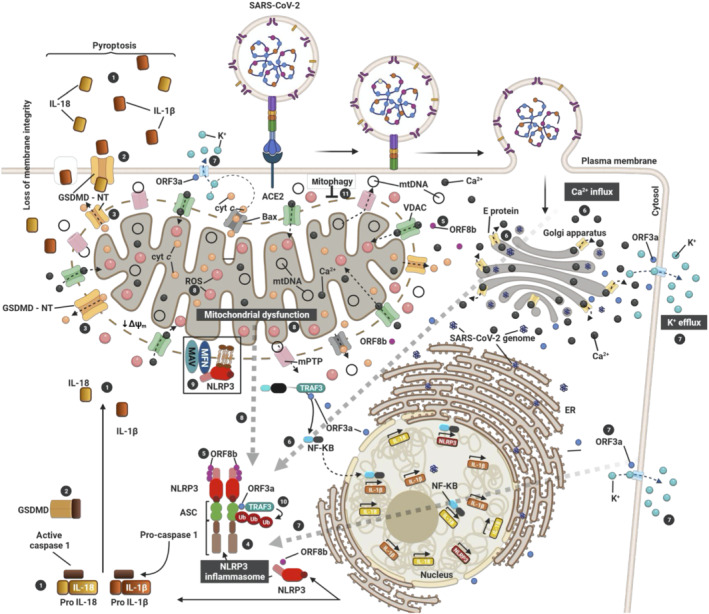
Activation of the NLRP3 inflammasome by SARS‐CoV‐2 via mitochondrial dysfunction. The three SARS‐CoV proteins (E, ORF3a and ORF8b) induce the activation of inflammasome (4). E protein (yellow) induces Ca2+ efflux through ERGIC/Golgi membranes to the cytosol (6). This induces influx into the mitochondria to generate mtROS (8). ORF3a (blue) induces K+ efflux to the extracellular space (7) and promotes inflammasome assembly (4) through TRAF3‐mediated ubiquitination of ASC (7; 10). On the other hand, TRAF3–ORF3a interaction is required for NF‐κβ activation, resulting in transcription of the pro–IL‐1β/IL‐18 and NLRP3 genes. ORF8b (violet) can interact directly with NLRP3 stimulating its activation (5). Consequent to inflammasome activation (4), gasdermin D (GSDMD) pores are formed on the plasma (2) and mitochondrial (3) membranes, causing IL‐1β/IL‐18 secretion (1), the cellular swelling associated with pyroptosis (1) and the induction of mitochondrial apoptotic pathway via Bax‐dependent release of cytochrome C into the cytosol. Additionally, activation of Bax can trigger NLRP3 activation via apoptotic caspases (dotted line) in a K+ efflux‐dependent manner (7). Thus, SARS‐CoV‐2 triggers NLRP3 inflammasome assembly and activation by damaging the mitochondria and inducing the production of mtROS (8) and the loss of mitochondrial membrane potential (ΔΨm) to release damaged mitochondrial DNA (mtDNA) in the cytosol through the mitochondrial pore transition (mPT). Therefore, mitophagy (11) stands as an important regulator of NLRP3 which is tethered on the mitochondria for activation in a mtROS‐dependent manner (black box)
Gasdermin D is also able to disrupt the mitochondrial membranes leading to the induction of apoptosis triggered by the release of cytochrome C (Zheng & Li, 2020) (Figure 1.3). SARS‐CoV‐2 infection therefore results in the activation of apoptosis as well as pyroptosis, and evidence also links the virus to the necroptotic type of cell death (Li et al., 2020). Infection by the virus can initiate the signalling cascade involving caspase 8 (Li et al., 2020), which is an acknowledged master regulator of various types of programmed cell death (Fritsch et al., 2019). It must be nonetheless stated that, even though crosstalk between different forms of programmed cell death is possible, infection by SARS‐CoV‐2 mostly activates pyroptosis, thus highlighting the inflammatory nature of the disease.
Pathogen‐driven pyroptosis requires the nucleotide‐binding oligomerization domain (NOD)‐like receptors (NLR) to assemble the inflammasome. The latter constitutes a platform for the recruitment and activation of caspase‐1 aided by the apoptosis‐associated speck‐like protein containing a caspase recruitment domain (ASC) which acts as a bridging molecule (Figure 1.4). The NOD‐like receptor (NLR) pyrin domain‐containing 3 (NLRP3) is the best characterized member of this family of receptors and has been implicated in a wide range of diseases spanning autoinflammatory disorders, including those involving the respiratory system, neurological conditions, virus‐associated illnesses and antiviral responses. Activation of the NLRP3 inflammasome is now confirmed in COVID‐19 patients in whom it represents a reliable indicator of the disease severity (Courjon et al., 2021; Toldo et al., 2021). The NLRP3 inflammasome is therefore a molecular signature which predicts release of inflammatory cytokines prodromal to the organ damage and deregulation of the immune system, which underlies the systemic COVID‐19 pathology.
SARS‐CoV‐2 (like its predecessors) expresses at least three proteins capable of engaging and activating the NLRP3 inflammasome: ORF8b (Figure 1.5), envelope (E) (Figure 1.6) and the ORF3a (Figure 1.7) (Yap et al., 2020). ORF8b directly activates the inflammasome via the leucine‐rich repeat (LRR) domain of the NLRP3 protein with which it co‐localizes (Shi et al., 2019) (Figure 1.5). The E protein is a viroporin (oligomeric complexes that act as ion channels) found on the membrane encapsulating the ER‐Golgi intermediate compartment (ERGIC) (Torres et al., 2007) which amplifies the inflammasome signalling by mobilizing Ca2+ in the cytosol to increase the production of cytokines and chemokines (Figure 1.6) (Murakami et al., 2012; Nieto‐Torres et al., 2015). Finally, ORF3a by acting as a K+ channel (Figure 1.7) (Chen et al., 2019) disrupts mitochondrial integrity causing an intracellular accumulation of ROS which facilitates the activation of the NLRP3 inflammasome (Figure 1.8).
Mitochondrial dysfunction, from which the ROS originates, is therefore linked with the NLRP3 activation and this link supports greater attention to the role of mitochondria in the pathogenesis of COVID‐19 (Figure 1.8). Even though engagement of pathogen‐associated molecular patterns is the priming signal to up‐regulate the transcription of the inflammasome complex subunits, NLRP3 and pro‐IL‐1β, the impaired mitochondrial function — as shown by the accumulation of the mitochondria‐derived ROS (mtROS)—is the second indispensable trigger for the functional assembly of the inflammasome.
Despite the mtROS being acknowledged to be essential for the stabilization of the inflammasome (Nakahira et al., 2011; Zhou et al., 2011), the hierarchy of molecular events which dictate, accompany or characterize the redox stress guiding the process, remains ill‐defined. Other mitochondrial proteins involved in inflammasome activation are the mitochondrial antiviral signalling (MAVS) proteins which directly associate with NLRP3 on the outer membrane (Ichinohe et al., 2013; Iyer et al., 2013) (Figure 1.9). MAVS proteins can also recruit, in a ROS‐dependent manner, the E3 ligase TRAF3 which amplifies the inflammasome activation by ubiquitinating ASC (Figure 1.10) (Guan et al., 2015; Siu et al., 2019). Ubiquitinating and de‐ubiquitinating events define mitochondrial quality control, but how these events contribute to the stabilization of the inflammasome and hence activation of pyroptosis is still not clear. At present, what is known is that promotion of mitochondrial quality control via selective autophagy (mitophagy) limits NLRP3 activation, by eliminating damaged or stressed mitochondria (Figure 1.11) (Lin et al., 2019; Zhong et al., 2016). Accordingly, de‐ubiquitination of mitochondrial proteins by ROS drives the NLRP3 inflammasome complex assembly by blocking mitophagy (Zhang et al., 2019), corroborating the prodromal role played by the loss of mitophagy. It is therefore clear that the precise upstream, mitochondrially based, molecular events that culminate in inflammasome stabilization remain to be fully described and thus limit the precision of targeting this process.
Over the years, molecules capable of controlling pyroptotic cell death through the inhibition of the inflammasome complex have been developed and some are now in clinical trials to treat inflammatory diseases. Among these are (i) resveratrol which acts as an inhibitor of thioredoxin‐interacting protein, thus decreasing inflammasome assembly (Cheng et al., 2019) and (ii) the FDA‐approved disulfiram which blocks pyroptosis by modifying gasdermin D, thereby preventing pore formation (Hu et al., 2020). Notably, all the pharmacological agents which hold the potential to integrate the therapeutic protocols for patients, counteract inflammasome stabilization by targeting the subunits of the supramolecular complex itself, rather than upstream events leading to NLRP3 assembly. Dysfunction of the mitochondria is one such upstream event of NLRP3 inflammasome assembly for which there are several advanced strategies of targeting (Singh et al., 2021).
Blocking the biochemical consensus for NRLP3 stabilization as well as curbing the associated signalling cascades (by reducing ROS, preventing morphological aberrations or increasing mitophagy) may therefore represent a viable strategy to pharmacologically prevent pyroptosis and hence ameliorate the inflammatory processes via effects on mitochondria.
Deciphering the role of mitochondrial dysfunction in SARS‐CoV‐2‐induced NLRP3 activation will be instrumental to identify these molecular checkpoints and so improve the pharmacological tools‐kit. An increased mechanistic awareness of the upstream processes of pyroptosis may therefore prevent the feed‐forward mechanisms which amplify the effects of the inflammasome.
Most of the current efforts to develop anti‐COVID‐19 therapeutic protocols are devoted to preventing the intracellular access of the virus via prophylaxis, with very few aimed at repressing and preventing pyroptosis. To this end, we are highlighting that the mitochondrial‐dependent paths to activation of the NLRP3 inflammasome may provide early molecular read‐outs to predict and/or counter the severity of toxicity in SARS‐CoV‐2 infected cells. This could form the basis of innovative treatments against the uncontrolled inflammation in COVID‐19 patients.
NOMENCLATURE OF TARGETS AND LIGANDS
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY (http://www.guidetopharmacology.org) and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Fabbro et al., 2019a, 2019b)
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
The research activities lead by M.C. on the topics of this script are supported by the European Research Council Consolidator Grant COG 2018‐819600_FIRM; AIRC‐MFAG 21903; the Petplan Charitable Trust; LAM‐Bighi Grant Initiative; and the CAST Grant.
Singh, A. , Strobbe, D. , & Campanella, M. (2022). Pyroptosis targeting via mitochondria: An educated guess to innovate COVID‐19 therapies. British Journal of Pharmacology, 179(10), 2081–2085. 10.1111/bph.15670
Funding information CAST Grant; LAM‐Bighi Grant Initiative; Petplan Charitable Trust; AIRC‐MFAG, Grant/Award Number: 21903; European Research Council Consolidator Grant, Grant/Award Number: COG 2018‐819600_FIRM
REFERENCES
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , & CGTP Collaborators . (2019a). The Concise Guide to PHARMACOLOGY 2019/20: Catalytic receptors. British Journal of Pharmacology, 176, S247–S296. 10.1111/bph.14751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , & CGTP Collaborators . (2019b). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, I. Y. , Moriyama, M. , Chang, M. F. , & Ichinohe, T. (2019). Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Frontiers in Microbiology, 10, 50. 10.3389/fmicb.2019.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, S. B. , Nakashima, A. , Huber, W. J. , Davis, S. , Banerjee, S. , Huang, Z. , Saito, S. , Sadovsky, Y. , & Sharma, S. (2019). Pyroptosis is a critical inflammatory pathway in the placenta from early onset preeclampsia and in human trophoblasts exposed to hypoxia and endoplasmic reticulum stressors. Cell Death & Disease, 10(12), 927–942. 10.1038/s41419-019-2162-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson, B. T. , & Brennan, M. A. (2001). Pro‐inflammatory programmed cell death. Trends in Microbiology, 9(3), 113–114. 10.1016/s0966-842x(00)01936-3 [DOI] [PubMed] [Google Scholar]
- Courjon, J. , Dufies, O. , Robert, A. , Bailly, L. , Torre, C. , Chirio, D. , & Boyer, L. (2021). Heterogeneous NLRP3 inflammasome signature in circulating myeloid cells as a biomarker of COVID‐19 severity. Blood Advances, 5(5), 1523–1534. 10.1182/bloodadvances.2020003918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, A. C. , Soares, V. C. , de Azevedo‐Quintanilha, I. G. , Dias, S. D. S. G. , Fintelman‐Rodrigues, N. , Sacramento, C. Q. , & Souza, T. M. L. (2021). SARS‐CoV‐2 engages inflammasome and pyroptosis in human primary monocytes. Cell Death Discovery, 7(1), 43–55. 10.1038/s41420-021-00428-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch, M. , Günther, S. D. , Schwarzer, R. , Albert, M. C. , Schorn, F. , Werthenbach, J. P. , Schiffmann, L. M. , Stair, N. , Stocks, H. , Seeger, J. M. , Lamkanfi, M. , Krönke, M. , Pasparakis, M. , & Kashkar, H. (2019). Caspase‐8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature, 575(7784), 683–687. 10.1038/s41586-019-1770-6 [DOI] [PubMed] [Google Scholar]
- Guan, K. , Wei, C. , Zheng, Z. , Song, T. , Wu, F. , Zhang, Y. , & Zhong, H. (2015). MAVS promotes inflammasome activation by targeting ASC for K63‐linked ubiquitination via the E3 ligase TRAF3. Journal of Immunology, 194(10), 4880–4890. 10.4049/jimmunol.1402851 [DOI] [PubMed] [Google Scholar]
- He, L. , Ding, Y. , Zhang, Q. , Che, X. , He, Y. , Shen, H. , & Jiang, S. (2006). Expression of elevated levels of pro‐inflammatory cytokines in SARS‐CoV‐infected ACE2+ cells in SARS patients: Relation to the acute lung injury and pathogenesis of SARS. The Journal of Pathology, 210(3), 288–297. 10.1002/path.2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , & Pöhlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280.e278. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojyo, S. , Uchida, M. , Tanaka, K. , Hasebe, R. , Tanaka, Y. , Murakami, M. , & Hirano, T. (2020). How COVID‐19 induces cytokine storm with high mortality. Inflammation and Regeneration, 40(1), 37–44. 10.1186/s41232-020-00146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, J. J. , Liu, X. , Xia, S. , Zhang, Z. , Zhang, Y. , Zhao, J. , Ruan, J. , Luo, X. , Lou, X. , Bai, Y. , Wang, J. , Hollingsworth, L. R. , Magupalli, V. G. , Zhao, L. , Luo, H. R. , Kim, J. , Lieberman, J. , & Wu, H. (2020). FDA‐approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nature Immunology, 21, 736–745. 10.1038/s41590-020-0669-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe, T. , Yamazaki, T. , Koshiba, T. , & Yanagi, Y. (2013). Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proceedings of the National Academy of Sciences of the United States of America, 110(44), 17963–17968. 10.1073/pnas.1312571110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer, S. S. , He, Q. , Janczy, J. R. , Elliott, E. I. , Zhong, Z. , Olivier, A. K. , & Sutterwala, F. S. (2013). Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity, 39(2), 311–323. 10.1016/j.immuni.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. I. , Kim, S. G. , Kim, S. M. , Kim, E. H. , Park, S. J. , Yu, K. M. , & Choi, Y. K. (2020). Infection and rapid transmission of SARS‐CoV‐2 in ferrets. Cell Host & Microbe, 27(5), 704–709.e702. 10.1016/j.chom.2020.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok, F. A. , Kruip, M. J. H. A. , van der Meer, N. J. M. , Arbous, M. S. , Gommers, D. A. M. P. J. , Kant, K. M. , Kaptein, F. H. J. , van Paassen, J. , Stals, M. A. M. , Huisman, M. V. , & Endeman, H. (2020). Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thrombosis Research, 191, 145–147. 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, S. K. P. , Lau, C. C. Y. , Chan, K. H. , Li, C. P. Y. , Chen, H. , Jin, D. Y. , & Yuen, K. Y. (2013). Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: Implications for pathogenesis and treatment. The Journal of General Virology, 94(Pt 12), 2679–2690. 10.1099/vir.0.055533-0 [DOI] [PubMed] [Google Scholar]
- Li, S. , Zhang, Y. , Guan, Z. , Li, H. , Ye, M. , Chen, X. , Shen, J. , Zhou, Y. , Shi, Z. L. , Zhou, P. , & Peng, K. (2020). SARS‐CoV‐2 triggers inflammatory responses and cell death through caspase‐8 activation. Signal Transduction and Targeted Therapy, 5, 235–245. 10.1038/s41392-020-00334-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Q. , Li, S. , Jiang, N. , Shao, X. , Zhang, M. , Jin, H. , & Ni, Z. (2019). PINK1‐parkin pathway of mitophagy protects against contrast‐induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biology, 26, 101254. 10.1016/j.redox.2019.101254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, B. , Brady, W. J. , Koyfman, A. , & Gottlieb, M. (2020). Cardiovascular complications in COVID‐19. The American Journal of Emergency Medicine, 38(7), 1504–1507. 10.1016/j.ajem.2020.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami, T. , Ockinger, J. , Yu, J. , Byles, V. , McColl, A. , Hofer, A. M. , & Horng, T. (2012). Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proceedings of the National Academy of Sciences of the United States of America, 109(28), 11282–11287. 10.1073/pnas.1117765109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira, K. , Haspel, J. A. , Rathinam, V. A. , Lee, S. J. , Dolinay, T. , Lam, H. C. , & Choi, A. M. (2011). Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature Immunology, 12(3), 222–230. 10.1038/ni.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto‐Torres, J. L. , Verdiá‐Báguena, C. , Jimenez‐Guardeño, J. M. , Regla‐Nava, J. A. , Castaño‐Rodriguez, C. , Fernandez‐Delgado, R. , & Enjuanes, L. (2015). Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology, 485, 330–339. 10.1016/j.virol.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, S. , Saxena, B. , & Mehta, P. (2021). Recent updates in the clinical trials of therapeutic monoclonal antibodies targeting cytokine storm for the management of COVID‐19. Heliyon, 7(2), e06158. 10.1016/j.heliyon.2021.e06158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa Cruz, A. , Mendes‐Frias, A. , Oliveira, A. I. , Dias, L. , Matos, A. R. , Carvalho, A. , & Silvestre, R. (2021). Interleukin‐6 is a biomarker for the development of fatal severe acute respiratory syndrome coronavirus 2 pneumonia. Frontiers in Immunology, 12, 613422. 10.3389/fimmu.2021.613422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, C.‐S. , Nabar, N. R. , Huang, N.‐N. , & Kehrl, J. H. (2019). SARS‐coronavirus open reading frame‐8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discovery, 5(1), 101–113. 10.1038/s41420-019-0181-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J. , Zhao, Y. , Wang, K. , Shi, X. , Wang, Y. , Huang, H. , & Shao, F. (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature, 526(7575), 660–665. 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- Singh, A. , Faccenda, D. , & Campanella, M. (2021). Pharmacological advances in mitochondrial therapy. eBioMedicine, 65, 103244. 10.1016/j.ebiom.2021.103244 Epub 2021 Feb 26. PMID: 33647769; PMCID: PMC7920826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu, K. L. , Yuen, K. S. , Castaño‐Rodriguez, C. , Ye, Z. W. , Yeung, M. L. , Fung, S. Y. , & Jin, D. Y. (2019). Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3‐dependent ubiquitination of ASC. The FASEB Journal, 33(8), 8865–8877. 10.1096/fj.201802418R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toldo, S. , Bussani, R. , Nuzzi, V. , Bonaventura, A. , Mauro, A. G. , Cannatà, A. , & Abbate, A. (2021). Inflammasome formation in the lungs of patients with fatal COVID‐19. Inflammation Research: Official Journal of the European Histamine Research Society … [et al.], 70(1), 7–10. 10.1007/s00011-020-01413-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, J. , Maheswari, U. , Parthasarathy, K. , Ng, L. , Liu, D. X. , & Gong, X. (2007). Conductance and amantadine binding of a pore formed by a lysine‐flanked transmembrane domain of SARS coronavirus envelope protein. Protein Science, 16(9), 2065–2071. 10.1110/ps.062730007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C. , Lu, W. , Zhang, Y. , Zhang, G. , Shi, X. , Hisada, Y. , & Li, Z. (2019). Inflammasome activation triggers blood clotting and host death through pyroptosis. Immunity, 50(6), 1401–1411.e1404. 10.1016/j.immuni.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Wang, H. , Kouadir, M. , Song, H. , & Shi, F. (2019). Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death & Disease, 10(2), 128–139. 10.1038/s41419-019-1413-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap, J. K. Y. , Moriyama, M. , & Iwasaki, A. (2020). Inflammasomes and pyroptosis as therapeutic targets for COVID‐19. The Journal of Immunologyji2000513, 205, 307–312. 10.4049/jimmunol.2000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, N.‐P. , Liu, X.‐J. , Xie, L. , Shen, X.‐Z. , & Wu, J. (2019). Impaired mitophagy triggers NLRP3 inflammasome activation during the progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis. Laboratory Investigation, 99(6), 749–763. 10.1038/s41374-018-0177-6 [DOI] [PubMed] [Google Scholar]
- Zheng, Z. , & Li, G. (2020). Mechanisms and therapeutic regulation of pyroptosis in inflammatory diseases and cancer. International Journal of Molecular Sciences, 21(4), 1456. 10.3390/ijms21041456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, Z. , Umemura, A. , Sanchez‐Lopez, E. , Liang, S. , Shalapour, S. , Wong, J. , & Karin, M. (2016). NF‐κB restricts inflammasome activation via elimination of damaged mitochondria. Cell, 164(5), 896–910. 10.1016/j.cell.2015.12.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. , Chu, H. , Li, C. , Wong, B. H. , Cheng, Z. S. , Poon, V. K. , & Yuen, K. Y. (2014). Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: Implications for pathogenesis. The Journal of Infectious Diseases, 209(9), 1331–1342. 10.1093/infdis/jit504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, R. , Yazdi, A. S. , Menu, P. , & Tschopp, J. (2011). A role for mitochondria in NLRP3 inflammasome activation. Nature, 469(7329), 221–225. 10.1038/nature09663 [DOI] [PubMed] [Google Scholar]


