To the Editor:
Relevant concerns are being raised regarding the weak serological response of solid organ transplant (SOT) recipients after receiving two doses of messenger RNA-based vaccine against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1 and some severe cases of coronavirus disease (COVID-19) occurring after vaccination.2 Thus, additional preventive booster doses are proposed to enhance anti-viral immunity.3 , 4
In this regard, recent reports have shown the immunological response to a third dose of the two main mRNA-based vaccines (BNT162b2; Pfizer–BioNTech or mRNA-1273; Moderna) leading to an increase in IgG seroconversion rates from 40% to 50%–70% 4 weeks after, especially among those with detectable antibodies after the two doses. Despite that no SARS-CoV-2 infections occurred, these data suggest the need of booster doses in this particularly vulnerable patient population. Nevertheless, while this evidence points toward an impaired adaptive immune response to vaccination in SOT patients,5 the short-term clinical scenario of the incidence of COVID-19 among SOT after two vaccine doses does not fully support this biological feature.
To investigate this question, we took advantage of the rapid vaccine prioritization of SOT patients in our country, which was done over a 3-week period starting late February 2021, and by early May, up to 90% of the total SOT population had received the two doses (http://trasplantaments.gencat.cat).
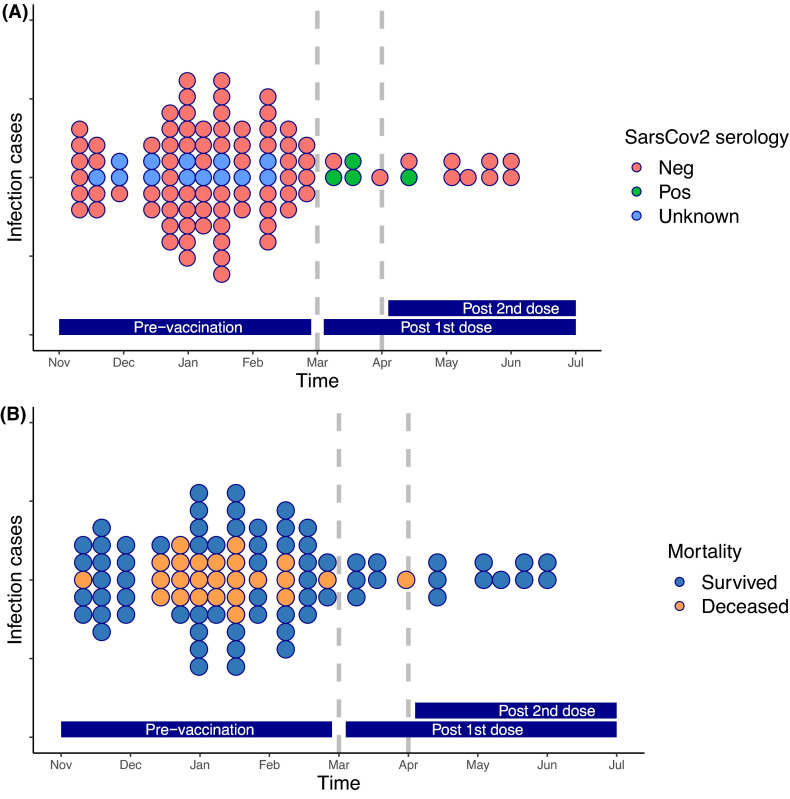
Remarkably, in our SOT center (the largest in Spain [http://www.ont.es/infesp/Paginas/Memorias.aspx]), the incidence of SARS-CoV-2 infection markedly dropped during the 4 months after vaccination (March–June) ( Figure 1, Table S1). Out of the total 117 detected infections over this 8-month period, 103 (88%) occurred before and only 14 (12%) after vaccination occurring 6/14 (42.8%) infections between the first and second dose. This difference was observed across all types of SOT and remained significant in a multivariate Poisson model accounting for age, gender, and type of SOT and immunosuppression (incidence ratio [IR] 0.211, p < .001) (Figure S2). To assess the independent effect of the two-dose vaccination, we compared COVID-19 rates between SOT and the general population in our region (www.dadescovid.cat) using a Poisson regression model analyzing the influence of the investigation period on COVID-19 incidences. Interestingly, the effect of vaccination era (before/after) had a significant impact on IRs of vaccination (0.50, p < .001) (Table S2), independently of the progressive reduction of infection rates observed over the same time period in the general population (Figure S1). Notably, the same adjusted incidences of patients requiring hospitalization, ICU admission, and deaths related to COVID-19 significantly decreased after vaccination (Table S2). Therefore, we did not make any recommendation for further vaccine booster aimed at improving this initial protection during this initial timeframe.
FIGURE 1.
SARS-CoV-2 infections over time, from November 1, 2020 to June 30, 2021, at Vall d’Hebron University Hospital. Each dot represents an infected patient. (A) Colors represent SARS-CoV2 Spike IgG serologies (blue: unknown serology [due to the lack of sample], red: non detectable SARS-CoV-2 IgG antibodies, and green: detectable SARS-CoV-2 Spike IgG antibodies). (B) Colors represent mortality (orange dots are patients deceased from COVID-19). Dashed lines represent the vaccination periods (first and second doses) [Color figure can be viewed at wileyonlinelibrary.com]
Interestingly, the reduction in COVID-19 infections, did also overlap with less restrictive community measures and a marked reduction of virus circulation, thus suggesting on the one hand a progressive generation of herd immunity (due to a widespread vaccination policy) and less viral infectivity, and on the other, the relative role of other protective immunological mechanisms such as virus-specific cellular immunity controlling viral replication. Altogether, while generalized booster vaccinations seem to be likely necessary among SOT in view of the low seroconversion rates, especially with the now-predominant Delta variant, the question is whether a vaccine should treat numbers or improve clinical outcomes, thus a better immune-risk stratification of SOT patients and the development of interventional clinical trials aiming at conferring immune protection, also involving these biomarkers, is highly warranted.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
Supplementary Material
REFERENCES
- 1.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali NM, Alnazari N, Mehta SA, et al. Development of COVID-19 infection in transplant recipients after SARS-CoV-2 vaccination. Transplant. 2021;105(9):e104–e106. doi: 10.1097/TP.0000000000003836. [DOI] [PubMed] [Google Scholar]
- 3.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326(11):1063. doi: 10.1001/jama.2021.12339. https://doi.org.10.1001/jama.2021.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rincon-Arevalo H, Choi M, Stefanski A-L, et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6(60):eabj1031. doi: 10.1126/sciimmunol.abj1031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material