CONFLICT OF INTEREST
LV received grants from Gilead Sciences. The other authors declare no conflict of interest.
To the Editor,
To date, several vaccines against severe acute respiratory coronavirus 2 (SARS‐CoV‐2) have proven to effectively reduce severe illness. 1 , 2 To promote herd immunity and reduce virus circulation, vaccines need to effectively reduce transmission risk. The nasal cavity is the first entrance point for SARS‐CoV‐2, and it has been suggested that viral replication is most efficient in the upper airways. 3 Local neutralizing antibodies (NAbs) in the nasal mucosa can play an important role in preventing SARS‐CoV‐2 infection and transmission by limiting viral replication and shedding. While induction of systemic neutralizing humoral responses has been shown for both natural infection and upon vaccination with BNT162b2 and ChAdOx1, the presence of NAbs in the nasal mucosa upon vaccination remains unclear. A recent not peer‐reviewed report described the potential of BNT162b2 to induce NAbs in the nasal cavity, but did not consider prior COVID‐19 as a potentiator of this response. 4 Local humoral responses after vaccination with viral vector‐based vaccines, another type of frequently used SARS‐CoV‐2 vaccines, have not been investigated. In the present study, we compared systemic and local immune responses in the serum and nasal secretions of 46 study subjects vaccinated with SARS‐CoV‐2 mRNA (BNT162b2) or viral vector‐based (ChAdOx1) vaccines.
Serum and nasal secretions from subjects visiting the COVID‐19 vaccination center at the University Hospital Ghent, Belgium were collected, just prior to the first SARS‐CoV‐2 vaccination and after the second dose of the same vaccine. Median time between second vaccine dose and sampling was 19 days (IQR: 15–23) for the BNT162b2 group and 18 days (IQ: 15–26) for the ChAdOx1 group. Collection of nasal secretions was performed as described previously. 5 SARS‐CoV‐2 NAbs in serum and nasal secretions were determined using the Elabsciene SARS‐CoV‐2 Neutralization Antibody ELISA kit (Gentaur) as per manufacturer's instructions. This surrogate virus neutralization test uses purified receptor‐binding domain (RBD) from the S protein and the host cell receptor ACE2 to mimic the virus‐host interaction. 6 This RBD‐ACE2 interaction is blocked by SARS‐CoV‐2 specific NAbs in patient samples. Inhibition rates are calculated based on the OD value of the negative control. A cutoff of 20% inhibition is determined as positive for the presence of NAbs by the manufacturer, based on testing 500 negative control sera.
Forty‐six subjects, mainly females, were included in the study (Table 1). Twenty‐four subjects were vaccinated with BNT162b2 and 22 with ChAdOx1. In both groups, half of the study subjects had a history of prior COVID‐19. NAbs were determined in serum and nasal secretions prior and post‐vaccination in all study subjects. Prior to vaccination, 16 subjects had NAbs in serum and 4 in nasal secretions. At second sampling, except for one, all subjects showed NAbs in their serum, regardless of the vaccine received (Figure S1). In nasal secretions, NAbs were observed in the majority of subjects (n = 23; 96%) vaccinated with BNT162b2 and in about half of the subjects (n = 13; 59%) vaccinated with ChAdOx1 at second sampling (p = 0.0032; Fisher's exact test) (Figure 1A). Moreover, the ACE2 binding inhibition in nasal secretion was higher in subjects vaccinated with BNT162b2 compared to those vaccinated with ChAdOx1 (p < 0.0001; 2‐way repeated‐measures ANOVA with Sidak's multiple comparisons test) (Figure 1D). Induction of NAbs occurred irrespective of prior SARS‐CoV‐2 infection or the presence of patient‐reported allergy to aeroallergens (pollen, animals and house dust mite) (Figure 1C‐F).
TABLE 1.
Subject baseline characteristics
| BNT162b2 | ChAdOx1 | |
|---|---|---|
| No (%) | No (%) | |
| n= | 24 | 22 |
| Sex | ||
| Female | 18 (75%) | 18 (82%) |
| Male | 6 (25%) | 4 (18%) |
| Age, median (IQR), y | 42.5 (38.5–49.0) | 36.0 (25.0–42.0) |
| BMI, median (IQR), kg/m2 | 25.0 (23.0–27.1) | 23.7 (20.6–26.5) |
| Current smoking | 1 (4%) | 2 (9%) |
| Prior COVID‐19 infection | 12 (50%) | 11 (50%) |
| Confirmed by RT‐PCR | 9 (75%) | 10 (91%) |
| Confirmed by serology | 1 (8%) | 1 (9%) |
| Self‐reported | 2 (17%) | 0 (0%) |
| Patient‐reported allergy to aeroallergens | ||
| Yes | 8 (33%) | 5 (23%) |
| No | 16 (67%) | 17 (77%) |
FIGURE 1.
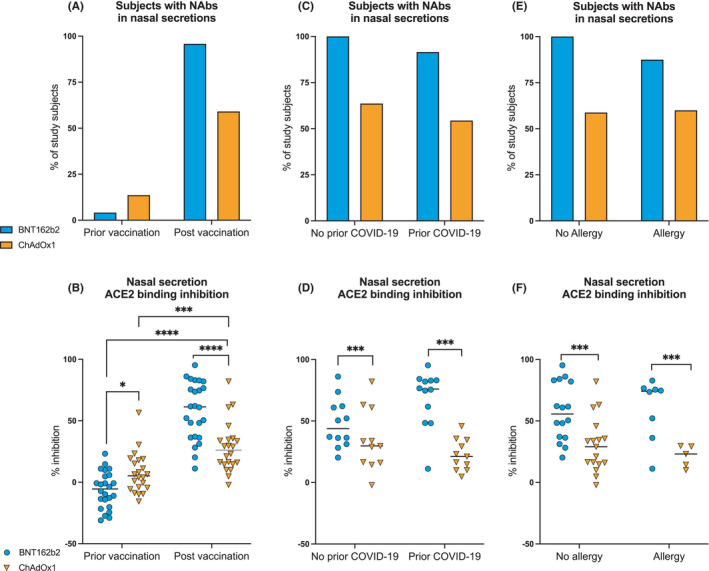
Effect of SARS‐CoV‐2 vaccination on the induction of nasal neutralizing antibodies. A‐B, Percentage of patients with SARS‐CoV‐2 neutralizing antibodies (A) and ACE2 binding inhibition rates (B) in nasal secretions prior and post‐vaccination with BNT162b2 and ChAdOx1. (C‐E), Percentage of patients with SARS‐CoV‐2 NAbs (C, E) and ACE2 binding inhibition rates (D, F) post‐vaccination in nasal secretions of patients respective to prior COVID‐19 (C, D) and to allergy to aeroallergens (E,F). Asterisks indicate statistical significance by two‐way repeated‐measures ANOVA followed by Sidak's multiple comparisons test for B and by ordinary two‐way ANOVA with Tukey's multiple comparisons test for D and F. *p < 0.05, **p < 0.001, ***p < 0.0002, ****p < 0.0001
Taken together, our study shows that both BNT162b2 and ChAdOx1 vaccines can induce nasal NAbs, albeit variable in the ChAdOx1 arm. Why only some subjects develop local NAbs in the former group is currently unclear. Differences in time between the two vaccine doses or other mechanisms of action of the vaccines might account for the observed differences. Further research is needed to fully understand the underlying immunological mechanisms. Longitudinal follow‐up of the described subjects is needed to see whether vaccines can induce long‐lasting neutralizing responses in the nasal mucosa. Failure to induce long‐lasting NAbs warrants rational booster design or other strategies, such as nasal vaccination. Based on our findings and given that mucosal NAbs might be key to prevent infection and viral shedding, we advocate for the inclusion of nasal mucosa NAbs measurements in vaccine efficacy trials and routine testing procedures.
Supporting information
Figure S1
ACKNOWLEDGEMENTS
The authors acknowledge professional support and committed efforts from various organizations and individuals, and thank all study subjects for participation to the trial. The clinical trial team of the Department of General Internal medicine at UZ Gent (Liesbeth Delesie, Lucas Van Dooren and Els De Leyn) were involved in sample collection. SV is supported by a senior postdoctoral fellowship from FWO Flanders (grant 1244321N). JD is supported by a doctoral fellowship from FWO Flanders (grant 11B7720N).
Jozefien Declercq and Els Tobback are shared co‐first authorship.
Philippe Gevaert and Vandekerckhove Linos are shared last authorship.
REFERENCES
- 1. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. NEJM 2020;383:2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Voysey M, Costa Clemens SA, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sungnak W, Huang N, Bécavin C, et al. SARS‐CoV‐2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020;26:681‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan RWY, Liu S, Cheung JY, et al. Study on the mucosal and serological immune response to the novel coronavirus (SARS‐CoV‐2) vaccines. MedRxiv preprint. 10.1101/2021.06.15.21256661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berings M, Arasi S, De Ruyck N, et al. Reliable mite‐specific IgE testing in nasal secretions by means of allergen microarray. J Allergy Clin Immunol 2017;140:301‐303. [DOI] [PubMed] [Google Scholar]
- 6. Tan CW, Chia WN, Qin X, et al. A SARS‐CoV‐2 surrogate virus neutralization test based on antibody‐mediated blockage of ACE2‐spike protein‐protein interaction. Nat Biotechnol 2020;38:1073‐1078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


