Abstract
Introduction
An increasing number of published reports on SARS‐CoV‐2 neurological manifestations have revealed a wide spectrum of symptoms, diagnostic features, and outcomes. We report a fatal case of a COVID‐19‐associated acute necrotizing encephalopathy (ANE).
Case report
We report a 70‐year‐old man brought to the hospital after a generalized tonic‐clonic seizure. He was confused and disoriented. Nasopharyngeal swab testing for SARS‐CoV‐2 was positive. A head computed tomography (CT) scan and cerebrospinal fluid (CSF) analysis showed no signs of acute pathology. After recurrent seizures, he was sedated and intubated. Throughout the days that followed he remained in a therapeutic coma. After discontinuation of sedatives, he remained unconscious. A repeated head CT scan showed signs of pontine edema, and brain magnetic resonance imaging (MRI) revealed inhomogeneous hyperintensities with microhemorrhages and small autonecrotic cavities in both thalami, brain stem, and cerebellar peduncles. With a high suspicion of a COVID‐19‐associated ANE, the patient was started on high‐dose glucocorticoids; however, he died the next day. The CSF tested negative for SARS‐CoV‐2.
Discussion
A variety of COVID‐19 neurological manifestations have been reported to date, including various forms of encephalitis and encephalopathy. In our patient, encephalopathy with seizures was the presenting symptom of SARS‐CoV‐2 infection. The radiological findings on days 8 and 9 were consistent with an ANE. The precise pathogenesis of ANE remains unclear; however, an immune‐mediated mechanism is suspected. Early diagnostics with prompt administration of immunomodulators may be lifesaving. Suspicion of a COVID‐19‐related encephalopathy/encephalitis should be raised in all patients with altered mental status, seizures, and/or coma.
Keywords: COVID‐19, necrotizing encephalopathy, neurological manifestations, SARS‐CoV‐2
A fatal case of COVID‐19‐associated acute necrotizing encephalopathy.
INTRODUCTION
An increasing number of published reports on SARS‐CoV‐2 neurological manifestations have revealed a wide spectrum of symptoms, diagnostic features, and outcomes [1, 2]. We report a fatal case of a COVID‐19‐associated acute necrotizing encephalopathy (ANE).
CASE REPORT
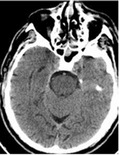
On 13 January 2021, a stuporous 70‐year‐old man was brought to the emergency department (ED). An hour earlier, he complained of a severe headache, and his blood pressure was 237/129 mmHg, after which he collapsed and had a generalized tonic‐clonic seizure. Upon arrival at the ED, he was confused and disoriented, without other obvious signs of focal neurological damage. The patient's medical records showed a history of poorly treated hypertension, heart failure, diabetes, and obesity, as well as a previous intracerebral hemorrhage (2017) and an ischemic left middle cerebral artery stroke (2018). A history of frequent alcohol consumption was also noted. A head computed tomography (CT) scan, CT angiography (Figure 1a), blood tests, and a lumbar puncture were performed, revealing no acute pathology or signs of acute inflammation. Nasopharyngeal swab testing for SARS‐CoV‐2 was positive and his chest CT scan demonstrated signs of bilateral pneumonia. After recurrent convulsive seizures, the patient was sedated, intubated, and hospitalized in the intensive care unit (ICU). The patient remained in a therapeutic coma throughout the days that followed, and had a continuous fever of up to 39°C. Blood tests showed elevated acute phase proteins (interleukin‐6 [IL‐6] 51.3 pg/mL, C‐reactive protein [CRP] 243.85 mg/L, and lactate dehydrogenase [LDH] 245.83 U/L), progressive pancytopenia, and a significant hyperferritinemia. Ferritin and LDH levels had a subsequent upward trend. After discontinuation of sedatives, the patient did not regain consciousness, and a repeat neurology consultation was required. On 20 January, he became bradycardic and hypotensive. His neurological status was consistent with a coma (Glasgow Coma Scale score of 3). A repeated head CT scan (Figure 1b) showed signs of edematous changes in the pons. An urgent brain magnetic resonance imaging (MRI) scan revealed edematous changes in both thalami, brain stem, and cerebellar peduncles (Figure 1c) with microhemorrhages (Figure 1e), small autonecrotic cavities, and restricted diffusion (Figure 1f). Also, some patchy contrast enhancement was observed in the brain stem (Figure 1d). With a high suspicion of a COVID‐19‐associated ANE, the patient was started on high‐dose glucocorticoids; however, his overall status deteriorated, and he died the next day. The previously obtained cerebrospinal fluid (CSF) specimen was tested for SARS‐CoV‐2, but no viral RNA was found.
FIGURE 1.
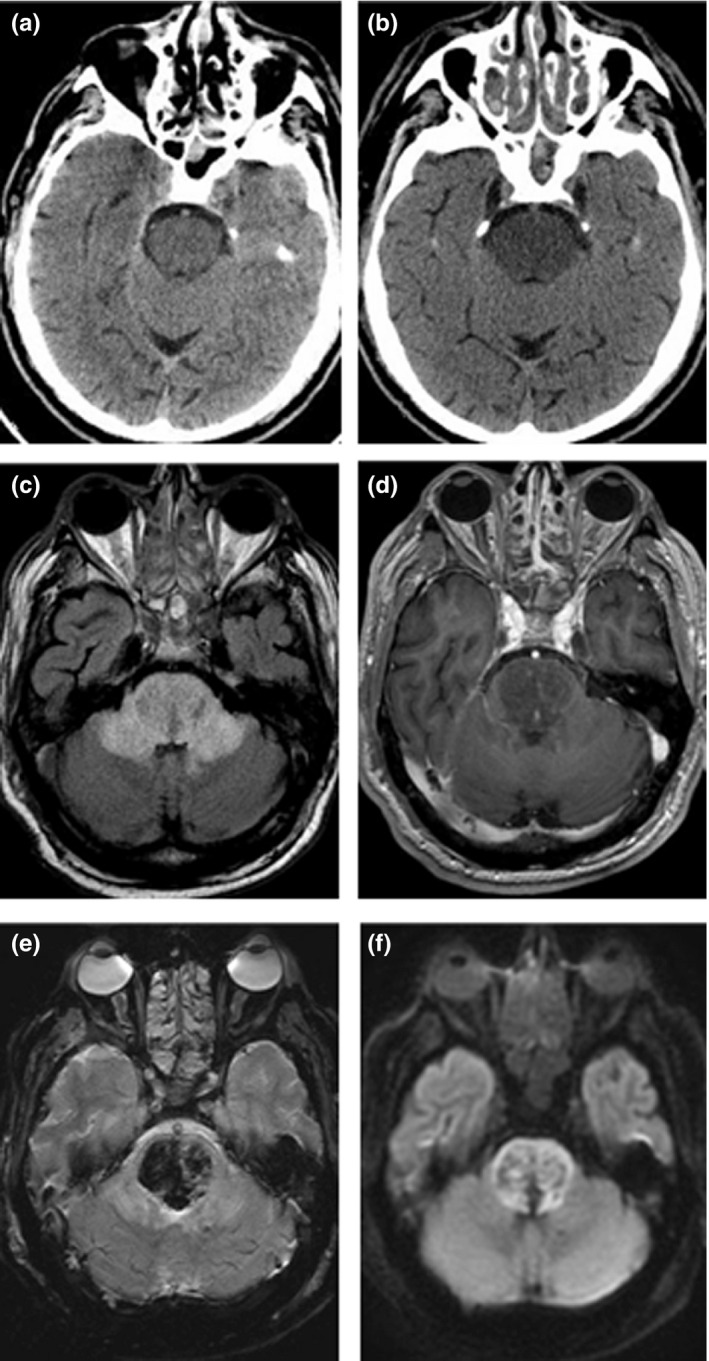
CT and MRI findings in a 70‐year‐old man with COVID‐19 associated acute necrotizing encephalopathy
DISCUSSION
The number of reports on SARS‐CoV‐2 extrapulmonary manifestations continues to grow, revealing a significant number of patients presenting with neurological symptoms. Central nervous system (CNS) involvement has been described in COVID‐19‐associated encephalopathy, acute disseminated encephalomyelitis, limbic encephalitis, myelitis, ANE, and stroke [2]. ANE is a rare type of rapidly progressing encephalopathy, with altered consciousness, seizures, and focal neurological deficits. Radiologically, it is characterized by multiple bilaterally distributed lesions within the thalamus, basal ganglia, brainstem, and subcortical white matter [3, 4]. ANE has been previously linked to various infectious pathogens, including influenza A and B, herpes simplex virus, and others. Our case adds to the few reports of ANE associated with SARS‐CoV‐2 infection (Table S1).
Our patient presented with altered mental status and recurrent seizures, without other focal neurological deficits. To the best of our knowledge, no respiratory symptoms were observed before the onset of neurological symptoms, suggesting that encephalopathy with seizures was the presenting symptom of SARS‐CoV‐2 infection.
Due to a suspected status epilepticus, the patient was sedated and kept in a therapeutic coma; however, the unavailability of electroencephalography for COVID‐19‐positive patients in our hospital limits the drawing of further conclusions. The head CT scan on admission showed no signs of cerebral damage; however, the head CT and MRI on days 8 and 9, accordingly, demonstrated prominent CNS involvement, highlighting a rapid progression of the disease. The radiological findings were consistent with ANE.
The precise pathogenesis of ANE remains unclear. However, an immune‐mediated mechanism involving pro‐inflammatory cytokines, rather than a direct invasion of a pathogen, is suspected [3]. In our case, such a mechanism can be supported by the elevated IL‐6, CRP, LDH, and ferritin found in the patient's serum. Reports of dramatic patient improvement after glucocorticoid or plasma exchange therapy support the theory of an immune‐mediated mechanism [5]. The CSF sample being negative for SARS‐CoV‐2 also contradicts the hypothesis of a direct CNS invasion. Unfortunately, the patient's CSF was not tested for other possible etiological factors, including autoimmune antibodies, and the patient's family refused an autopsy, thus limiting further conclusions.
Although it has been proven to be a challenge, early diagnosis with prompt administration of immunomodulatory therapy may be lifesaving [5]. The clinical suspicion of a COVID‐19‐related encephalopathy/encephalitis should be raised in all patients with COVID‐19, altered mental status, seizures, and/or coma.
DATA AVAILABILITY STATEMENT
All supporting data are available from the corresponding author upon reasonable request.
ETHICAL APPROVAL
Written patient consent was not obtained on account of the patient's death. Lack of written consent was waived, and the publication of this case report was approved by the local Research Ethics Committee of Riga Stradiņš University.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Dace Ziemele: Conceptualization (lead); Data curation (lead); Formal analysis (equal); Investigation (equal); Methodology (equal); Project administration (lead); Resources (equal); Supervision (equal); Validation (equal); Visualization (supporting); Writing‐original draft (equal); Writing‐review & editing (lead). Gundega Ķauķe: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Project administration (supporting); Resources (equal); Supervision (supporting); Validation (equal); Visualization (supporting); Writing‐original draft (equal); Writing‐review & editing (supporting). Krista Skrējāne: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Project administration (equal); Resources (equal); Visualization (supporting); Writing‐original draft (equal); Writing‐review & editing (supporting). Līga Jaunozoliņa: Conceptualization (supporting); Data curation (supporting); Formal analysis (equal); Methodology (equal); Resources (equal); Software (equal); Visualization (lead); Writing‐original draft (supporting); Writing‐review & editing (supporting). Guntis Karelis: Conceptualization (equal); Supervision (lead); Validation (equal); Writing‐review & editing (lead).
Supporting information
Tab S1
Ziemele D, Ķauķe G, Skrējāne K, Jaunozoliņa L, Karelis G. A fatal case of COVID‐19‐associated acute necrotizing encephalopathy. Eur J Neurol. 2021;28:3870–3872. 10.1111/ene.14966
REFERENCES
- 1. Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID‐19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104‐3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pilotto A, Masciocchi S, Volonghi I, et al. Clinical presentation and outcomes of severe acute respiratory syndrome coronavirus 2‐related encephalitis: the ENCOVID multicenter study. J Infect Dis. 2021;223(1):28‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID‐19‐associated acute hemorrhagic necrotizing encephalopathy: Imaging features. Radiology. 2020;296(2):E119‐E120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dixon L, Varley J, Gontsarova A, et al. COVID‐19‐related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflammation. 2020;7(5):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao A, Rohaut B, Le Guennec L, et al. Severe COVID‐19‐related encephalitis can respond to immunotherapy. Brain. 2020;143(12):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tab S1
Data Availability Statement
All supporting data are available from the corresponding author upon reasonable request.


