Patients with haematological malignancies are at increased risk of severe disease and death from coronavirus disease 2019 (COVID‐19). 1 Vaccination is essential to increase population immunity and decrease disease burden. The first COVID‐19 vaccines were authorised in the UK after phase III trials, which showed that both the BNT162b2 (Pfizer‐BioNTech) and ChAdOx1 nCoV‐19 (Oxford‐AstraZeneca) vaccines were effective at preventing symptomatic disease and hospitalisation. 2 , 3 Whilst both vaccines have demonstrated robust immune responses in healthy volunteers, patients with haematological malignancies were excluded from clinical trials. Emerging data suggest such patients are less likely to mount a humoral immune response to COVID‐19 vaccination, with those who have received Bruton tyrosine kinase inhibitors (BTKi) or cluster of differentiation (CD)20‐directed therapies for B‐cell malignancies a particularly high‐risk group. 4 , 5 , 6 , 7
We report interim results from 55 participants recruited to our ongoing COV‐VACC study, exploring the immune response to COVID‐19 vaccination in patients with B‐cell malignancies (South Central Berkshire B Research Ethics Committee and UK Health Research Authority approval IRAS number: 294547). Patients on treatment or treated within the last 24 months for a B‐cell malignancy and receiving either the BNT162b2 (Pfizer‐BioNTech; n = 41) or ChAdOx1 nCoV‐19 (Oxford‐AstraZeneca; n = 14) vaccines were recruited. The median (range) age of participants was 60 (27–82) years and 50% were receiving systemic anti‐cancer therapy (SACT) at the time of vaccination (Fig 1A, B).
Fig 1.
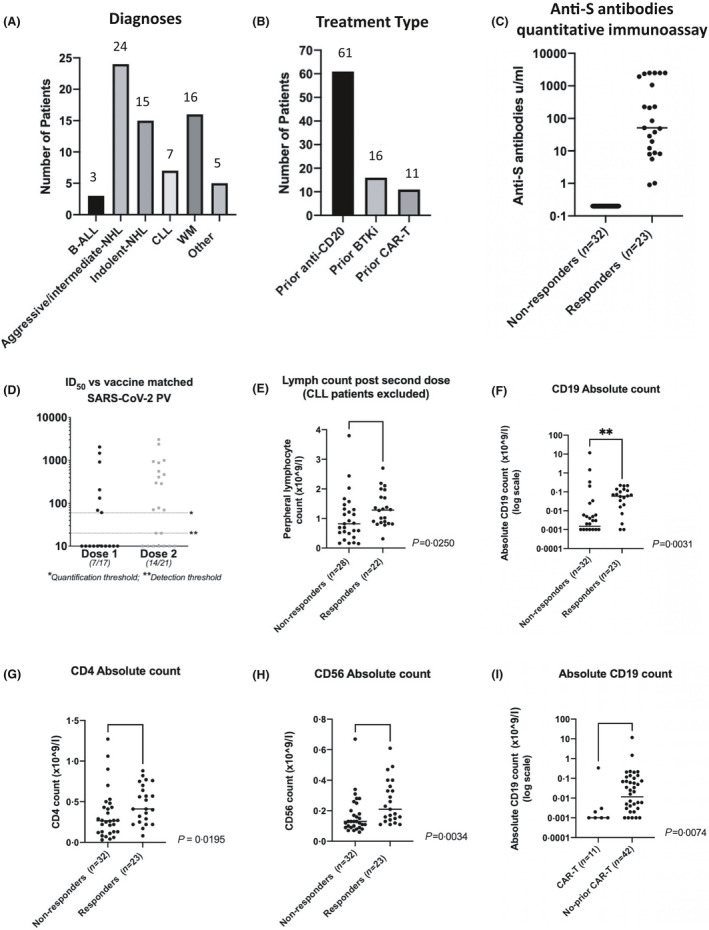
(A) Number of patients by diagnostic group recruited to the study to date (n = 70); (B) Number of patients (whole cohort) exposed to common therapeutic modalities; (C) Anti‐S antibody levels 1 month after second vaccination quantified by Elecsys Roche anti‐SARS‐CoV‐2 S assay (Spike); (D) ID50s of serum (from seropositive patients) able to neutralise SARS‐CoV‐2 pseudotyped virus after first dose (seven of 17) and second dose (14/21); (E) Peripheral lymphocyte count (excluding patients with CLL) in responders (n = 22) and non‐responders (n = 28) after second vaccination (P = 0·0250); (F) Peripheral CD19 counts in responders (n = 23) and non‐responders (n = 32) after second vaccination (P = 0·031); (G) Peripheral blood CD4 count in responders (n = 23) and non‐responders (n = 32) after second vaccination (P = 0·00195); (H) Peripheral blood CD56 count in responders (n = 23) and non‐responders (n = 32) after second vaccination (P = 0·0034); (I) Peripheral CD19 count in patients who had received CAR‐T (n = 11) versus those who had received a different SACT (n = 42) (P = 0·0074). ‘Responders’ = seropositive with anti‐S antibody level >0·4 µ/ml. ALL, acute lymphoblastic leukaemia; BTKi, Bruton tyrosine kinase inhibitors; CAR‐T, chimeric antigen receptor T‐cell; CD, cluster of differentiation; CLL, chronic lymphocytic leukaemia; ID50, 50% inhibitory dilution; S, Spike protein; NHL, non‐Hodgkin lymphoma; SACT, systemic anti‐cancer therapy; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2; WM, Waldenström macroglobulinaemia.
Blood samples were taken before vaccination and 1 month after the first and second vaccine doses. At each time‐point, a full blood count and enumeration of whole blood lymphocyte subsets (CD3, CD4, CD19, CD56) by flow cytometry (Aquios flow cytometers; Beckman Coulter, Brea, CA, USA) were performed. Serum samples were screened for anti‐severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) antibodies using quantitative double‐antigen sandwich immunoassays for both the nucleocapsid (N) antigen and the spike (S) protein receptor binding domain (RBD) (both Roche, Basel, Switzerland). Samples from participants with detectable anti‐S antibodies were then assessed to determine if these antibodies were able to neutralise SARS‐CoV‐2 infection in vitro using a luciferase encoding lentivirus pseudotyped with the SARS‐CoV‐2 spike as previously described. 8 , 9 Groups were compared using logistic regression, chi‐squared/Fisher’s exact tests and Wilcoxon–Mann–Whitney tests.
After a single dose of either BNT162b2 (Pfizer‐BioNTech; n = 41) or ChAdOx1 nCoV‐19 (Oxford‐AstraZeneca; n = 14) vaccine, 36% overall had detectable anti‐S antibodies (15/41 Pfizer‐BioNTech and five of 14 Oxford‐AstraZeneca) and 42% (23/55) after a second dose (Fig 1C). Three participants had serological evidence of previous infection with SARS‐CoV‐2.
Where available, sera from seropositive participants after the first or second dose were then used to assess neutralisation activity in vitro. Of the seropositive patients after the first dose (n = 17), just 41% were able to neutralise SARS‐CoV‐2 pseudotyped virus with a 50% inhibitory dilution (ID50) of >1:50. After two doses (n = 23) 57% of the seropositive patients had detectable neutralisation activity [median (range) ID50 of 1:469 (1:70–1:3056)] (Fig 1D).
Total blood lymphocyte, CD19, CD4, and CD56 counts all showed a significant association with seropositivity (Fig 1E–H). For a 1 log increase in each lymphocyte subset, the odds of developing antibodies in response to vaccination were 1·32 [95% confidence interval (CI) 1·05–1·66, P = 0·013], 2·5 (95% CI 1·12–5·55, P = 0·025) and 4·47 (95% CI 1·46–13·06, P = 0·0008) times higher respectively for CD19, CD4 and CD56 counts (Table I). Timing of vaccination in relation to SACT was important (P = 0·0126), with participants vaccinated >6 months after completing therapy more likely to develop antibodies; odds ratio (OR) 5·33 (95% CI1·14–24·90). Patients on or within 6 months of treatment had significantly lower CD56 and CD19 counts (P = 0·003 and P = 0·014) and a trend towards lower CD4 (P = 0·11). Chimeric antigen receptor (CAR) T‐cell recipients had very low rates of seropositivity (two of nine, 22·2%; Table I). No difference was seen for patients treated with CD20 antibody therapies or BTKis (Supplementary Table S1).
Table I.
Logistic regression analysis.
| All patients: anti‐S Ab positivity | Seropositive patients only: neutralising activity | |||||
|---|---|---|---|---|---|---|
| Seropositive, n/N | OR (95% CI) | P | Neutralising, n/N | OR (95% CI) | P | |
| Lymphocyte subsets | ||||||
| CD19 (1 log increase) | 23/54 | 1·32 (1·06–1·66) | 0·013 | 12/17 | 2·04 (0·99–4·22) | 0·054 |
| CD4 (1 log increase) | 23/53 | 2·50 (1·12–5·55) | 0·025 | 12/19 | 1·23 (0·28–5·39) | 0·78 |
| CD56 (1 log increase) | 23/53 | 4·47 (1·46–13·06) | 0·008 | 12/19 | 6·79 (0·62–73·92) | 0·12 |
| Treatments | ||||||
| Rituximab | ||||||
| No | 1/4 | 1·00 | 0·49 | 0/1 | — | 0·37* |
| Yes | 22/51 | 2·28 (0·22–23·39) | 12/18 | — | ||
| BTKi | ||||||
| No | 17/42 | 1·00 | 0·72 | 11/16 | 1·00 | 0·54 |
| Yes | 6/13 | 1·26 (0·36–4·41) | 2/4 | 0·57 (0·05–4·67) | ||
| CAR‐T | ||||||
| No | 221/46 | 1·00 | 0·21 | 13/18 | — | 0·37* |
| Yes | 2/9 | 0·34 (0·06–1·82) | 0/1 | — | ||
| Vaccine time‐point | ||||||
| On treatment | 9/25 | 1·00 | 0·026 | 3/7 | — | 0·034* |
| Within 6 months of treatment | 5/18 | 0·68 (0·18–2·55) | 2/5 | — | ||
| >6 months from treatment | 9/12 | 5·33 (1·14–24·90) | 7/7 | — | ||
Ab, antibody; BTKi, Bruton tyrosine kinase inhibitors; CAR‐T, chimeric antigen receptor T‐cell; CD, cluster of differentiation; CI, confidence interval; OR, odds ratio; S, Spike protein.
OR not estimable, Fisher’s exact test used (P = 0·017) for on treatment/within 6 months versus >6 months.
Seropositive patients could be divided into those whose sera did or did not demonstrate neutralising activity. Neutralising activity was associated with higher median anti‐S antibody levels (P = 0·0005). Further, both higher CD56 and CD19 counts showed trends towards increased odds of developing neutralising antibodies; OR 6·79 (95% CI 0·62–73·9), P = 0·12 and 2·04 (95% CI 0·99–4·22), P = 0·054. All seropositive patients (seven of seven) who were >6 months from treatment had neutralising antibodies compared to five of 12 on or within 6 months of treatment (Fisher’s exact, P = 0·017).
This interim analysis adds to increasing evidence that immunocompromised patients are less likely to produce robust immune responses after COVID‐19 vaccination. 4 , 5 , 6 , 7 In our cohort, 42% had detectable anti‐S antibodies after two doses of an approved vaccine compared to 91–100% in healthy individuals in phase I/II trials. 2 , 10 Even when seroconversion occurs, the protective humoral response may be limited. Just 23% of the cohort (n = 56; 57% of seropositive participants) neutralised virus in vitro. Others have shown neutralising antibody levels to be highly predictive of immune protection from symptomatic infection. 11 Our data identifies several factors associated with vaccine response such as peripheral blood lymphocyte, CD19, CD4 and CD56 counts, which if validated in larger cohorts may enable the identification of patients unlikely to respond to vaccination.
These data provide further evidence that patients on SACT are less likely to produce antibodies following COVID‐19 vaccination. 6 Anti‐S seropositivity does not necessarily correlate with serum neutralisation and is unlikely predictive of an effective antibody response based on current estimates of correlates of protection. Urgent validation in larger cohorts is required, as many patients with B‐cell malignancies may remain at high risk of infection regardless of anti‐S antibody status. Clinically vulnerable patients, regardless of vaccination status, should be considered for neutralising monoclonal antibody therapies if they develop COVID‐19. 12 , 13
Urgent consideration needs to be given to provision of booster doses or full re‐vaccination to this group of patients, particularly if they have been vaccinated within 6 months of active therapy. The correlation between peripheral blood lymphocyte, CD19, CD4 and CD56 counts suggest that booster doses or vaccination may be most effective if given when an individual has recovered lymphocytes and are ≥6 months following SACT.
This interim analysis is limited by cohort size and heterogeneity. However, we demonstrate a disconnect between seropositivity and virus neutralisation in vitro, following vaccination against COVID‐19.
Supporting information
Table SI. Anti‐S antibody results and corresponding in vitro neutralisation data for all 55 patients who had samples available for analysis after two doses of vaccine.
Acknowledgements
This study is supported by the National Institute for Health Research (NIHR) University College London Hospitals NHS Foundation Trust (UCLH) Biomedical Research Centre and Blood Cancer UK. Robert S. Heyderman is a NIHR Senior Investigator. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
References
- 1. Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, et al. Outcomes of patients with hematologic malignancies and COVID‐19: a systematic review and meta‐analysis of 3377 patients. Blood. 2020;136:2881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 Vaccine. N Engl J Med. 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voysey M, Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tzarfati KH, Gutwein O, Apel A, Rahimi‐Levene N, Sadovnik M, Harel L, et al. BNT162b2 COVID‐19 Vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol. 2021;96:1195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lim SH, Campbell N, Johnson M, Joseph‐Pietras D, Collins GP, O'Callaghan A, et al. Antibody responses after SARS‐CoV‐2 vaccination in patients with lymphoma. Lancet Haematol. 2021;8:e542–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vijenthira A, Gong I, Betschel SD, Cheung M, Hicks LK. Vaccine response following anti‐CD20 therapy: a systematic review and meta‐analysis of 905 patients. Blood Adv. 2021;5:2624–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brouwer PJ, Caniels TG, van der Straten K, Snitselaar JL, Aldon Y, Bangaru S, et al. Potent neutralizing antibodies from COVID‐19 patients define multiple targets of vulnerability. Science. 2020;369:643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJ, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS‐CoV‐2 infection in humans. Nat Microbiol. 2020;5:1598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij‐Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet. 2020;396:467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27:1205–11. [DOI] [PubMed] [Google Scholar]
- 12. Dougan M, Nirula A, Azizad M, Mocherla B, Gottlieb RL, Chen P, et al. Bamlanivimab plus etesevimab in mild or moderate Covid‐19. N Engl J Med. 2021. (Online ahead of print). 10.1056/NEJMoa2102685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN‐COV2, a neutralizing antibody cocktail, in outpatients with Covid‐19. N Engl J Med. 2021;384:238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Anti‐S antibody results and corresponding in vitro neutralisation data for all 55 patients who had samples available for analysis after two doses of vaccine.


