Summary
Patients affected by lymphoid malignancies (LM) are frequently immune‐compromised, suffering increased mortality from COVID‐19. This prospective study evaluated serological and T‐cell responses after complete mRNA vaccination in 263 patients affected by chronic lymphocytic leukaemia, B‐ and T‐cell lymphomas and multiple myeloma. Results were compared with those of 167 healthy subjects matched for age and sex. Overall, patient seroconversion rate was 64·6%: serological response was lower in those receiving anti‐cancer treatments in the 12 months before vaccination: 55% vs 81·9% (P < 0·001). Anti‐CD20 antibody plus chemotherapy treatment was associated with the lowest seroconversion rate: 17·6% vs. 71·2% (P < 0·001). In the multivariate analysis conducted in the subgroup of patients on active treatment, independent predictors for seroconversion were: anti‐CD20 treatment (P < 0·001), aggressive B‐cell lymphoma diagnosis (P = 0·002), and immunoglobulin M levels <40 mg/dl (P = 0·030). The T‐cell response was evaluated in 99 patients and detected in 85 of them (86%). Of note, 74% of seronegative patients had a T‐cell response, but both cellular and humoral responses were absent in 13·1% of cases. Our findings raise some concerns about the protection that patients with LM, particularly those receiving anti‐CD20 antibodies, may gain from vaccination. These patients should strictly maintain all the protective measures.
Keywords: COVID‐19, lymphoid malignancies, T‐cell immune response, Seroconversion, anti‐CD20 antibody
Introduction
Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS‐CoV‐2) infection and the resulting coronavirus disease 2019 (COVID‐19) have had devastating consequences worldwide. Patients affected by lymphoid malignancies (LM) are at an increased risk for severe COVID‐19 and have an exceedingly high mortality rate. 1 , 2 , 3 Recently, two mRNA‐based vaccines were approved for the general population to prevent against COVID‐19. However, individuals with LM were not included in clinical trials, and the immune response elicited by SARS‐CoV‐2 vaccines in this immune‐compromised population is largely unknown.
The phase 3 trials of mRNA‐1273 (Moderna) and BNT162b2 (Pfizer BioNTech), the first mRNA‐based vaccines, which target the spike protein to elicit protective immunity, demonstrated an efficacy at preventing COVID‐19 in healthy individuals of 94% and 95%, respectively. 4 , 5 Seroconversion occurred in almost all vaccinated individuals. 6 , 7 These results suggested potential beneficial effects also in LM patients, although the seroconversion rate was expected to be lower than in the general population as it happens already after the infection. 8
In 2009, the emergence of H1N1 influenza led to the development of an inactivated virus‐based vaccine. Some data showed decreased seroconversion in LM patients, particularly in those treated with antibodies targeting CD20 antigen, 9 whereas the T‐cell mediated response was similar to that in healthy individuals. 10 Nonetheless, viral vaccines are routinely recommended in LM patients. 11 , 12 , 13 , 14
In Italy, the indication from healthcare authorities was to use mRNA vaccines in LM patients, because of a supposed higher activity and better safety profile. The aim of this study was to evaluate the humoral and cellular response to mRNA‐1273 and BNT162b2 vaccines in patients with LM.
Methods
This prospective study assessed the efficacy of two doses of either mRNA‐1273 or BNT162b2 vaccines administered 28 days apart, according to the national healthcare system’s indications in order to increase the vaccine’s availability in the first phases of the national vaccination plan and contrast the risk of vaccine shortage. We included adult (age≥18 years) consecutive patients who were vaccinated at the Istituto Nazionale dei Tumori, Milan, Italy. According to national healthcare system indications, priority to vaccination was given to frail patients, defined by the presence of one of the following: presence of active disease; ongoing treatments or within 12 months from last therapy; active graft‐versus‐host disease; allogeneic stem cell transplantation (allo‐HSCT) or chimaeric antigen receptor‐modified (CAR‐) T‐cell therapy within 3 to 12 months from administration of the first dose of vaccine. After the completion of vaccination of this high‐priority population, we included also patients in remission who had completed their treatment more than 12 months prior to vaccination.
The control group consisted of age‐ and sex‐matched healthcare workers (HCW), who were enrolled in the prospective study INT65/20 and, based on the local availability, received the BNT162b2 vaccine at the Istituto Nazionale dei Tumori, Milan, Italy. The trial was approved by the Institutional Review Board of Istituto Nazionale dei Tumori, Milan, Italy, and written informed consent was collected from all patients (INT112/21).
The primary end‐point of the study was the seroconversion rate among LM patients after full‐dose vaccination. Anti‐SARS‐CoV‐2 S levels were monitored before the first dose, at the time of second dose administration, and two weeks later. Patients with a positive basal anti‐SARS‐CoV‐2 S titre were excluded from this study.
Among patients who received chemotherapy with or without anti‐CD20 antibody, immune modulatory drugs (IMIDs) or novel oral agents within the last 12 months, we selected a cohort of 99 patients and evaluated their SARS‐CoV‐2‐specific T‐cell response two weeks after the second dose independently from their serological status after vaccination. The control group for the T‐cell response consisted of 99 HCW, who received the BNT162b2 vaccine and whose T‐cell response was evaluated two weeks after the second dose at the National Institute for Infectious Diseases “Lazzaro Spallanzani”, Rome, Italy.
Briefly, the Roche Elecsys® Anti‐SARS‐CoV‐2 S (Roche S tAb, Roche Diagnostics International Ltd, Rotkreuz, Switzerland) was used to measure antibodies directed against the receptor‐binding domain (RBD) of the viral spike (S) protein. T‐cell responses were evaluated through measurement of in vitro T‐helper cell type 1 (Th1)‐associated cytokine release [interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α, interleukin (IL)‐2] in plasma samples after stimulation with S peptides using an automatic enzyme‐linked immunosorbent assay (ELISA; ELLA, Protein Simple, San Jose, CA). 15 Variable definitions, complete laboratory and statistical methods are provided in Data S1.
Results
Patient characteristics
Between 15 March 2021 and 10 May 2021, 263 patients were vaccinated at our institution: 243 (93·4%) received two doses mRNA‐1273 and 20 (7·6%) BNT162b2.
Fifty‐nine patients (22·4%) had B‐cell aggressive lymphoma, 111 (42·2%) B‐cell indolent lymphoma or B‐cell chronic lymphocytic leukaemia (CLL), 33 (12·6%) Hodgkin lymphoma, 52 (19·8%) multiple myeloma, and 8 (3%) T‐cell lymphoma. A detailed description of patients’ characteristics is available in Table I.
Table I.
Clinical characteristics of patients and seroconversion rate at four and six weeks after first dose.
| Characteristic | No. (%) | Serology | |
|---|---|---|---|
| Seroconversion week 4 No. (%) | Seroconversion week 6 No. (%) | ||
| Overall population | 263 (100%) | 131(49·8%) | 170 (64·6%) |
| Sex — no. (%) | |||
| Female | 123 (46·7%) | 61 (49·6%) | 78 (63·4%) |
| Male | 140 (53·3%) | 70 (50%) | 92 (65·7%) |
| Median age (range), years | |||
| Age – no. (%) | |||
| ≥ 65 years | 121 (46%) | 58 (47·9%) | 81 (66·9%) |
| < 65 years | 142 (54%) | 73 (51·4%) | 89 (62·6%) |
| Type of haematologic malignancy — no. (%) | |||
| B‐cell aggressive lymphomas | 59 (22·4%) | 14 (23·7%) | 21 (35·5%) |
| B‐cell indolent lymphomas or B‐cell lymphocytic leukaemia | 111 (42·2%) | 49 (44·1%) | 68 (61·2%) |
| Hodgkin lymphoma | 33 (12·6%) | 18 (54·5%) | 26 (78·8%) |
| Multiple myeloma | 52 (19·8%) | 46 (88·4%) | 49 (94·2%) |
| T‐cell lymphomas | 8 (3%) | 4 (50%) | 6 (75%) |
| Disease status — no. (%) | |||
| Active | 179 (68%) | 93 (51·9%) | 119 (66·4%) |
| Remission | 84 (32%) | 38 (45·2%) | 51 (60·7%) |
| Last therapy ≤ 12 months prior — no. (%) | 169 (64·2%) | 67 (39·6%) | 93 (55%) |
| ≤ 6 months prior — no. (%) | 140 (53·2%) | 52 (37·1%) | 75 (53·6%) |
| > 6 to ≤12 months prior — no. (%) | 29 (11%) | 15 (51·7%) | 18 (62%) |
| Anti CD20 antibody plus chemotherapy | 51 (19·3%) | 3 (5·9%) | 9 (17·6%) |
| ≤6 months prior — no. (%) | 40 (15·2%) | 1 (2·5%) | 4 (10%) |
| >6 to ≤12 months prior — no. (%) | 11 (4·1%) | 2 (18·2%) | 5 (45·4%) |
| Chemotherapy | 36 (13·6%) | 17 (47·2%) | 25 (69·4%) |
| ≤6 months prior — no. (%) | 33 (12·5%) | 15 (45·5%) | 22 (66·6%) |
| >6 to ≤12 months prior — no. (%) | 3 (1·1%) | 2 (66·7%) | 3 (100%) |
| IMIDs | 26 (9·9%) | 21 (80·7%) | 22 (84·6%) |
| Oral target therapy (ibrutinib or venetoclax) | 21 (8%) | 5 (23·8%) | 11 (52·3%) |
| CAR‐T cell therapy or HSCT | 21 (8%) | 11 (52·3%) | 12 (57·1%) |
| ≤6 months prior — no. (%) | 6 (2·2%) | 0 (0%) | 1 (17%) |
| >6 to ≤12 months prior — no. (%) | 15 (5·8%) | 11 (73%) | 11 (73%) |
| Other therapies | 14 (5·4%) | 10 (71·4%) | 14 (100%) |
| Watch‐and‐wait strategy or last therapy more than12 months prior — no. (%) | 94 (35·8%) | 64 (68·1%) | 77 (81·9%) |
| ALC — no. (%) | |||
| ≥800 cells/μl | 190 (72·3%) | 114 (60%) | 140 (73·6%) |
| <800 cells/μl | 49 (18·6%) | 11 (22·4%) | 21 (42·8%) |
| Not evaluable | 24 (9·1%) | 6 (25%) | 9 (37,5%) |
| ANC — no. (%) | |||
| ≥1500 cells/μl | 243 (92·4%) | 124 (51%) | 158 (65%) |
| <1500 cells/μl | 20 (7·6%) | 7 (35%) | 12 (60%) |
| IgG — no. (%) | |||
| ≥600 mg/dl | 133 (50·5%) | 67 (50·3%) | 88 (66·1%) |
| <600 mg/dl | 56 (21·3%) | 24 (42·8%) | 30 (53·5%) |
| Not evaluable or not available | 74 (28·2%) | 40 (54%) | 52 (70·3%) |
| IgA — no. (%) | |||
| ≥80 mg/dl | 114 (43·3%) | 59 (51·7%) | 77 (67·5%) |
| <80 mg/dl | 75 (28·5%) | 33 (44%) | 43 (57·3%) |
| Not evaluable or not available | 74 (28·2%) | 39 (52·7%) | 50 (67·5%) |
| IgM — no. (%) | |||
| ≥40 mg/dl | 97 (36·9%) | 53 (54·6%) | 69 (71·1%) |
| <40 mg/dl | 85 (32·3%) | 34 (40%) | 44 (51·7%) |
| Not evaluable or not available | 81 (30·8%) | 43 (53·1%) | 56 (69·1%) |
ALC, absolute lymphocytic count; ANC, absolute neutrophilic count; HSCT, allogeneic stem cell transplants; CAR‐T, chimaeric antigen receptor T cells; IMIDs, immunomodulatory drugs.
In all, 140 patients (53·2%) received therapies for the lymphoid malignancy within six months, whereas 29 patients (11%) received their last treatment seven to 12 months prior to the first dose of vaccine. Overall, 169 patients (64·2%) received therapies for the LM within 12 months before the administration of the first dose of vaccine and were defined as being on active treatment. They were further stratified according to their last therapy: 51 patients (19·3%) received anti‐CD20 antibody plus chemotherapy, of whom 40 (15·2%) in the last six months; 36 (13·6%) received chemotherapy alone, of whom 33 (12·5%) in the last six months; 26 (9·9%) IMIDs, 21 (8%) novel oral agents (ibrutinib or venetoclax without rituximab in the last 12 months), 21 patients (8%) CAR‐T cells or HSCT, of whom six (2·2%) in the last six months, and 14 (5·3%) other therapies. Patients treated with autologous HSCT were considered as treated with chemotherapy alone or anti‐CD20 antibody plus chemotherapy if they received rituximab before chemotherapy conditioning. The remaining 94 (35·8%) patients were on “watch and wait” or follow‐up strategies, since they had not received treatment for their malignancy yet or they had been treated more than 12 months before vaccination.
SARS‐CoV‐2 antibody response after vaccination
Overall, 131 (49·8%; 95% CI 43·6%–56·0%) patients seroconverted four weeks after the first dose and 39 [14·8%; 95% confidence interval (CI) 11·0%–19·6%] two weeks after the second one, for a total of 170 (64·6%; 95% CI 58·5%–70·4%), as shown in Table I. The median antibody titre at two weeks after the second dose was 175 U/ml [interquartile range (IQR) 0·44–2 600]. We compared the seroconversion rate and the antibody titres of 167 LM patients and 167 HCW matched for age and sex (73 female, median age 56 years, range: 46–62 in both groups). In the HCW group, the seroconversion rate was 99·4% (95% CI 96·7%–100%) as compared to 64·1% (95% CI 56·3%–71·3%) in the matched LM group (P < 0·001). Interestingly, we observed lower antibody titres in LM patients compared to the healthy subjects (median = 1 078 U/ml, IQR 643–1 841 U/ml vs median = 207·5 U/ml, IQR 0·44–3 062 U/ml, P < 0·001; Fig 1A).
Fig 1.
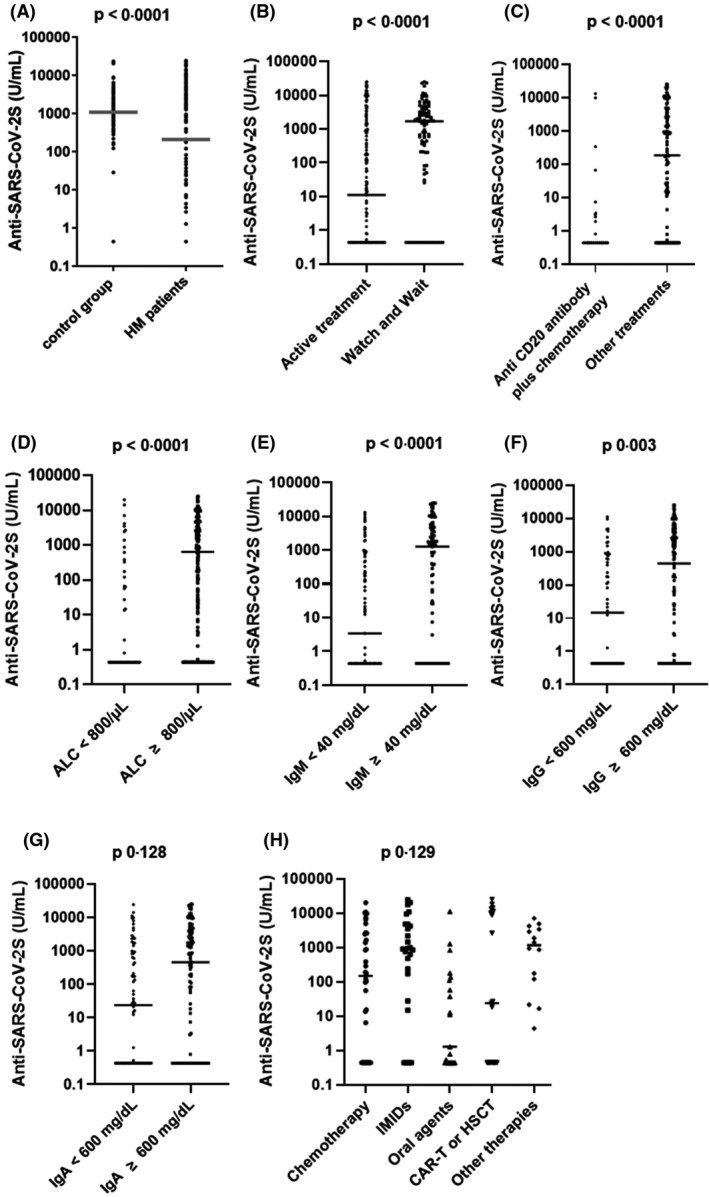
Anti‐SARS‐CoV‐2 S titres in lymphoid malignancy (LM) patients and healthy subjects. Comparison between LM patients and age‐ and sex‐matched healthy controls (A). Anti‐SARS‐CoV‐2 levels in LM patients seronegative at baseline according to: treatment status (B), treatment with anti‐CD20 plus chemotherapy or other therapies (C), absolute lymphocyte count (ALC) at the moment of the first dose (D), IgM (E), IgG (F), and IgA (G) levels at the moment of the first dose and according to other treatments (H).
At two weeks after the second dose, the seroconversion rate was similar among patients who had received their last treatment within six months and in those who had been treated seven to 12 months prior the first dose of vaccine (53·6%, 95% CI 45·3%–61·6% vs 62%, 95% CI 44%–77·3%, P = 0·42 respectively), suggesting a long‐lasting immunosuppressive effect due to anti‐lymphoma treatments. Considering the lack of statistical difference, we decided to consider only treatment in the last 12 months for univariable and multivariable analyses.
At univariable analysis by binary logistic models, the variables significantly associated with the lack of serological response included: treatment in the last 12 months, type of LM, lymphopenia (<800 cells/μl), IgM levels < 40 mg/dl, and, among patients on treatment, the administration of anti‐CD20 antibody plus chemotherapy in the last 12 months (Table II).
Table II.
Univariate analysis for serologic response rate in HM patients.
| Variable | Odds ratio | 95% CI | P |
|---|---|---|---|
| Treatment status | |||
| Last therapy ≤ 12 months prior | 1 | <0·001 | |
| Watch and wait strategy or last therapy > 12 months prior | 3·7 | 2·02–6·79 | |
| Type of treatment* | |||
| Other treatments | 1 | <0·001 | |
| Anti CD20 plus chemotherapy | 0·09 | 0·04–0·2 | |
| Sex | |||
| Male | 1 | 0·697 | |
| Female | 0·9 | 0·55–1·5 | |
| Age | |||
| <65 years | 1 | 0·471 | |
| ≥65 years | 1·21 | 0·72–2·01 | |
| Diagnosis | |||
| Aggressive B‐cell lymphomas | 1 | <0·001 | |
| Indolent B‐cell lymphomas or B‐cell lymphocytic leukaemia | 2·86 | 1·49–5·51 | |
| Hodgkin lymphoma | 6·72 | 2·5–18·09 | |
| Multiple myeloma | 29·56 | 1–29·32 | |
| T‐cell lymphomas | 5·43 | 8·2–106·49 | |
| Disease Status | |||
| Remission | 1 | 0·362 | |
| Active | 1·28 | 0·75–2·19 | |
| ALC | |||
| <800 cells/μl | 1 | <0·001 | |
| ≥800 cells/μl | 3·73 | 1·95–7·16 | |
| Not evaluable | 0·8 | 0·29–2·18 | |
| ANC | |||
| <1500 cells/μl | 1 | 0·652 | |
| ≥1500 cells/μl | 1·24 | 0·49–3·15 | |
| IgG | |||
| <600 mg/dl | 1 | 0·129 | |
| ≥600 mg/dl | 1·69 | 0·9–3·2 | |
| Not evaluable or not available | 2·05 | 0·99–4·23 | |
| IgA | |||
| <80 mg/dl | 1 | 0·296 | |
| ≥80 mg/dl | 1·55 | 0·85–2·83 | |
| Not evaluable or not available | 1·55 | 0·79–3·02 | |
| IgM | |||
| <40 mg/dl | 1 | 0·011 | |
| ≥40 mg/dl | 2·3 | 1·25–4·23 | |
| Not evaluable or not available | 2·21 | 1·17–4·19 |
ALC, absolute lymphocytic count; ANC, absolute neutrophilic count; CI, confidence interval.
This analysis was conducted only among patients who had received therapy in the last 12 months.
The rate of seroconversion and the antibody titre at two weeks after the second dose were lower for subjects on active treatment compared to those untreated or in follow‐up [55% vs 81·9% respectively, odds ratio (OR) 3·7, 95% CI 2·02–6·79, P < 0·001; median = 11 U/ml, IQR 0·44–1 320 U/ml vs median=1 686 U/ml, IQR 83·43‐3 965 U/ml, P < 0·0001; Fig 1B)]. Among actively treated patients, those who had received anti‐CD20 antibody plus chemotherapy had a lower seroconversion rate (17·6%) and antibody titres (median = 0·44 U/ml, IQR 0·44–0·44 U/ml) compared to patients receiving other treatments (71·2%, OR 0·09, 95% CI 0·04–0·20, P < 0·001; and median=183 U/ml, IQR 0·44–2 993 U/ml, P < 0·0001, respectively; Fig 1C). Moreover, seven patients received the anti‐CD20 antibody alone: five were on maintenance therapy whereas two received anti‐CD20 as their last no‐maintenance therapy. In the maintenance subgroup, the seroconversion rate was 40% (two out of five), whereas none of the remaining two patients developed a humoral response. A low seroconversion rate was reported also among patients treated with chemotherapy alone (69·4%) and among those treated with novel oral agents (52·3%). Interestingly, we observed a higher seroconversion rate in myeloma patients treated with IMIDs (84·6%). A seroconversion rate of 100% was observed in 10 myeloma patients treated with anti‐CD38 antibody (daratumumab) in monotherapy or in combination with lenalidomide or bortezomib. Among subjects who were treated with CAR‐T cells or HSCT the seroconversion rate was 57·1%. Specifically, the seroconversion rate of the 12 allo‐HSCT recipients was 83·3% whereas among the nine patients treated with CAR‐T cells, the seroconversion rate was 22%. Patients without lymphopenia had a higher seroconversion rate (73·6%) and higher antibody levels (median = 639 U/ml; IQR 0·44–3 324 U/ml) compared to those with lymphopenia (42·8%, OR 3·73, 95% CI 1·95–7·16, P < 0·001; median = 0·44 U/ml, IQR 0·44–253, P < 0·0001; Fig 1D). We also observed a lower seroconversion rate (51·7%) and antibody titres (median = 3·42 U/ml; IQR 0·44–557 U/ml) among subjects with IgM levels < 40 mg/dl compared to those with IgM levels ≥ 40 mg/dl (71·1%, OR 2·3, 95% CI 1·25–4·23, P < 0·011; median = 1 267 U/ml, IQR 0·44–4 731, P < 0·001; Fig 1E).
Age, sex, disease status, absolute neutrophil count at the time of the first dose, IgG and IgA levels had no statistically significant association with seroconversion rate. Nonetheless, we observed a higher antibody titre in patients with IgG levels ≥ 600 mg/dl compared to those with IgG < 600 mg/dl (median = 437 U/ml; IQR 0·44–3 675 U/ml; median = 14·5 U/ml; IQR 0·44–719·8 U/ml, respectively, P = 0·003; Fig 1F) and in subjects with IgA levels ≥ 80 mg/dl compared to those with IgA < 80 mg/dl (median = 456 U/ml; IQR 0·44 3 890 U/ml; median = 23·8 U/ml; IQR 0·44–939 U/ml, respectively, P = 0·0128; Fig 1G). The median antibody titre differed significantly among patients treated with chemotherapy (median = 149 U/ml; IQR 0·44–2258 U/ml), IMIDs (median = 918 U/ml; IQR 135–4 701 U/ml), novel oral agents (median = 1·28 U/ml; IQR 0·44–123 U/ml), CAR‐T‐cell therapy or HSCT (median = 23·8 U/ml; IQR 0·44–10 725 U/ml), or other therapies (median = 1159 U/ml; IQR 0·44 3 580 U/ml, P = 0·129; Fig 1H).
In the multivariable logistic model (Table III), the independent predictors for seroconversion were: type of treatment (P < 0·001), type of LM (P < 0·001), absolute lymphocytic count (ALC) < 800 cells/ml (P = 0·001) and IgM levels < 40 mg/dl (P = 0·004; Table III). In the multivariate analysis conducted in the subgroup of patients on active treatment, the independent predictors of humoral response were: type of treatment (P < 0·001), type of malignancy (P = 0·002), and IgM levels < 40 mg/dl (P = 0·030; Table IV).
Table III.
Multivariate analysis for serologic response in HM patients.
| Variable | Odds ratio | 95% CI | P |
|---|---|---|---|
| Sex | |||
| Male | 1 | 0·239 | |
| Female | 0·65 | 0·32–1·33 | |
| Age | |||
| <65 years | 1 | 0·679 | |
| ≥65 years | 0·85 | 0·40–1·83 | |
| Diagnosis | |||
| Hodgkin lymphoma | 1 | <0·001 | |
| Aggressive B‐cell lymphomas | 0·54 | 0·15–1·91 | |
| Indolent B‐cell lymphomas or B‐cell chronic lymphocytic leukaemia | 1·33 | 0·36–4·93 | |
| Multiple myeloma | 28·15 | 4·43–178·95 | |
| T‐cell lymphomas | 0·5 | 0·06–3·82 | |
| Disease status | |||
| Remission | 1 | 0·095 | |
| Active | 0·44 | 0·17–1·15 | |
| Type of treatment | |||
| Other therapies | 1 | <0·001 | |
| Anti‐CD20 antibody plus chemotherapy | 0·07 | 0·02–0·22 | |
| Watch and wait or last therapy > 12 months prior | 2·84 | 0·96–8·36 | |
| ALC | |||
| <800 cells/μl | 1 | 0·001 | |
| ≥800 cells/μl | 1·66 | 0·65–4·25 | |
| Not evaluable | 0·17 | 0·04–0·77 | |
| ANC | |||
| <1500 cells/μl | 1 | 0·884 | |
| ≥1500 cells/μl | 1·10 | 0·31–3·96 | |
| IgG | |||
| <600 mg/dl | 1 | 0·510 | |
| ≥600 mg/dl | 1·44 | 0·47–4·47 | |
| Not evaluable or not available | 0·68 | 0·18–2·65 | |
| IgA | |||
| <80 mg/dl | 1 | 0·548 | |
| ≥80 mg/dl | 1·5 | 0·50–4·50 | |
| Not evaluable or not available | 0·49 | 0·06–3·77 | |
| IgM | |||
| <40 mg/dl | 1 | 0·004 | |
| ≥40 mg/dl | 4·31 | 1·52–12·24 | |
| Not evaluable or not available | 17·54 | 1·93–159·03 |
ALC, absolute lymphocytic count; ANC, absolute neutrophilic count; CI, confidence interval.
Table IV.
Multivariate analysis for serologic response in HM patients in active treatment.
| Variable | Odds ratio | 95% CI | P |
|---|---|---|---|
| Sex | |||
| Male | 1 | 0·100 | |
| Female | 0·48 | 0·20–1·15 | |
| Age | |||
| <65 years | 1 | 0·812 | |
| ≥65 years | 0·89 | 0·35–2·30 | |
| Diagnosis | |||
| Hodgkin lymphoma | 1 | 0·002 | |
| Aggressive B‐cell lymphomas | 0·77 | 0·2–3·01 | |
| Indolent B‐cell lymphomas or B‐cell chronic lymphocytic leukaemia | 1·38 | 0·32–5·90 | |
| Multiple myeloma | 28·23 | 3·83–207·97 | |
| T‐cell lymphomas | 0·71 | 0·09–5·58 | |
| Disease status | |||
| Remission | 1 | 0·251 | |
| Active | 0·56 | 0·21–1·51 | |
| Type of treatment | |||
| Other therapies | 1 | <0·001 | |
| Anti‐CD20 antibody plus chemotherapy | 0·07 | 0·02–0·24 | |
| ALC | |||
| <800 cells/μl | 1 | 0·055 | |
| ≥800 cells/μl | 2·54 | 0·98–6·57 | |
| ANC | |||
| <1500 cells/μl | 1 | 0·961 | |
| ≥1500 cells/μl | 0·97 | 0·26–3·54 | |
| IgG | |||
| <600 mg/dl | 1 | 0·940 | |
| ≥600 mg/dl | 1·26 | 0·35–4·54 | |
| Not evaluable or not available | 1·11 | 0·20–6·19 | |
| IgA | |||
| <80 mg/dl | 1 | 0·577 | |
| ≥80 mg/dl | 1·69 | 0·44–6·45 | |
| Not evaluable or not available | 0·53 | 0·05–5·38 | |
| IgM | |||
| <40 mg/dl | 1 | 0·030 | |
| ≥40 mg/dl | 4·20 | 1·18–14·93 | |
| Not evaluable or not available | 13·31 | 1·00–177·50 |
ALC, absolute lymphocytic count; ANC, absolute neutrophilic count; CI, confidence interval; HM, haematological malignancy.
With a median follow‐up of three months after two doses of mRNA vaccines, we did not observe any case of SARS‐CoV‐2 infection.
T‐cell‐mediated immune response
Considering the importance of T‐cell immunity in COVID‐19, 16 we assessed the anti‐spike T‐cell response two weeks after the second dose, in 99 patients on active treatment. Forty‐eight of them were seropositive after vaccination whereas 51 were seronegative. We compared the response rate and the IFN‐γ, IL‐2 and TNF‐α levels of the 99 LM patients (median age 60 years, IQR 50–70) and 99 HCW (median age 51 years, IQR 45–56). IFN‐γ levels were higher than 12 pg/ml in 85 (86%) LM patients, supporting the presence of a detectable anti‐spike T‐cell‐mediated immune response. Among the 14 non‐responding patients, 10 showed a good response to staphylococcal enterotoxin B (SEB) stimulation, comparable with that observed in HCW (IFN‐γ > 2000 pg/ml), three showed a positive, but weak response (30 pg/ml < IFN‐γ < 100 pg/ml) and one showed no response (IFN‐γ = 0·3 pg/ml). In the control group, the T‐cell response was detected in all 99 subjects (100%; P < 0·001). Th1‐cytokine levels of HCW before and after vaccination and analysis method are detailed in Figure S1 and Data S1. Moreover, when compared to the control group, the ppike‐specific T‐cell response from LM patients showed a lower median value of IFN‐γ (median = 179·5 U/ml, IQR 28·4–369·6 pg/ml vs median = 309·0 pg/ml, IQR 181·3–662·8 pg/ml, P < 0·0001) and of TNF‐α (median=32·71 pg/ml, IQR 6·2‐92·5 pg/ml vs median=104·0 pg/ml, IQR 17·4‐199·0 pg/ml, P < 0·0001; Fig 2A). Unexpectedly, we reported an increased median value of IL‐2 among LM patients compared to healthy subjects (median=553·6 pg/ml, IQR 90·2‐1039·0 pg/ml vs. median=196·0 pg/ml, IQR 99·9‐303·8 pg/ml, P < 0·0001; Fig 2A) that would require further investigations in a larger cohort.
Fig 2.
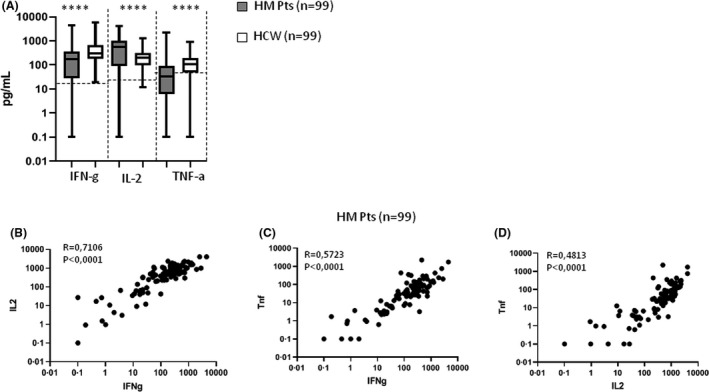
T‐cell‐mediated response in haematological malignancy (HM) patients and healthcare workers (HCW). Comparison of interferon (IFN)‐γ, interleukin (IL)‐2 and tumour necrosis factor (TNF)‐α in HM patients and HCW at two weeks after the second vaccine dose (A). Linear correlation between IFN‐γ and IL‐2 (B), IFN‐γ and TNF‐α (C), and IL‐2 and TNF‐α (D) in HM patients.
IFN‐γ values were directly correlated with IL‐2 and TNF‐α levels (R = 0·5723, P < 0·0001 and R = 0·4813, P < 0·0001, respectively; Fig 2B, C). Moreover, we also confirmed a positive correlation between IL‐2 and TNF‐α levels (R = 0·4813, P < 0·0001; Fig 2D). Th1‐associated cytokine release was not significantly correlated with serological responses, neither when evaluating IFN‐γ levels (R = 0·071, P > 0·05) nor IL‐2 levels (R = 0·048, P > 0·05). T‐cell immune response was detectable in 47 (98%) and 38 (74%) seropositive and seronegative patients, respectively. IFN‐γ levels were lower among subjects who received chemotherapy with or without anti‐CD20 antibody in the last month (75%) compared to those who received chemotherapy with or without anti‐CD20 antibody more than two months before vaccination (90%). Cytokine release was higher in patients on active treatment with IMIDs (94%) or with novel oral agents (ibrutinib or venetoclax; 91%). Thirteen individuals (13%) were defined as “double negative” with neither T‐cell nor humoral responses after a complete vaccination schedule. Among these non‐responders, 11 received chemotherapy with or without anti‐CD20 antibody in the last month, one was on treatment with lenalidomide (IMID) and one with ibrutinib. This represents a population potentially at high risk for COVID‐19 infection.
Discussion
Among individuals affected by COVID‐19, LM patients represent a high‐risk population due to their immunosuppressed state and have a mortality rate ranging from 30% to 37%. 1 , 3 , 17 It is therefore essential to implement an effective vaccination strategy. For these reasons, in Italy, LM patients have been among the first categories vaccinated with mRNA‐based SARS‐CoV‐2 vaccines. To the best of our knowledge, this is the first report comparing both humoral and T‐cellular responses after mRNA full‐dose vaccination in LM and healthy individuals.
As reported in other studies evaluating the efficacy of seasonal vaccines among cancer patients, 10 , 18 we observed a seroconversion rate in LM patients which is significantly lower than in healthy individuals. Interestingly, the seroconversion rate increased from 49·8% to 64·6% after the second dose, emphasising the importance of receiving both doses and complying with the official guidelines regarding the timing of administration of the second dose. Our results are in line with those from other groups who reported a lower seroconversion rate in LM after only one dose. 19 , 20 , 21 , 22 The main negative predictive factor for the seroconversion was an active treatment, however even in the watch‐and‐wait cohort we observed a lower response compared to the healthy individuals, supporting the notion that patients can be immune‐compromised by the disease itself.
Consistent with previous reports on pneumococcal and H1N1 vaccination, 9 , 23 we detected an extremely low seroconversion rate among patients who received anti‐CD20 antibody plus chemotherapy in the 12 months preceding vaccination. This finding can be explained by the known prolonged half‐life of rituximab which is detectable in the serum at lympholytic levels for up to six months after therapy completion and by the subsequent long‐lasting B‐cell depletion. 24 , 25 The B‐cell depletion is more durable in patients receiving rituximab maintenance, raising the problem of the more appropriate timing for vaccination in this setting.
We observed a low seroconversion rate among patients treated with novel oral agents. Sun et al. have also reported low antibody response rates after influenza vaccine in CLL patients treated with Bruton Tyrosine kinase (BTK) inhibitors, 26 due to the alteration of the B‐cell receptor signalling pathway. Our results are slightly better than those recently reported by Herishanu et al., 22 probably because we considered CLL patients treated with venetoclax without the anti‐CD20 antibody. Furthermore, we found a decreased seroconversion rate among patients who received CAR‐T therapy, especially in those treated in the six months preceding vaccination, but this is expected considering the impaired immune reconstitution reported after these procedures. 27 , 28 The high seroconversion among allo‐HSCT recipients was unexpected, but it is probably due to the small number of patients evaluated and to the fact that all these patients had suspended their immunosuppressive therapy.
Interestingly, we observed that myeloma patients receiving IMIDs had an increased antibody response relative to subjects receiving other therapies. Our finding is in line with other reports highlighting the potentially immune‐adjuvant role of IMIDs after pneumococcal or H1N1 vaccinations. 29 The elevated response rate reported in patients treated with daratumumab is in contrast with other recent studies showing a negative impact on humoral response. 20 , 30 It is possible that small numbers and concomitant treatment with IMIDs are confounding factors; nevertheless further investigations are required to clarify this aspect.
Previous studies have reported the impact of low IgM serum levels on the incidence of non‐neutropenic infections in patients treated with rituximab‐based regimens. 31 , 32 This particular form of hypogammaglobulinaemia probably is a manifestation of B‐cell compartment perturbation secondary to rituximab‐containing regimens and the lymphoid malignancy itself.
Although the precise definition of all factors responsible for protection against COVID‐19 remains to be fully determined, it is known that both neutralising antibodies and antigen‐specific T cells play important roles. 16 , 33 , 34 , 35 Given the rather low seroconversion rate among patients who received their last treatment in the 12 months preceding vaccination, we decided to investigate their T‐cell response.
We documented the in vitro release of Th1‐associated cytokines upon SARS‐CoV‐2 spike stimulation, suggestive of an effective T‐cell‐mediated immune response, in an unexpectedly high rate of patients receiving active treatment. This was confirmed in almost every patient with a serological response after two doses of vaccine, as T lymphocytes are necessary for the production of high‐affinity antibodies and generation of memory B‐cells. Interestingly, cytokine release was detected in 74% of seronegative patients. This finding is novel and relevant, as it highlights the potential protection against infection provided by T lymphocytes in seronegative patients. Finally, we also described a small fraction of “double‐negative” patients with neither serological nor cellular responses, who mostly received chemotherapy with or without anti‐CD20 in the month preceding vaccination. This subgroup is at high risk of infection and potentially life‐threatening complications.
Although our study shows a disappointingly low seroconversion rate among patients on active treatment with anti‐CD20 antibody plus chemotherapy, the intriguing data on the T‐cell‐specific response suggest a potential benefit for vaccination despite the lack of fseroconversion.
Additionally, the preliminary data in recipients of a solid organ would imply that a third vaccine dose can be a reasonable option to improve seroconversion rates in particularly fragile settings. 36 Thus, although prospective studies are required to assess the efficacy of T‐cell immunity in preventing morbidity and mortality of COVID‐19, our data may be in favour of trials evaluating the administration of a third vaccine dose to patients with evidence of at least T‐cell immune response after the first two doses. On the other hand, in double‐negative subjects the rationale for a third dose seems weak and the prophylactic administration of second‐generation neutralising monoclonal anti‐spike antibodies should be tested in clinical trials.
Our study was not able to clearly identify an optimal time for vaccination for LM patients that could probably depend on the last treatment. Our serological findings support the idea that all LM patients should receive vaccination prior to therapy at disease onset or at disease relapse and should wait at least 12 months after any treatment containing anti–CD20 antibody and at least six months after chemotherapy alone. However, the analysis of the T‐cell response showed more encouraging data, suggesting a possible benefit of the vaccination even early after treatment discontinuation. Thus, further investigations will be needed to define the best accurate timing for vaccination.
In conclusion, we demonstrated a lower seroconversion rate among LM patients compared to healthy individuals, in particular among patients on treatment with anti‐CD20 antibody and chemotherapy and a better than expected T‐cell‐mediated response. These results confirm the value of vaccination strategy, but indicate the need for more effective measures for LM patients, to prevent serious SARS‐CoV‐2 infections and reduce their exceedingly high mortality rate.
Funding
This work was funded by Ministero della Salute (Ricerca Corrente ‐ Linea 1) and Associazione Italiana Leucemie (AIL Milano).
Author contributions
VM, CC, CA, and PC designed the study and wrote the manuscript; AG, LF, GA and GI helped design the study; CC, MM and DM conducted the experiments; RM made statistical analyses and wrote the paper; SL and PV made statistical analyses; LC wrote the manuscript; FS enrolled patients; all authors provided final approval of the manuscript.
Conflicts of interest
The authors declare no competing financial interests.
Supporting information
Fig S1. Levels of T‐helper cell type 1 (Th1) cytokines before and after vaccination in healthcare workers (HCW).
Data S1. Supplementary methods.
Acknowledgements
The authors wish to thank the patients who made this research possible, Ilaria Lo Russo and Chiara Ghidoli for the collection of blood samples and the study coordinators. Open Access Funding provided by Universita degli Studi di Milano within the CRUI‐CARE Agreement. [Correction added on 4 June 2022, after first online publication: CRUI funding statement has been added.]
These authors contributed equally to the study as last author
References
- 1. Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, et al. Clinical characteristics and risk factors associated with COVID‐19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7(10):e737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Piñana JL, Martino R, García‐García I, Parody R, Morales MD, Benzo G, et al. Risk factors and outcome of COVID‐19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, et al. Outcomes of patients with hematologic malignancies and COVID‐19: a systematic review and meta‐analysis of 3377 patients. Blood. 2020;136(25):2881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA vaccine against SARS‐CoV‐2 — preliminary report. N Engl J Med. 2020;383(20):1920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li J, Hui A, Zhang X, Yang Y, Tang R, Ye H, et al. Safety and immunogenicity of the SARS‐CoV‐2 BNT162b1 mRNA vaccine in younger and older Chinese adults: a randomized, placebo‐controlled, double‐blind phase 1 study. Nat Med. 2021;27:1062–70. [DOI] [PubMed] [Google Scholar]
- 8. Passamonti F, Romano A, Salvini M, Merli F, Della PMG, Bruna R, et al. COVID‐19 elicits an impaired antibody response against SARS‐CoV‐2 in patients with haematological malignancies. Br J Haematol. 2021; 10.1111/bjh.17704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yri OE, Torfoss D, Hungnes O, Tierens A, Waalen K, Nordøy T, et al. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood. 2011;118(26):6769–71. [DOI] [PubMed] [Google Scholar]
- 10. Mariotti J, Spina F, Carniti C, Anselmi G, Lucini D, Vendramin A, et al. Long‐term patterns of humoral and cellular response after vaccination against influenza A (H1N1) in patients with hematologic malignancies. Eur J Haematol. 2012;89(2):111–9. [DOI] [PubMed] [Google Scholar]
- 11. Cheuk DKL, Chiang AKS, Lee TL, Chan GCF, Ha SY, Lau YL. Vaccines for prophylaxis of viral infections in patients with hematological malignancies. Cochrane Database Syst Rev. 2007;3:1465–858. [DOI] [PubMed] [Google Scholar]
- 12. Mikulska M, Viscoli C, Orasch C, Livermore DM, Averbuch D, Cordonnier C, et al. Aetiology and resistance in bacteraemias among adult and paediatric haematology and cancer patients. J Infect. 2014;68(4):321–31. [DOI] [PubMed] [Google Scholar]
- 13. Cordonnier C, Einarsdottir S, Cesaro S, Di Blasi R , Mikulska M, Rieger C, et al. Vaccination of haemopoietic stem cell transplant recipients: guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19(6):e200–12. [DOI] [PubMed] [Google Scholar]
- 14. Mikulska M, Cesaro S, de Lavallade H , Di Blasi R , Einarsdottir S, Gallo G, et al. Vaccination of patients with haematological malignancies who did not have transplantations: guidelines from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19(6):e188–99. [DOI] [PubMed] [Google Scholar]
- 15. Agrati C, Castilletti C, Goletti D, Meschi S, Sacchi A, Matusali G, et al. Coordinate induction of humoral and spike specific T‐cell response in a cohort of italian health care workers receiving BNT162b2 mRNA vaccine. Microorganisms. 2021;9(6):1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bange EM, Han NA, Wileyto P, Kim JY, Gouma S, Robinson J, et al. CD8+ T cells contribute to survival in patients with COVID‐19 and hematologic cancer. Nat Med. 2021;27(7):1280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wood WA, Neuberg DS, Thompson JC, Tallman MS, Sekeres MA, Sehn LH, et al. Outcomes of patients with hematologic malignancies and COVID‐19: a report from the ASH Research Collaborative Data Hub. Blood Adv. 2020;4(23):5966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mazza JJ, Yale SH, Arrowood JR, Reynolds CE, Glurich I, Chyou P‐H, et al. Efficacy of the influenza vaccine in patients with malignant lymphoma. Clin Med Res. 2005;3(4):214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Monin L, Laing AG, Muñoz‐Ruiz M, McKenzie DR, Barrio DMD, I, Alaguthurai T, et al. Safety and immunogenicity of one versus two doses of the COVID‐19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pimpinelli F, Marchesi F, Piaggio G, Giannarelli D, Papa E, Falcucci P, et al. Fifth‐week immunogenicity and safety of anti‐SARS‐CoV‐2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol. 2021;14(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bird S, Panopoulou A, Shea RL, Tsui M, Saso R, Sud A, et al. Response to first vaccination against SARS‐CoV‐2 in patients with multiple myeloma. Lancet Haematol. 2021;3026(21):19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Souza KJ, Ferro RS, Prestes‐Carneiro LE, Carrilho PAM, de Vasconcelos DM. Infectious diseases and immunological markers associated with patients with non‐Hodgkin lymphoma treated with rituximab. Immunopharmacol Immunotoxicol. 2018;40(1):13–7. [DOI] [PubMed] [Google Scholar]
- 24. Salles G, Barrett M, Foà R, Maurer J, O’Brien S, Valente N, et al. Rituximab in B‐cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017;34(10):2232–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li J, Levi M, Charoin J‐E, Frey N, Kheoh T, Ren S, et al. Rituximab exhibits a long half‐life based on a population pharmacokinetic analysis in non‐hodgkin’s lymphoma (NHL) patients. Blood. 2007;110(11):2371.17515402 [Google Scholar]
- 26. Sun C, Gao J, Couzens L, Tian X, Farooqui MZ, Eichelberger MC, et al. Seasonal influenza vaccination in patients with chronic lymphocytic leukemia treated with ibrutinib. JAMA Oncol. 2016;2(12):1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ogonek J, Kralj Juric M, Ghimire S, Varanasi PR, Holler E, Greinix H, et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front Immunol. 2016;17:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strati P, Varma A, Adkins S, Nastoupil LJ, Westin J, Hagemeister FB, et al. Hematopoietic recovery and immune reconstitution after axicabtagene ciloleucel in patients with large B‐cell lymphoma. Haematologica. 2020;106(10):2667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Noonan K, Rudraraju L, Ferguson A, Emerling A, Pasetti MF, Huff CA, et al. Lenalidomide‐induced immunomodulation in multiple myeloma: impact on vaccines and antitumor responses. Clin Cancer Res. 2012;18(5):1426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Terpos E, Gavriatopoulou M, Ntanasis‐Stathopoulos I, Briasoulis A, Gumeni S, Malandrakis P, et al. The neutralizing antibody response post COVID‐19 vaccination in patients with myeloma is highly dependent on the type of anti‐myeloma treatment. Blood Cancer J. 2021;11(8):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bedognetti D, Zoppoli G, Massucco C, Zanardi E, Zupo S, Bruzzone A, et al. Impaired response to influenza vaccine associated with persistent memory B cell depletion in non‐hodgkin’s lymphoma patients treated with rituximab‐containing regimens. J Immunol. 2011;186(10):6044–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cabanillas F, Liboy I, Pavia O, Rivera E. High incidence of non‐neutropenic infections induced by rituximab plus fludarabine and associated with hypogammaglobulinemia: a frequently unrecognized and easily treatable complication. Ann Oncol. 2006;17(9):1424–7. [DOI] [PubMed] [Google Scholar]
- 33. Mathew D, Giles JR, Baxter AE, Greenplate AR, Wu JE, Alanio C, et al. Deep immune profiling of COVID‐19 patients reveals patient heterogeneity and distinct immunotypes with implications for therapeutic interventions. Science. 2020;369(6508):eabc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dagotto G, Yu J, Barouch DH. Approaches and challenges in SARS‐CoV‐2 vaccine development. Cell Host Microbe. 2020;28(3):364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen Z, John WE. T cell responses in patients with COVID‐19. Nat Rev Immunol. 2020;20(9):529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid‐19 vaccine in solid‐organ transplant recipients. N Engl J Med. 2021;385(7):661–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Levels of T‐helper cell type 1 (Th1) cytokines before and after vaccination in healthcare workers (HCW).
Data S1. Supplementary methods.


