Abstract
Background
Veno‐venous extracorporeal membrane oxygenation (V‐V ECMO) support is increasingly used in the management of COVID‐19‐related acute respiratory distress syndrome (ARDS). However, the clinical decision‐making to initiate V‐V ECMO for severe COVID‐19 still remains unclear. In order to determine the optimal timing and patient selection, we investigated the outcomes of both COVID‐19 and non‐COVID‐19 patients undergoing V‐V ECMO support.
Methods
Overall, 138 patients were included in this study. Patients were stratified into two cohorts: those with COVID‐19 and non‐COVID‐19 ARDS.
Results
The survival in patients with COVID‐19 was statistically similar to non‐COVID‐19 patients (p = .16). However, the COVID‐19 group demonstrated higher rates of bleeding (p = .03) and thrombotic complications (p < .001). The duration of V‐V ECMO support was longer in COVID‐19 patients compared to non‐COVID‐19 patients (29.0 ± 27.5 vs 15.9 ± 19.6 days, p < .01). Most notably, in contrast to the non‐COVID‐19 group, we found that COVID‐19 patients who had been on a ventilator for longer than 7 days prior to ECMO had 100% mortality without a lung transplant.
Conclusions
These findings suggest that COVID‐19‐associated ARDS was not associated with a higher post‐ECMO mortality than non‐COVID‐19‐associated ARDS patients, despite longer duration of extracorporeal support. Early initiation of V‐V ECMO is important for improved ECMO outcomes in COVID‐19 ARDS patients. Since late initiation of ECMO was associated with extremely high mortality related to lack of pulmonary recovery, it should be used judiciously or as a bridge to lung transplantation.
Keywords: artificial organs, circulatory support devices, COVID‐19, outcomes, V‐V ECMO
The survival in patients with COVID‐19 was statistically similar compared to non‐COVID‐19 patients, but the mortality of COVID‐19 patients who had been on a ventilator for longer than 7 days prior to the initiation of ECMO approached 100%.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by the novel SARS‐CoV‐2 virus, initially appeared in late 2019 and has rapidly evolved into a global pandemic. While most patients with COVID‐19 develop mild to moderate respiratory symptoms, a significant portion progress to respiratory failure requiring intubation and mechanical ventilation. Unfortunately, the mortality associated with COVID‐19 patients requiring mechanical ventilation is high. 1 Veno‐venous extracorporeal membrane oxygenation (V‐V ECMO) is a life‐support technique that is frequently used for patients with respiratory or circulatory failure. 2 Indeed, V‐V ECMO is used routinely used as a bridge to recovery in patients with severe acute respiratory distress syndrome (ARDS) due to the H1N1 influenza virus and more recently has been the breakthrough treatment for respiratory failure associated with coronavirus disease 2019. 3 , 4 , 5
Although adoption of V‐V ECMO is rapidly evolving, 6 various adverse effects have been associated with V‐V ECMO, such as nosocomial infections and bacteremia. 7 However, little is known about the potential adverse effects in patients undergoing V‐V ECMO due COVID‐19 associated respiratory failure. One case series of critically ill patients demonstrated favorable outcomes in a patient who underwent five days of V‐V ECMO. 8 In contrast, in another study examining clinical characteristics of severe COVID‐19 patients, five out of six patients receiving ECMO died. 9 Similarly, other studies have reported a dismal 100% mortality for ECMO patients. 10 , 11 Despite the small sample sizes of these studies, their findings raise concern for the benefits of ECMO therapy for COVID‐19. Recently, an international study of COVID‐19 patients, involving the ELSO registry demonstrated that the estimated mortality 90 days after receiving ECMO was roughly 37%. 12 Furthermore, various studies have described higher incidence of a multitude of complications associated with V‐V ECMO use in COVID‐19 patients such as pneumothorax, hemothorax, bleeding, and thrombotic events. 12 , 13 , 14 , 15 , 16
In this study, our aim was to evaluate the clinical characteristics and outcomes for patients undergoing V‐V ECMO due to COVID‐19 respiratory failure, and to determine if there are any differences compared to non‐COVID patients that may improve clinical management. Additionally, to compare the outcomes in the two cohorts, we also analyzed the incidence of complications including pneumothorax, hemothorax, bleeding events, thrombotic events, neurologic dysfunction, acute kidney injury (AKI), pump malfunction, and oxygenator dysfunction.
2. MATERIALS AND METHODS
2.1. Study subjects
Patient data was collected retrospectively using the electronic medical record and kept in our ECMO database for the purposes of the study. Adult patients placed on V‐V ECMO at our medical center between January 2015 and September 2020 were included in the study. A total of 18 patients were excluded from this study to avoid confounding effects. We excluded patients who required conversion to veno‐arterial ECMO or veno‐arterial‐veno ECMO. In the COVID‐19 group, confirmation of SARS‐CoV‐2 was determined via either nasopharyngeal swabs or bronchoalveolar lavage at the time of admission. Real‐time reverse‐transcription polymerase chain reaction (RT‐PCR) assays were performed to confirm the presence of COVID‐19. Patients did not receive continuous anticoagulation unless there was specific indication such as DVT or PE and were not monitored with bleeding parameters such as ACT or aPTT, consistent with our recent report. 17 All patients not receiving continuous systemic anticoagulation received 5000 U subcutaneous unfractionated heparin every 8 h for deep venous thrombosis prophylaxis. Flow was maintained at least 3.0–3.5 L/min consistent with our recent reports demonstrating the feasibility of using V‐V ECMO without anticoagulation. 17 , 18 , 19 This was done in order to reduce thrombotic complications in the ECMO circuit. For both groups, transfusions were administered if any of the following criteria were met: Platelets < 50 000/ml, Hemoglobin < 7 g/dl, or hemodynamic instability in the setting of active blood loss. Different cannulation strategies [Internal jugular vein—femoral vein cannulation vs ProtekDuo® cannulation (CardiacAssist Inc, Pittsburgh, PA, USA)] were used in patients depending on the surgeon preference. The V‐V ECMO circuit included Quadrox iD adult (7.0) oxygenator (MAQUET Holding BV & Co. KG, Germany) and Rotaflow pump (MAQUET Holding BV & Co. KG, Germany). Except for the cannulas, the other components of the circuit had a heparin coating.
Patients with respiratory failure were considered for ECMO if they failed to achieve satisfactory gas exchange (PaO2 > 55 mm Hg, Oxygen saturations > 88%, pH > 7.2, with plateau pressures less than 35) despite lung protective mechanical ventilation and recruitment maneuvers with neuromuscular blockade. The decision to cannulate was made by a multidisciplinary ECMO team. This study was approved by the Northwestern University Institutional Review Board (STU00207250). However, the need for patient consent for data collection was waived by the IRB as this was a retrospective study.
2.2. Definitions of complications
Post‐cannulation complications were determined using the following definitions. Gastrointestinal bleeding with one or more of the following: guaiac‐positive stool, hematemesis, melena, active bleeding at the time of endoscopy or colonoscopy, or blood within the stomach at endoscopy or colonoscopy. Hemothorax was defined as the presence of blood in the chest cavity, typically confirmed via chest X‐ray or CT scan. Hemothorax occurring as a result of surgery was exempt from this definition. Oral and nasal bleedings were defined as bleeding from the mouth or nose that required wound packing by an otorhinolaryngologist. Diffuse alveolar hemorrhage was defined as hemorrhage in the alveoli, confirmed via bronchoscopy. Retroperitoneal bleeding was confirmed via CT scan. DVT and PE were determined by duplex ultrasonography and pulmonary CT angiograms, respectively. Ischemic fingers were determined by vascular surgeons with clinical symptoms. Sepsis was defined as bacteremia confirmed via blood cultures. Neurological dysfunction (ND) was a new neurological deficit associated with abnormal neuroimaging findings. This was further divided into ischemic or hemorrhagic based on imaging findings. AKI was defined using the Risk, Failure, Loss of kidney function and End‐stage kidney disease (RIFLE) classification. 20
2.3. Statistical analysis
Statistical analyses were performed using Stata/MP14 (StataCorp, College Station, TX). Patient demographics, post‐ECMO complications, and outcomes were compared between the non‐COVID‐19 and COVID‐19 groups. Continuous variables were compared using t‐test and reported as means. Categorical variables were compared using chi‐square test and reported as a number (percentage). Contal and O’Quigley analysis was performed to statistically determine the cutoff of the days of ventilation and the number of times proning prior to V‐V ECMO for worse overall survival outcomes. p‐Values < .05 were accepted as statistically significant. Cox proportional hazard regression was used to derive hazard ratios and 95% confidence intervals. To build our models, we first performed a univariate analysis of all variables. Then, the variables with a p value less than .20 in the univariate Cox analysis were included in our final multivariate model to identify predictors of overall postoperative mortality. We performed Gronnesby and Borgan tests to assess the overall goodness of fit. The Kaplan‐Meier method was used to estimate survival and a log‐rank test was performed to compare survival between the two groups. Propensity score model was created to match the non‐Covid‐19 group with the COVID‐19 group. We used EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics. 21
3. RESULTS
3.1. Study population
During the study period, 138 patients were placed on V‐V ECMO (Table 1). Table 1 shows pre‐V‐V ECMO characteristics of the study cohort. Overall, 112 patients were placed on V‐V ECMO due to non‐COVID‐19 pneumonia while 26 had COVID‐19 pneumonia. There were no significant differences in patient characteristics between the two groups, except for BMI, BSA (28.8 ± 8.9 vs 33.4 ± 5.9, p < .01, 2.0 ± 0.3 vs 2.1 ± 0.2, p < .01). Non‐COVID‐19 patients’ group has lower sodium (137.8 ± 6.6 vs 140.3 ± 4.9, p = .04) and lower HCO3 (26.5 ± 7 vs 31.5 ± 6.6, p < .01). While creatinine (1.4 ± 1.9 vs 0.9 ± 0.5, p = .02), albumin (3.1 ± 0.7 vs 2.7 ± 0.5, p < .01), INR (1.3 ± 0.5 vs 1.2 ± 0.2, p = .04), PaO2 (108.4 ± 88.9 vs 72.9 ± 21.1, p < .01) were higher in the non‐COVID‐19 group.
TABLE 1.
Characteristics of veno‐venous extracorporeal membrane oxygenation in study cohort
| Variable | Overall (n = 138) | Non‐COVID‐19 (n = 112) | COVID‐19 (n = 26) | p value |
|---|---|---|---|---|
| Age, years | 47.8 ± 14.5 | 47.8 ± 15.3 | 47.6 ± 10.9 | .92 |
| Female | 56 (40.6%) | 49 (43.8%) | 7 (26.9%) | .12 |
| BMI, kg/m2 | 29.7 ± 8.6 | 28.8 ± 8.9 | 33.4 ± 5.9 | <.01 |
| BSA, m2 | 2.0 ± 0.3 | 2.0 ± 0.3 | 2.1 ± 0.2 | <.01 |
| Hypertension | 46 (33.3%) | 41 (36.6%) | 5 (19.2%) | .11 |
| Diabetes mellitus | 34 (24.6%) | 27 (24.1%) | 7 (26.9%) | .80 |
| Smoking history | 46 (33.3%) | 41 (36.6%) | 5 (19.2%) | .11 |
| Chronic obstructive pulmonary disease | 13 (9.4%) | 12 (10.7%) | 1 (3.8%) | .46 |
| CKD | 18 (13%) | 18 (16.1%) | 0 (0%) | .02 |
| Dialysis | 17 (12.3%) | 15 (13.4%) | 2 (7.7%) | .74 |
| Laboratory | ||||
| Hemoglobin, g/dl | 11.3 ± 2.6 | 11.2 ± 2.7 | 11.7 ± 2 | .35 |
| WBC, 1000/mm3 | 13.2 ± 7.3 | 13.5 ± 7.5 | 11.9 ± 6.4 | .30 |
| Platelets, 1000/mm3 | 232.2 ± 118.7 | 224.7 ± 120.8 | 264.8 ± 105.2 | .10 |
| Sodium, mEq/L | 138.3 ± 6.3 | 137.8 ± 6.6 | 140.3 ± 4.9 | .04 |
| Creatinine, mg/dl | 1.3 ± 1.8 | 1.4 ± 1.9 | 0.9 ± 0.5 | .02 |
| BUN, mg/dl | 25.2 ± 16.7 | 25.2 ± 16.8 | 24.9 ± 16.6 | .94 |
| AST, U/L | 56 ± 96.2 | 57.5 ± 105.7 | 50.1 ± 39.5 | .58 |
| ALT, U/L | 52.5 ± 84.1 | 52.5 ± 91.3 | 52.4 ± 45.8 | .99 |
| Total bilirubin, mg/dl | 1.3 ± 3.9 | 1.4 ± 4.4 | 0.8 ± 0.6 | .15 |
| Albumin, g/dl | 3 ± 0.7 | 3.1 ± 0.7 | 2.7 ± 0.5 | <.01 |
| INR | 1.3 ± 0.5 | 1.3 ± 0.5 | 1.2 ± 0.2 | .04 |
| ABG (at cannulation) | ||||
| pH | 7.3 ± 0.1 | 7.3 ± 0.1 | 7.3 ± 0.1 | .91 |
| PaCO2 | 58.7 ± 20.4 | 58.8 ± 22.1 | 58.2 ± 10.3 | .85 |
| PaO2 | 101.7 ± 81.7 | 108.4 ± 88.9 | 72.9 ± 21.1 | <.001 |
| HCO3 | 27.4 ± 7.1 | 26.5 ± 7 | 31.5 ± 6.6 | <.01 |
| Lactate | 3.1 ± 3.1 | 3.2 ± 3.2 | 2.1 ± 1.5 | .05 |
Continuous data are shown as means ± standard deviation (SD).
Abbreviations: ABG, arterial blood gas; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BSA, body surface area; BUN, blood urea nitrogen; CKD, chronic kidney disease; INR, international normalized ratio; WBC, white blood cell.
3.2. Complication rates and mortality
We compared post‐cannulation complications between patients with non‐COVID‐19 and COVID‐19. After V‐V ECMO initiation, there was no significant difference in the incidence of AKI, dialysis, tracheostomy, ND, oxygenator exchange, and/or sepsis between two groups (Table 2). However, the COVID‐19 group had significantly higher incidence of bleeding and thrombotic complications (p = .03 and p < .001 respectively). In particular, hemothorax, oral/nasal bleeding, and DVT were higher in the COVID‐19 group (p ≤ .001, .04, <.001, respectively, Table 2).
TABLE 2.
Incidence of post‐cannulation complications
| Event | Non‐COVID‐19 (n = 112; 1781 days) | COVID‐19 (n = 26; 756 days) | p value | ||||
|---|---|---|---|---|---|---|---|
| Patients | Events | EPPD | Patients | Events | EPPD | ||
| AKI | 48 (42.9%) | – | – | 14 (53.8%) | – | – | .17 |
| Dialysis | 36 (32.1%) | – | – | 9 (34.6%) | – | – | .82 |
| Tracheostomy | 63 (56.3%) | – | – | 18 (69.2%) | – | – | .22 |
| Neurological dysfunction | 6 (5.3%) | 6 | 0.003 | 0 (0%) | 0 | 0.000 | .19 |
| Oxygenator exchange | 37 (33%) | 41 | 0.021 | 11 (42.3%) | 11 | 0.056 | .37 |
| Sepsis | 16 (13.5%) | 18 | 0.009 | 5 (19.2%) | 6 | 0.031 | .75 |
| Bleeding complication | 61 (54.4%) | 68 | 0.034 | 12 (46.1%) | 15 | 0.076 | .03 |
| Hemothorax | 14 (12.5%) | 14 | 0.007 | 4 (15.3%) | 4 | 0.020 | <.001 |
| Oral/Nasal bleeding | 19 (16.9%) | 19 | 0.010 | 7 (26.9%) | 7 | 0.036 | .04 |
| GI bleeding | 15 (13.3%) | 18 | 0.009 | 3 (11.5%) | 3 | 0.015 | .34 |
| HND | 4 (3.5%) | 4 | 0.002 | 1 (3.4%) | 1 | 0.005 | .94 |
| DAH | 11 (9.8%) | 11 | 0.006 | 0 (0%) | 0 | 0.000 | .09 |
| Retroperitoneal bleeding | 2 (1.7%) | 2 | 0.001 | 0 (0%) | 0 | 0.000 | .49 |
| Thrombotic complications | 27 (24.1%) | 30 | 0.015 | 12 (46.1%) | 13 | 0.066 | <.001 |
| DVT | 21 (18.7%) | 21 | 0.011 | 12 (46.1%) | 12 | 0.061 | <.001 |
| PE | 2 (1.7%) | 2 | 0.001 | 0 (0%) | 0 | 0.000 | .49 |
| Ischemic fingers | 5 (4.4%) | 5 | 0.003 | 1 (3.4%) | 1 | 0.005 | .88 |
Abbreviations: AKI, acute kidney injury; DAH, diffuse alveolar hemorrhage; DVT, deep venous thrombosis; EPPD, event per patient‐day; GI bleeding; gastrointestinal bleeding; HND, hemorrhagic neurological dysfunction; IND, ischemic neurological dysfunction; PE, pulmonary embolism.
In the COVID‐19 group, patients supported with mechanical ventilator over 7 days prior to the initiation of V‐V ECMO had 100% mortality, while patients with less than 7 days had 63.1% mortality. Figure 1 further demonstrates the distribution of mortality based on pre‐ECMO ventilator days in the COVID‐19 cohort. However, there was no specific cut off for increased mortality associated with pre‐ECMO ventilator support in the non‐COVID‐19 patients. Indeed, patients who were placed on V‐V ECMO after 7 days showed only a 30.7% mortality (p = .01). Given that COVID‐19 patients undergo multiple proning episodes, we next analyzed whether increased proning was associated with post‐ECMO mortality in this cohort. Figure S1 demonstrates the number of times proning was attempted prior to V‐V ECMO for patients in the COVID‐19 group. We did not find any specific cut‐offs for the number of proning episodes prior to initiation of ECMO and post‐ECMO mortality, as evident by a Contal and O’Quigley analysis.
FIGURE 1.
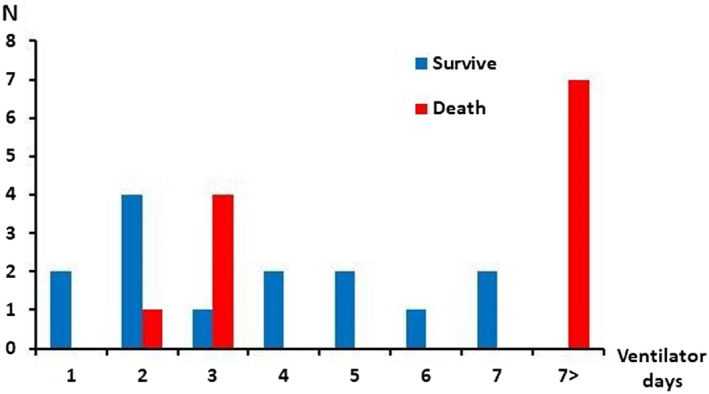
Length of ventilator use prior to ECMO in COVID‐19 group [Color figure can be viewed at wileyonlinelibrary.com]
Next, we compared mortality between COVID‐19 versus non COVID‐19 patients. The mortality rates at 30 days, 90 days, 180 days, 365 days after V‐V ECMO initiation were not significantly different between the two groups (p = .16, Figure 2).
FIGURE 2.
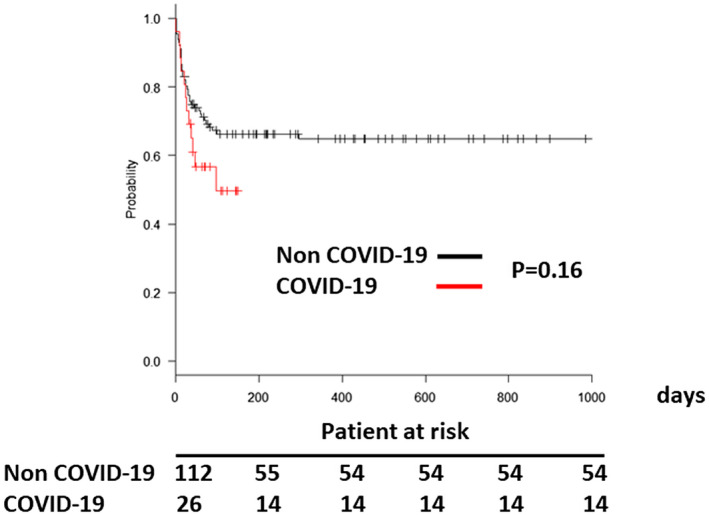
Survival of patients who underwent veno‐venous extracorporeal membrane oxygenation for lung failure [Color figure can be viewed at wileyonlinelibrary.com]
Finally, we did propensity matching analysis due to size difference between 2 groups (Table S1). In this model, the mortality rates at 30 days, 90 days, 180 days, 365 days after V‐V ECMO support were also not significantly different between the two groups (p = .28, Figure 3).
FIGURE 3.
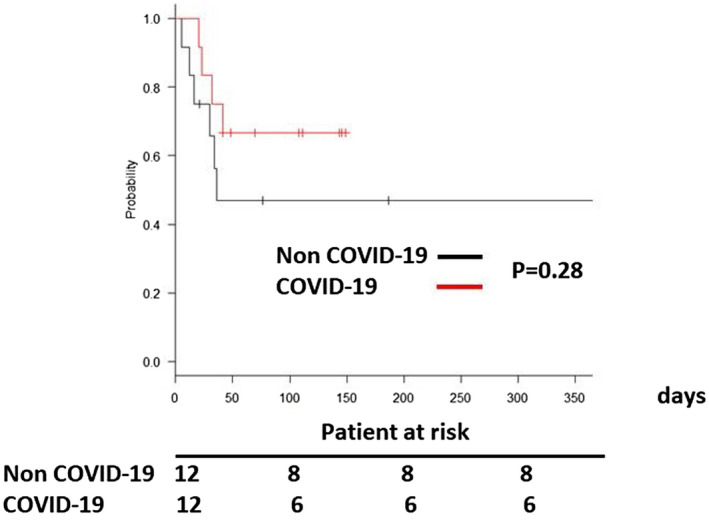
Survival of patients who underwent veno‐venous extracorporeal membrane oxygenation for lung failure after matching [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Cox multivariable logistic regression analysis of association between V‐V ECMO and outcome
We first performed a univariate analysis of all variables (Table S2). We found that total bilirubin level of prior to initiation of V‐V ECMO were independent predictors of post‐cannulation survival from multivariate Cox analysis (Table 3). We performed the same cox analysis for each group. For non‐COVID‐19 patients, BSA, RESP score, and platelets were independent predictors of post‐cannulation survival (Tables S3 and S4). On the other hand, for COVID‐19 patients, INR was the only independent predictors of post‐cannulation survival (Tables S5 and S6).
TABLE 3.
Cox multivariable logistic regression analysis: Predictors of post‐cannulation mortality
| Variable | HR | p value | 95% CI |
|---|---|---|---|
| COVID | 1.16 | .77 | 0.40–3.34 |
| Laboratory | |||
| WBC, 1000/mm3 | 0.96 | .77 | 0.91–1.01 |
| Total bilirubin, mg/dl | 1.08 | <.001 | 1.02–1.14 |
| Lactate | 1.06 | .25 | 0.95–1.19 |
Abbreviations: COVID, coronavirus disease 2019; WBC, white blood cell.
4. DISCUSSION
In this study, we found that adult COVID‐19 patients supported with V‐V ECMO had higher incidence of bleeding and thrombotic complications consistent with prior studies, 22 , 23 but there was no significant difference of survival rate between non COVID‐19 and COVID‐19 groups. Thrombosis is a known complication of ECMO and is thought to result due to contact between blood and non‐endothelial surfaces of the ECMO circuitry which leads in clotting factor activation and complement‐mediated inflammatory response. Additionally, COVID‐19 can also cause hypercoagulability. These provide a possible explanation for the observed increase in thrombotic complications. Paradoxically, bleeding complications were also higher in the COVID‐19 group, despite the fact that our center does not regularly anticoagulate patients undergoing V‐V ECMO unless they have a specific indication such as DVT or PE. 17 Bleeding is also a known complication of V‐V ECMO which worsens mortality. 24 In patients being bridged to transplantation, bleeding results in blood transfusions that increase sensitization towards histocompatibility antigens, posing immunological challenges. Furthermore, while COVID‐19 is hypothesized to result in a prothrombotic state, some articles have suggested that it may also increase risk of bleeding and coagulopathy. 25 , 26 Hence, it appears that the COVID‐19 patients are heterogeneous and the decision to anticoagulate or not should be made on a case‐by‐case basis.
While patients in the COVID‐19 group did have longer duration of cannulation, our findings suggest that ECMO remains a viable option for the treatment of COVID‐19‐associated ARDS given that mortality rates between the two study cohorts remained similar following V‐V ECMO implantation. These results are in contrast with initial studies that suggest use of V‐V ECMO in COVID‐19 patients is associated with increased mortality. 9 , 10 , 11 Our overall survival was 53.8%, which was compatible to the data from a EuroELSO international survey. 27
Most notably, we found that use of a ventilator for longer than 7 days prior to initiation of V‐V ECMO was associated with 100% mortality. This data should prove useful when deciding whether a COVID‐19 patient may benefit from V‐V ECMO. These results seem to answer one of the questions raised by Uemura et al, 13 in which they debate whether COVID‐19 patients would benefit from either early or late initiation of ECMO following mechanical ventilation. While increased use of ventilator did correlate with increased mortality, an increase in proning attempts prior to initiation of ECMO did not affect outcome. We postulate that initiation of V‐V ECMO beyond 7 days of mechanical ventilation should be made in exceptional cases or when lung transplant is a possibility if lung recovery does not occur. 28 , 29 Patients in our study were managed using the ARDsnet protocol for the ventilation. Nevertheless, in a future study, it may be necessary to investigate the role of various ventilator settings and pulmonary compliance in post‐ECMO outcomes.
Given the rapid development of the pandemic, there is conflicting information on the clinical characteristics of COVID‐19 who should be supported with V‐V ECMO. This has led to a large degree of ambiguity regarding the adoption of V‐V ECMO for these patients. Although most of the patients with COVID‐19 present with mild symptoms, about 14% of the patients develop severe cases, 5% of them with critical illness, and mechanical ventilation alone may not be enough to resolve severe hypoxemia. Some studies 30 , 31 have shown that early use of V‐V ECMO in respiratory distress may reduce pulmonary and systemic inflammation as well as severe multi‐organ dysfunction, suggesting that ECMO could be a potential option for COVID‐19 patients not responding to conventional interventions. 32 Our data supports these studies as patients with ventilator over 7 days prior to V‐V ECMO support had very high mortality. In concordance, current CDC guidelines suggest that in settings where ECMO is available, it should be considered as a potential therapy as part of the standard management algorithm of COVID‐19‐associated ARDS patients. 33 However, there have been concerns about adopting ECMO as a tool in treating refractory COVID‐19 pneumonia. A discussion in April 2020 34 among ELSO leaders suggested that ECMO is not a therapy that should be placed at the forefront for COVID‐19 due to its low availability and difficulties with referral and management. A review of the use of ECMO during past outbreaks such as MERS and H1N1 offers similar suggestions: ECMO may not be a therapy that can be implemented broadly across the globe given its resource constraints, but judicious use in appropriately chosen patients may be highly effective. 35 While resource utilization may be argued to limit the use of ECMO in the circumstances of the pandemic, emerging data continues to support its efficacy in COVID‐19 patients. More recently, results from the international ELSO registry involving 1035 ECMO‐supported patients from 36 countries demonstrate support for the use of ECMO in COVID‐19 related ARDS, strengthening the notion that centers experienced in ECMO treatment should strongly consider its use in COVID‐19 respiratory failure. 12 These findings in combination with our data contrast those of earlier articles which led some to suggest withholding ECMO support for patients with COVID‐19. 36 , 37
ECMO can provide lung rest and minimize or abolish the possible harm caused by mechanical ventilation. COVID‐19‐associated ARDS patients have a form of injury that is similar to that of classical ARDS, characterized by decreased compliance and increased lung weight. 38 The duration of mechanical ventilation and the length of intensive care unit stay are longer, especially compared with that reported in cohorts of patients with ARDS due to other causes, 39 , 40 which is consistence with our data. Long‐term mechanical ventilation can cause lung barotrauma. Extracorporeal support can reduce the ventilator‐induced lung injury and allows an enhancement of lung‐protective ventilator strategies while awaiting improvement of respiratory failure caused by COVID‐19. Additionally, ECMO can be used as a bridge to lung transplantation even for severe COVID‐19 patients, as demonstrated by our recent reports. 28 , 41
Our study has some limitations. We studied patients at a single center which may limit the generalizability of our conclusions. Also, the number of patients were small which may reduce statistical power. Furthermore, our study was conducted retrospectively and was not a randomized controlled trial. Nevertheless, our data indicate that for patients supported with V‐V ECMO, there is no difference in post‐cannulation complication rates between COVID‐19 and non‐COVID‐19 groups. In addition, we demonstrate that while COVID‐19 patients required longer ECMO support days, this was not associated with an increase in mortality. Notably, we also demonstrate that an increased length of ventilator use prior to initiation of ECMO is a strong predictor of mortality. While we do not have data on COVID‐19 patients requiring long ventilator uses without ECMO, this may be a future topic of investigation. Given the rapidly developing nature of the COVID‐19 pandemic, it is understandable that there remains much ambiguity regarding this topic, but we hope that our study would provide some clarity in the judicious use of ECMO for COVID‐19 patients.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Concept/design, data analysis/interpretation, drafting article: Chitaru Kurihara. Data analysis: Adwaiy Manerikar and Viswajit Kandula. Data collection: Azad Karim. Drafting article: Catherine Aiyuan Gao, Satoshi Watanabe, Alexandra Klonis, Vanessa Hoppner, Mark Saine, David D. Odell, Kalvin Lung, Rafael Garza‐Castillon, Samuel S. Kim, James McCauley Walter, Richard G. Wunderink, and G. R. Scott Budinger. Drafting article and approval of article: Ankit Bharat.
HUMAN STUDIES AND SUBJECTS
This study was approved by the Northwestern University Institutional Review Board (STU00207250). However, the need for patient consent for data collection was waived by the IRB as this was a retrospective study.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Ms Elena Susan, Ms Colleen McNulty, and Mrs Kweli Pryor for administrative assistance in the submission of this manuscript.
Kurihara C, Manerikar A, Gao CA, Watanabe S, Kandula V, Klonis A, et al. Outcomes after extracorporeal membrane oxygenation support in COVID‐19 and non‐COVID‐19 patients. Artif Organs. 2022;46:688–696. 10.1111/aor.14090
Funding information
Ankit Bharat is supported by National Institutes of Health HL145478, HL147290, and HL147575
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raman L, Dalton HJ. Year in review 2015: extracorporeal membrane oxygenation. Respir Care. 2016;61:986–91. [DOI] [PubMed] [Google Scholar]
- 3. Ramanathan K, Antognini D, Combes A, Paden M, Zakhary B, Ogino M, et al. Planning and provision of ECMO services for severe ARDS during the COVID‐19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020;8:518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang L, Zhang W, Yang Y, Wu W, Lu W, Xue H, et al. Application of extracorporeal membrane oxygenation in patients with severe acute respiratory distress syndrome induced by avian influenza A (H7N9) viral pneumonia: national data from the Chinese multicentre collaboration. BMC Infect Dis 2018;18:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li X, Guo Z, Li B, Zhang X, Tian R, Wu W, et al. Extracorporeal membrane oxygenation for coronavirus disease 2019 in Shanghai, China. ASAIO J. 2020;66:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith M, Vukomanovic A, Brodie D, Thiagarajan R, Rycus P, Buscher H. Duration of veno‐arterial extracorporeal life support (VA ECMO) and outcome: an analysis of the Extracorporeal Life Support Organization (ELSO) registry. Crit Care 2017;21:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fan E, Gattinoni L, Combes A, Schmidt M, Peek G, Brodie D, et al. Venovenous extracorporeal membrane oxygenation for acute respiratory failure. Intensive Care Med 2016;42: 712–24. [DOI] [PubMed] [Google Scholar]
- 8. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA 2020;323:1582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46: 846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395: 1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal membrane oxygenation support in COVID‐19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet 2020;396:1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Uemura T, Matsuda W, Ogawa T. Concerns about the timing and settings of initiating extracorporeal membrane oxygenation in patients with severe coronavirus disease 2019 pneumonia. Crit Care Med 2020;48:e1357–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang X, Cai S, Luo Y, Zhu F, Hu M, Zhao Y, et al. Extracorporeal membrane oxygenation for coronavirus disease 2019‐induced acute respiratory distress syndrome: a multicenter descriptive study. Crit Care Med 2020;48:1289–95. [DOI] [PubMed] [Google Scholar]
- 15. Zayat R, Kalverkamp S, Grottke O, Durak K, Dreher M, Autschbach R, et al. Role of extracorporeal membrane oxygenation in critically Ill COVID‐19 patients and predictors of mortality. Artif Organs 2021;45:E158–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID‐19: a retrospective cohort study. Lancet Respir Med. 2020;8:1121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kurihara C, Walter JM, Karim A, Thakkar S, Saine M, Odell DD, et al. Feasibility of veno‐venous extracorporeal membrane oxygenation without systemic anticoagulation. Ann Thorac Surg 2020;110:1209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tomasko J, Prasad SM, Dell DO, DeCamp MM, Bharat A. Therapeutic anticoagulation‐free extracorporeal membrane oxygenation as a bridge to lung transplantation. J Heart Lung Transplant 2016;35:947–8. [DOI] [PubMed] [Google Scholar]
- 19. Kurihara C, Walter JM, Singer BD, Cajigas H, Shayan S, Al‐Qamari A, et al. Extracorporeal membrane oxygenation can successfully support patients with severe acute respiratory distress syndrome in lieu of mechanical ventilation. Crit Care Med 2018;46:e1070–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Helms J, Tacquard C, Severac F, Leonard‐Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;46:1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hékimian G, Lebreton G, Bréchot N, Luyt C‐E, Schmidt M, Combes A. Severe pulmonary embolism in COVID‐19 patients: a call for increased awareness. Crit Care 2020;24:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 2018;378:1965–75. [DOI] [PubMed] [Google Scholar]
- 25. Iba T, Levy JH, Levi M, Thachil J. Coagulopathy in COVID‐19. J Thromb Haemost 2020;18:2103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Al‐Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, et al. COVID‐19 and coagulation: bleeding and thrombotic manifestations of SARS‐CoV‐2 infection. Blood 2020;136:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mang S, Kalenka A, Broman LM, Supady A, Swol J, Danziger G, et al. Extracorporeal life support in COVID‐19‐related acute respiratory distress syndrome: a EuroELSO international survey. Artif Organs 2021;45:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bharat A, Querrey M, Markov NS, Kim S, Kurihara C, Garza‐Castillon R, et al. Lung transplantation for patients with severe COVID‐19. Sci Transl Med 2020;12:eabe4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bharat A, Machuca TN, Querrey M, Kurihara C, Garza‐Castillon R Jr, Kim S, et al. Early outcomes after lung transplantation for severe COVID‐19: a series of the first consecutive cases from four countries. Lancet Respir Med. 2021;9:487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bein T, Weber‐Carstens S, Goldmann A, Müller T, Staudinger T, Brederlau J, et al. Lower tidal volume strategy (≈3 ml/kg) combined with extracorporeal CO2 removal versus ‘conventional’ protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent‐study. Intensive Care Med 2013;39:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rozencwajg S, Guihot A, Franchineau G, Lescroat M, Bréchot N, Hékimian G, et al. Ultra‐protective ventilation reduces biotrauma in patients on venovenous extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Crit Care Med 2019;47:1505–12. [DOI] [PubMed] [Google Scholar]
- 32. Boyle AJ, Sklar MC, McNamee JJ, Brodie D, Slutsky AS, Brochard L, et al. Extracorporeal carbon dioxide removal for lowering the risk of mechanical ventilation: research questions and clinical potential for the future. Lancet Respir Med. 2018;6:874–84. [DOI] [PubMed] [Google Scholar]
- 33. CDC . Coronavirus Disease 2019 (COVID‐19). Centers for Disease Control and Prevention; 2020. [Google Scholar]
- 34. MacLaren G, Fisher D, Brodie D. Preparing for the most critically ill patients with COVID‐19: the potential role of extracorporeal membrane oxygenation. JAMA 2020;323:1245–6. [DOI] [PubMed] [Google Scholar]
- 35. Cho HJ, Heinsar S, Jeong IS, Shekar K, Li Bassi G, Jung JS, et al. ECMO use in COVID‐19: lessons from past respiratory virus outbreaks—a narrative review. Crit Care 2020;24:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID‐19): pooled analysis of early reports. J Crit Care 2020;58:27–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ñamendys‐Silva SA. ECMO for ARDS due to COVID‐19. Heart Lung 2020;49:348–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grasselli G, Tonetti T, Protti A, Langer T, Girardis M, Bellani G, et al. Pathophysiology of COVID‐19‐associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020;8:1201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020;323:1574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cypel M, Feld JJ, Galasso M, Pinto Ribeiro RV, Marks N, Kuczynski M, et al. Prevention of viral transmission during lung transplantation with hepatitis C‐viraemic donors: an open‐label, single‐centre, pilot trial. Lancet Respir Med. 2020;8:192–201. [DOI] [PubMed] [Google Scholar]
- 41. Bharat A, Machuca TN, Querrey M, Kurihara C, Garza‐Castillon R Jr, Kim S, et al. Early outcomes after lung transplantation for severe COVID‐19: a series of the first consecutive cases from four countries. Lancet Respir Med. 2021;9:487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


