Abstract
We report a case of severe pseudomembranous colitis due to a toxin A− B+ strain of Clostridium difficile in an immunosuppressed patient and discuss the implications for diagnostic testing in suspected C. difficile-associated diarrhea.
CASE REPORT
A 60-year-old man was admitted to the hospital for evaluation of crampy abdominal pain and severe diarrhea. The patient's underlying medical conditions included chronic immunosuppression following liver transplantation 5 years prior to admission, chronic hepatitis C infection, end-stage renal disease requiring hemodialysis, hypothyroidism, and hypertension. His medications included prednisone, tacrolimus, mycophenolate mofetil, levothyroxine, isradipine, ranitidine, cisapride, metoprolol, and clonidine.
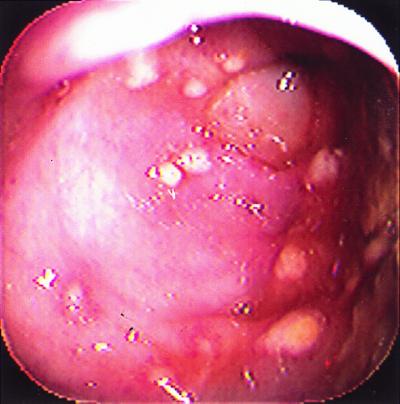
He was well until 3 weeks prior to admission, when sinusitis was diagnosed, and he was treated with a 10-day course of oral trimethoprim-sulfamethoxazole. Several days after completion of trimethoprim-sulfamethoxazole therapy, the patient developed crampy abdominal pain accompanied by fever and 10 to 15 watery stools per day without blood or mucus. The patient was admitted to the hospital for evaluation. On physical examination, he was febrile (101.5°F) and appeared ill. Moderate right-lower abdominal tenderness was noted. Relevant laboratory studies included a leukocyte count of 31,000 cells/μl (with toxic granulation), and a stool gram stain showed many leukocytes and fecal flora. An abdominal computed tomography scan showed right colon thickening but no abscess. Stool specimens for enteric pathogens and Clostridium difficile toxin were obtained, and empiric therapy with 500 mg of metronidazole orally every 6 h and 250 mg of levofloxacin intravenously every 24 h was begun. A colonoscopy to evaluate for cytomegalovirus or other opportunistic causes of colitis in this immunocompromised patient was also done, and it showed numerous whitish plaques (Fig. 1) and friable erythematous mucosa consistent with pseudomembranous colitis. All subsequent stool cultures for enteric pathogens (including Salmonella spp., Shigella spp., Yersinia spp., Campylobacter spp., Vibrio spp., Escherichia coli O157:H7, Aeromonas spp., and Plesiomonas spp.) remained negative. Likewise, three separate stool ovum and parasite exams were negative. Colon biopsies showed no histopathologic evidence of viral inclusions, and viral cultures remained negative. Although the stool toxin A enzyme immunoassay (EIA) (TechLab Tox A Test; TechLab, Blacksburg, Va.) was negative, C. difficile toxin was detected in stool by cytotoxicity assay (confirmed by neutralization with polyclonal antibody against C. difficile toxin) and by culture. On the basis of his severe clinical symptoms, detection of C. difficile toxin from stool, endoscopic findings of pseudomembranous colitis (PMC), and negative studies for other pathogens, a definitive diagnosis of C. difficile PMC was made, and therapy was switched from oral metronidazole to 125 mg of vancomycin orally every 6 h. The patient responded to therapy, with a decrease in leukocytosis and resolution of his abdominal pain and diarrhea and was discharged on the 4th hospital day in good condition. He has had no evidence of recurrence after 5 months of follow-up.
FIG. 1.
PMC. Colonoscopy photograph demonstrating multiple yellowish patches (“pseudomembranes”) and erythematous, friable mucosa.
C. difficile was cultured from stool under anaerobic conditions in brain heart infusion broth (BHI) supplemented with cefoxitin (30 mg/liter) and incubated at 37°C for 3 to 5 days. Colonial morphology on blood agar was characterized by nonhemolytic, grayish-translucent colonies. The isolate was identified as C. difficile based on biochemical reactions according to the An-Ident system (bioMérieux Vitek, Inc., Hazelwood, Mo.).
Filtrates from BHI dialysis flasks were prepared and analyzed by enzyme-linked immunosorbent assay (ELISA) and tissue culture as previously described (5). The filtrates were negative by the TechLab Tox A test. In the Tox A/B test, the filtrate was strongly positive (A450 >3.00), with an ELISA titer of 103. The filtrate was positive in the tissue culture assay and had a cytotoxic titer of 104. The cytotoxic activity was completely neutralized by polyclonal C. difficile antitoxin.
PCR using primers flanking the repeating units of the toxin A gene was done according to an in-house procedure developed at TechLab, Inc. The results indicated that the C. difficile strain from our patient contained a deletion of approximately 1.7 kb in the toxin A gene. This is the portion of the gene that encodes the epitope that reacts with the monoclonal antibody used in the diagnostic EIA kits for detection of toxin A (PCG-4 epitope). This most likely explains the toxin A− B+ nature of our patient's C. difficile isolate.
C. difficile is an important cause of antibiotic-associated diarrhea and is the causative agent of PMC (2, 6, 10). It was previously thought that toxigenic strains of C. difficile always produced both toxin A and toxin B, but recent studies have documented the presence of toxin A− B+ strains among clinical isolates (3, 4, 5, 7). However, the clinical significance and pathogenicity of toxin A− B+ strains of C. difficile are incompletely understood (5). In a recent report from Canada, al-Barrak and colleagues documented an outbreak of diarrhea associated with toxin A− B+ C. difficile at a tertiary care hospital (1). However, the molecular characterization of the C. difficile strains from these patients was not reported. In the present report, we have reported the detailed clinical course of an immunocompromised patient with severe PMC due to a toxin A− B+ strain of C. difficile and have described the molecular characterization of the strain.
The patient described herein had severe PMC due to a toxin A− B+ strain of C. difficile, with other potential causes excluded. It is possible that the patient's underlying immunosuppression allowed for severe disease to occur even with a toxin A− strain of C. difficile. Although the definitive roles of toxins A and B in C. difficile diarrhea are unknown, it is generally believed that toxin A plays the major role in C. difficile diarrhea (8). Consistent with this hypothesis, in a rabbit ileal loop assay, clinical strains of toxin A− B+ C. difficile do not demonstrate enteropathogenicity (5). Since the C. difficile strain from our patient was not tested in such an assay, it is possible that either toxin B alone was capable of causing diarrhea or the strain contained other factors capable of causing diarrhea. Furthermore, it is unknown whether a “mutant” toxin A is produced by toxin A− B+ strains and, if it is, whether such a toxin is enteropathogenic. Since previous reports have also described toxin A− B+ strains of C. difficile from symptomatic patients, it would be of interest to specifically determine whether the natures of the toxin A deletion are similar among such strains. Further analysis of such phenotypically similar strains by either additional sequencing or other methods for determining relatedness may help to address the possibility that such isolates represent a particular pathogenic clone.
Current diagnostic methods for C. difficile include culture, detection of organism-specific glutamate dehydrogenase, detection of toxin B by cell culture or cytotoxicity assay, and detection of toxin A and/or B from stool by immunoassay. Several of the widely used diagnostic tests for C. difficile rely on the detection of toxin A. The monoclonal antibody used in these assays (PCG-4) reacts with an epitope encoded by a portion of the toxin A gene that is deleted in toxin A− B+ strains (9). PCR with primers specific for the repeating subunits of toxin A demonstrated a 1.7-kb deletion in the portion of the toxin A gene that encodes the PCG-4-reacting epitope in our patient's C. difficile isolate, and this most likely explains the toxin A− B+ nature of our isolate.
Although the clinical significance of toxin A− B+ strains of C. difficile has not been well defined, an outbreak of toxin A− B+ C. difficile-associated diarrhea at a Canadian tertiary care hospital has recently been reported (1). In this report, a presumptive case was defined when stool studies were negative for toxin A by a toxin A EIA (Prima System EIA; Bartels, Inc.), but positive by either cytotoxicity assay or a combination toxin A-toxin B EIA (Tox A/B test; TechLab). If additional characterization of C. difficile strains from this outbreak confirms their toxin A− B+ nature, this report will add strong support to the hypothesis that such strains are fully pathogenic in humans.
Given our findings that a toxin A− B+ C. difficile strain is capable of causing PMC, clinical laboratories that use diagnostic methods that rely solely on detection of toxin A need to be aware that results may be falsely negative. If C. difficile-associated diarrhea is clinically suspected and toxin A is not detected, then the possibility of a toxin A− B+ strain should be considered, and further diagnostic testing should be performed. The relative frequency of toxin A− B+ clinical strains and their relative pathogenicity compared to that of toxin A+ B+ strains warrant further study.
Acknowledgments
We thank Limin Zheng, Laurie Neville, and David Lyerly of TechLab, Inc., Blacksburg, Va., for EIA testing, cytotoxicity testing, and PCR analysis.
REFERENCES
- 1.al-Barrak A, Embil J, Dyck B, Olekson K, Nicoll D, Alfa M, Kabani A. An outbreak of toxin A negative, toxin B positive Clostridium difficile-associated diarrhea in a Canadian tertiary-care hospital. Can Commun Dis Rep. 1999;25:65–69. [PubMed] [Google Scholar]
- 2.Bongaerts G P A, Lyerly D M. Role of bacterial metabolism and physiology in the pathogenesis of Clostridium difficile disease. Microb Pathog. 1997;2:253–256. doi: 10.1006/mpat.1996.0119. [DOI] [PubMed] [Google Scholar]
- 3.Brazier J S. The diagnosis of Clostridium difficile-associated disease. J Antimicrob Ther. 1998;41(Suppl. C):29–40. doi: 10.1093/jac/41.suppl_3.29. [DOI] [PubMed] [Google Scholar]
- 4.Kato H, Kato N, Katow S, Maegawa T, Nakamura S, Lyerly D M. Deletions in the repeating sequences of the toxin A gene of toxin A-negative, toxin B-positive Clostridium difficile strains. FEMS Microbiol Lett. 1999;175:197–203. doi: 10.1111/j.1574-6968.1999.tb13620.x. [DOI] [PubMed] [Google Scholar]
- 5.Kato H, Kato N, Watanabe K, Iwai N, Nakamura H, Yamamoto T, Suzuki K, Kim S-M, Chong Y, Wasito E B. Identification of a toxin A-negative, toxin B-positive Clostridium difficile by PCR. J Clin Microbiol. 1998;36:2178–2182. doi: 10.1128/jcm.36.8.2178-2182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knoop F C, Owens M, Crocker I C. Clostridium difficile: clinical disease and diagnosis. Clin Microbiol Rev. 1993;6:251–265. doi: 10.1128/cmr.6.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyerly D M, Barroso L A, Wilkins T D, Depitre C, Corthier G. Characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile. Infect Immun. 1992;60:4633–4639. doi: 10.1128/iai.60.11.4633-4639.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyerly D M, Krivan H C, Wilkins T D. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988;1:1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyerly D M, Neville L M, Evans D T, Fill J, Allen S, Greene W, Sautter R, Hnatuck P, Torpey D J, Schwalbe R. Multicenter evaluation of the Clostridium difficile TOX A/B Test. J Clin Microbiol. 1998;36:184–190. doi: 10.1128/jcm.36.1.184-190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surawicz C M, McFarland L V. Pseudomembranous colitis: causes and cures. Digestion. 1999;60:91–100. doi: 10.1159/000007633. [DOI] [PubMed] [Google Scholar]