Abstract
Background and purpose
The experience gained during the first COVID‐19 wave could have mitigated the negative impact on stroke care in the following waves. Our aims were to analyze the characteristics and outcomes of patients with stroke admitted during the second COVID‐19 wave and to evaluate the differences in the stroke care provision compared with the first wave.
Methods
This retrospective multicenter cohort study included consecutive stroke patients admitted to any of the seven hospitals with stroke units (SUs) and endovascular treatment facilities in the Madrid Health Region. The characteristics of the stroke patients with or without a COVID‐19 diagnosis were compared and the organizational changes in stroke care between the first wave (25 February to 25 April 2020) and second wave (21 July to 21 November 2020) were analyzed.
Results
A total of 550 and 1191 stroke patients were admitted during the first and second COVID‐19 waves, respectively, with an average daily admission rate of nine patients in both waves. During the second wave, there was a decrease in stroke severity (median National Institutes of Health Stroke Scale 5 vs. 6; p = 0.000), in‐hospital strokes (3% vs. 8.1%) and in‐hospital mortality (9.9% vs. 15.9%). Furthermore, fewer patients experienced concurrent COVID‐19 (6.8% vs. 19.1%), and they presented milder COVID‐19 and less severe strokes. Fewer hospitals reported a reduction in the number of SU beds or deployment of SU personnel to COVID‐19 dedicated wards during the second wave.
Conclusions
During the second COVID‐19 wave, fewer stroke patients were diagnosed with COVID‐19, and they had less stroke severity and milder COVID‐19.
Keywords: COVID‐19, intracerebral hemorrhage, ischaemic stroke, organized stroke care, outcomes
During the second COVID‐19 wave in the Madrid Region, fewer stroke patients admitted to the seven hospitals with stroke units and endovascular treatment facilities were diagnosed with COVID‐19, and they had less stroke severity and milder COVID‐19.

INTRODUCTION
The first coronavirus disease 2019 (COVID‐19) pandemic wave challenged stroke care provision worldwide, with reduced stroke admissions and rates of intravenous thrombolysis (IVT) and mechanical thrombectomy (MT) [1, 2]. This has been partly attributed to the overload of emergency medical systems (EMS) and hospitals with patients infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), with many of the human and material resources usually dedicated to stroke care reallocated to treat patients with COVID‐19 [3].
Several expert‐based recommendations on stroke care have been released to provide timely and effective care for stroke whilst ensuring stroke teams’ safety and minimizing their risk of infection [4, 5, 6, 7, 8]. Therefore, the experience gained during the first COVID‐19 wave could have mitigated the negative impact on stroke care in the following pandemic waves. In fact, a recovery in stroke hospitalization volumes was reported in the final period of the first COVID‐19 wave [1], and a smaller decline in hospitalizations occurred during the second wave than during the first compared with two pre‐pandemic periods in Germany [9]. Moreover, it has been reported that strokes experienced by patients infected with SARS‐CoV‐2 are more severe and had poorer outcomes [10, 11], but it is possible that the treatment protocols for COVID‐19, refined thanks to the results of clinical trials and observational studies conducted during the first wave, together with better organization of stroke care for infected patients, might have resulted in better outcomes.
The Madrid Stroke Network provides acute stroke care for approximately 6.5 million inhabitants, and seven hospitals are MT‐ready on a weekly rotation basis, ensuring that three hospitals provide full‐time coverage every day. During the first wave of the pandemic, some organizational changes to secure stroke care provision were arranged [12, 13]. Briefly, they included protocols to ensure access to hospital care; measures for the early recognition of COVID‐19‐positive patients; the organization of specific pathways for infected and non‐infected patients; the avoidance of unnecessary diagnostic procedures that could increase the risk of contagion; and early discharge.
Our aims were to analyze the characteristics and outcomes of patients with acute stroke admitted during the second COVID‐19 wave and to evaluate the differences in stroke care provision compared with the first wave of the pandemic in the Madrid Health Region.
METHODS
A retrospective, multicenter cohort study was conducted that included all consecutive acute stroke patients admitted to any of the seven hospitals equipped with stroke units (SUs) and endovascular treatment facilities in the Madrid Health Region [14]. The number and characteristics of stroke patients with or without a diagnosis of SARS‐CoV‐2 infection were compared and the organizational changes implemented for stroke care between the first (25 February to 25 April 2020) [10] and second (21 July to 21 November 2020) pandemic waves were recorded. The dates defining the two waves were selected considering the beginning of the rise in the incidence and the flattening of the downward slope of the curve according to the official data on the daily incidence of COVID‐19 in the Madrid Health Region [15]. No exclusion criteria, other than stroke mimics, were applied, to ensure the complete capture of all patients with acute stroke admitted to the participating hospitals.
Demographic data, risk factors, stroke characteristics and management, workflow metrics (times from onset to arrival, door‐to‐imaging, door‐to‐needle if IVT, door‐to‐puncture if MT) and stroke severity (assessed using the National Institutes of Health Stroke Scale [NIHSS] score) were recorded. The time elapsed between stroke and SARS‐CoV‐2 infection diagnosis, treatment received, chest computed tomography (CT) and laboratory data were recorded, as well as in‐hospital complications and modified Rankin Scale score at discharge.
Confirmed diagnoses of COVID‐19 disease were based on detecting SARS‐CoV‐2 nucleic acid by polymerase chain reaction (PCR) assay from nasopharyngeal/oropharyngeal swabs or detecting immunoglobulin G (IgG) or IgM serum antibodies in selected patients with high level of suspicion of COVID‐19 and a negative PCR test [16, 17]. Given the deficiency of PCR and immunoglobulin assays during the first wave, some patients might have been classified as suspected COVID‐19 cases based on their clinical symptoms, blood assessments and chest CT findings [18]. The clinical severity of COVID‐19 was classified as mild (mild symptoms), moderate (evidence of lower respiratory disease during clinical assessment or imaging with oxygen saturation [SpO2] ≥94% on room air or with low supplemental oxygen requirements) or severe (high oxygen requirements, non‐invasive or invasive ventilation or other cause of intensive care unit admission).
Lastly, a survey amongst the SU coordinators from the participating centers was conducted to analyze the organizational changes implemented for stroke care provision during the first and second COVID‐19 waves. Questions were focused on changes in infrastructure and resources, stroke code pathways and rehabilitation, and on the provision of educational and research activities.
Data management and statistical analysis
Study data were collected and managed using Research Electronic Data Capture (REDCap) [19] tools hosted at IdiPAZ Health Research Institute. IBM SPSS Statistics v21 and Stata 12.1 (Stata Corp LP) were used for the statistical analysis. Data are shown as absolute and relative frequencies for categorical variables or median and interquartile ranges (IQRs) for numerical variables. Data were compared using the chi‐squared test, Fisher's exact test, Student's t test or the Mann–Whitney U test, as appropriate.
The data recorded during the first and second COVID‐19 waves were compared and the differences between the patients with confirmed SARS‐CoV‐2 infection in both waves were analyzed. The relationship between the COVID‐19 diagnoses and stroke outcomes (death or dependence) during the second wave was analyzed using multivariate logistic regression models to adjust for confounders. Temporal trends in stroke admissions were analyzed by the autoregressive integrated moving average. Statistical significance was considered when p values were <0.05.
This study was approved by the Ethics Committee of La Paz University Hospital. As a retrospective study, the committee exempted it from the need for patient consent.
RESULTS
A total of 550 and 1191 patients with acute stroke were admitted during the first [10] and second COVID‐19 waves, with a daily admission rate (median, IQR) of 9 (5) and 9 (4), respectively. Patients admitted during the second wave were more frequently smokers and had a higher frequency of prior stroke, namely prior cerebral infarction (13.7% vs. 8.9%; p = 0.002) (Table 1).
TABLE 1.
Comparison of demographics and baseline data of patients with acute stroke admitted during the first and second COVID‐19 waves
|
First wave N = 550 |
Second wave N = 1190 |
p | |
|---|---|---|---|
| Male patients, n (%) | 311 (56.5) | 650 (54.6) | 0.243 |
| Median age, years (IQR) | 73 (61;82) | 75 (62;84) | 0.099 |
| Hypertension, n (%) | 169 (69.3) | 813 (68.6) | 0.413 |
| Diabetes, n (%) | 143 (26.5) | 317 (26.8) | 0.462 |
| Dyslipidemia, n (%) | 286 (52) | 611 (51.3) | 0.413 |
| Ischaemic cardiopathy, n (%) | 60 (10.9) | 128 (10.8) | 0.503 |
| AF, n (%) | 114 (20.8) | 270 (22.8) | 0.192 |
| COPD, n (%) | 49 (9) | 96 (8.1) | 0.303 |
| Tobacco use, n (%) | 93 (16.9) | 242 (20.6) | 0.041 |
| Alcohol abuse, n (%) | 43 (7.8) | 72 (6.1) | 0.111 |
| Prior stroke, n (%) | 84 (15.4) | 234 (19.9) | 0.014 |
| Type of stroke (final diagnosis), n (%) | |||
| TIA | 60 (10.9) | 158 (13.3) | 0.167 |
| Cerebral infarction | 406 (73.8) | 863 (72.5) | 0.553 |
| Intracerebral hemorrhage | 77 (14) | 145 (12.2) | 0.288 |
| Subarachnoid hemorrhage | 4 (0.7) | 10 (0.8) | 1 |
| Cerebral venous thrombosis | 3 (0.5) | 15 (1.3) | 0.20 |
| Type of hospital arrival, n (%) | |||
| Emergency medical services | 290 (54.3) | 559 (47.9) | 0.014 |
| Patient/relative personal transportation | 136 (25.5) | 396 (33.9) | 0.000 |
| In‐hospital stroke | 43 (8.1) | 35 (3.0) | 0.000 |
| Transfer from another hospital | 65 (12.2) | 177 (15.2) | 0.101 |
| Stroke severity | |||
| NIHSS, median (IQR) | 6 (2–16) | 5 (1–13) | 0.000 |
| Median metrics in stroke management, min (IQR) | |||
| Stroke onset to hospital arrivala | 132 (85–295) | 149 (85–323) | 0.403 |
| DTI time | 29 (17–53) | 29 (18–60) | 0.630 |
| Ward at first admission, n (%) | |||
| Acute stroke unit | 397 (72.4) | 955 (80.8) | 0.000 |
| Neurology ward | 23 (4.2) | 60 (5.1) | 0.426 |
| Non COVID‐19 medical ward | 4 (0.7) | 16 (1.4) | 0.337 |
| COVID‐19 medical ward | 52 (9.5) | 11 (0.9) | 0.000 |
| ICU | 40 (7.3) | 114 (9.6) | 0.111 |
| Emergency department wait longer than 24 h | 30 (5.5) | 17 (1.4) | 0.000 |
| Outcomes on discharge (if alive) | |||
| In‐hospital mortality, n (%) | 87 (15.9) | 118 (9.9) | 0.000 |
| Death or dependency (mRS 3–6), n (%) | 264 (48.2) | 491 (41.3) | 0.007 |
| Median stay, days (IQR) | 5 (3–10) | 5 (3–10) | 0.246 |
| Destination on discharge (if alive), n (%) | |||
| Home | 343 (62.7) | 760 (64.3) | 0.522 |
| Nursing home | 19 (3.5) | 33 (2.8) | 0.440 |
| Another hospital including rehabilitation facilities | 98 (17.9) | 271 (22.9) | 0.018 |
Abbreviations: AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; COVID‐19, coronavirus disease 2019; DTI, door‐to‐imaging; ICU, intensive care unit; IQR, interquartile range; mRS, modified Rankin Scale; NIHSS; National Institutes of Health Stroke Scale; TIA, transient ischaemic attack.
aData on 806 patients with known stroke onset date who arrived at the hospital by emergency medical systems or their own transport (255 in the first wave and 601 in the second wave). Data on patients with stroke during the first pandemic wave have been reported previously [10].
COVID‐19 was confirmed in 105 (19.1%) and 81 (6.8%) acute stroke patients during the first and second COVID‐19 waves (p < 0.001). Confirmation was based on PCR in 101 (96.2%) and 78 (96.3%) patients in the first and second waves, respectively. Other diagnostic tests such as antibody tests were less frequently used as the only confirmation test (3.8% and 3.7% in each wave). All patients in the second wave had a confirmed diagnosis, and none was managed based on clinical suspicion, whilst 19 (3.5%) patients were managed as clinically suspected COVID‐19 cases during the first wave [11].
Acute stroke management and global outcomes
Figure 1 shows the total number of new PCR‐confirmed COVID‐19 patients according to the official data in the Madrid Health Region (bottom figure) and the temporal trend in the number of stroke admissions in the participating hospitals in both waves. To note, the incidence of COVID‐19 during the first wave might be underestimated since PCR tests were restricted to symptomatic patients, whilst the second wave also included asymptomatic PCR‐confirmed COVID‐19 patients. Interestingly, in contrast to the first wave [10], the rate of stroke admissions remained stable throughout the second wave.
FIGURE 1.

Total number of new PCR‐confirmed COVID‐19 patients according to the official data in the Madrid Health Region (bottom figure) and temporal trends in the number of stroke admissions: upper left box, first COVID‐19 wave; upper right box, second COVID‐19 wave. Shaded areas represented the study periods. The y‐axis indicates the number per day
The prehospital stroke code was activated for 47.9% of patients during the second wave, a rate significantly lower than that of the first wave [10], with a higher proportion of patients arriving at hospital using their own personal transport and no differences in the percentage of secondary transfers. Interestingly, in‐hospital strokes significantly decreased during the second wave (Table 1). Figure 2 shows the temporal trends in the transport methods for arriving at the hospital during the second wave. There was a slight increase in transfers to hospitals by the EMS throughout the study period, whilst secondary transfers and arrivals by personal transportation remained stable.
FIGURE 2.
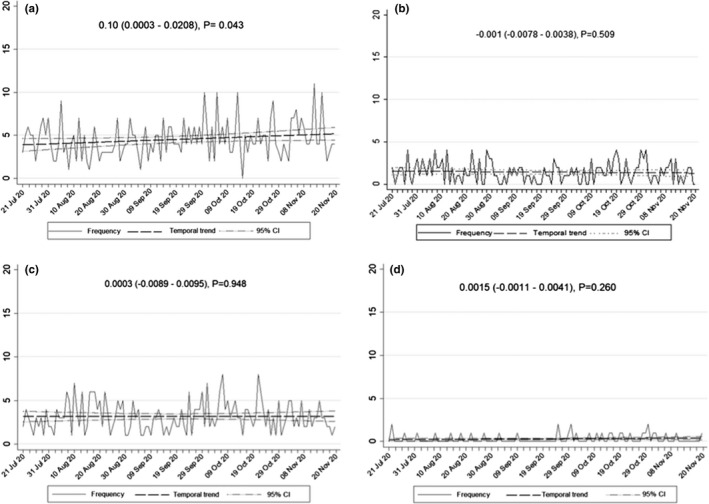
Temporal trends. (a) Stroke admissions transferred by EMS. (b) Stroke admissions transferred from other hospitals. (c) Stroke admissions arriving at the hospital by their own transport. (d) In‐hospital strokes. The y‐axis indicates the number per day
There were no significant differences in the time from stroke onset to hospital arrival, even amongst EMS transfers (median time [IQR] 117 min [76–199.75] vs. 122.5 min [83.25–209.75]; p = 0.565) or in other workflow time metrics (Table 1).
During the second wave, significantly fewer patients waited longer than 24 h in the emergency department to be admitted to a hospital ward (1.4% vs. 5.5%), and a higher proportion were first admitted to the SU (80.8% vs. 72.4%). As a result of the decrease in the number of patients with stroke with a COVID‐19 diagnosis, fewer patients were admitted to a dedicated COVID‐19 ward (Table 1). Significantly fewer chest CTs were performed during the second wave (8% vs. 52.1%; p = 0.000).
Figure 3 shows the distribution of NIHSS scores at admission and the modified Rankin Scale scores at discharge by COVID‐19 wave. Overall, stroke severity was lower during the second wave in terms of lower NIHSS score, as were in‐hospital mortality and the rate of death or dependence at discharge. The proportions of stroke types in the final diagnosis were similar (Table 1).
FIGURE 3.

Stroke severity and stroke outcomes during the first and second COVID‐19 waves. (a) Histogram and kernel density estimates of the NIHSS scores at admission. (b) Distribution of the modified Rankin Scale scores at discharge; p = 0.006
COVID‐19 characteristics
COVID‐19 was diagnosed prior to stroke in approximately half of the infected patients, with no differences between the first and second waves (45.2% vs. 54.5%, p = 0.232). During the second wave, more patients with COVID‐19 and stroke were transferred from another hospital, with the differences in other arrival methods like those of the overall sample (Table 2). Patients with COVID‐19 presented less severe strokes and milder COVID‐19 during the second wave, with significantly higher oxygen saturation at stroke onset and lower levels of acute phase reactants (Table 2). Of note, fewer patients underwent drug therapies empirically targeted against COVID‐19. Forty‐two percent and 10% of patients were administered corticosteroids and remdesivir, respectively, during the second wave; unfortunately, these data were not collected in the first wave. A higher proportion of patients were treated with MT alone during the second wave (Table 2). Overall, there was a decrease in in‐hospital mortality for patients with stroke and COVID‐19 during the second wave. The main cause of death was related to COVID‐19 pneumonia, but its proportion was significantly lower in the second wave than in the first (11.1% vs. 26.7%, p = 0.009).
TABLE 2.
Characteristics of patients with confirmed COVID‐19 diagnosis
|
First wave N = 105 |
Second wave N = 81 |
p | |
|---|---|---|---|
| Demographic data, risk factors and comorbidities | |||
| Male patients, n (%) | 66 (62.9) | 49 (60.5) | 0.429 |
| Median age, years (IQR) | 74 (63; 81.5) | 71 (59.5; 84) | 0.526 |
| Hypertension, n (%) | 78 (74.3) | 51 (63.8) | 0.083 |
| Diabetes, n (%) | 28 (28.3) | 25 (32.1) | 0.352 |
| Dyslipidemia, n (%) | 52 (49.5) | 36 (44.4) | 0.295 |
| Ischaemic cardiopathy, n (%) | 10 (9.5) | 10 (12.3) | 0.351 |
| AF, n (%) | 21 (20) | 22 (27.2) | 0.165 |
| COPD, n (%) | 17 (16.3) | 8 (9.9) | 0.144 |
| Tobacco use, n (%) | 12 (11,4) | 10 (12.5) | 0.499 |
| Alcohol abuse, n (%) | 10 (9.5) | 4 (4.9) | 0.186 |
| Prior stroke, n (%) | 10 (9.6) | 9 (11.1) | 0.462 |
| Type of hospital arrival, n (%) | |||
| Emergency medical services | 51 (49.5) | 28 (35) | 0.049 |
| Patient/relative personal transportation | 15 (14.6) | 26 (32.5) | 0.004 |
| In‐hospital stroke | 29 (28.2) | 9 (11.2) | 0.005 |
| Transfer from another hospital | 8 (7.8) | 17 (21.2) | 0.008 |
| Stroke and COVID‐19 clinical severity | |||
| NIHSS, median (IQR) | 11.5 (4 – 18.75) | 5 (2 – 15) | 0.004 |
| Mild COVID‐19, n (%) | 34 (32.7) | 43 (56.6) | 0.001 |
| Moderate COVID‐19, n (%) | 35 (33.7) | 15 (19.7) | 0.039 |
| Severe COVID‐19, n (%) | 35 (33.7) | 18 (23.7) | 0.147 |
| Baseline vital signs and laboratory findings, median (IQR) | |||
| Body temperature, °C | 36.6 (36.1–37.1) | 36.6 (36.3–36.9) | 0.963 |
| O2 saturation, % | 95 (92–97) | 96 (95–98) | 0.004 |
| Platelet count | 257,000 (180,000–345,500) | 224,000 (178,000–265,000) | 0.010 |
| Fibrinogen | 599 (492.2–740) | 490 (349.7–571.7) | <0.001 |
| D‐dimer | 1973 (850–4294) | 760 (280–1538) | <0.001 |
| C‐reactive protein | 18.7 (4–86) | 5.8 (1.1–19.9) | 0.001 |
| Type of stroke, n (%) | |||
| TIA | 3 (2.9) | 6 (7.4) | 0.181 |
| Cerebral infarction | 85 (81) | 60 (74.1) | 0.262 |
| Intracerebral hemorrhage | 14 (13.3) | 13 (16) | 0.602 |
| Subarachnoid hemorrhage | 2 (1.9) | 0 (0) | 0.506 |
| Cerebral venous thrombosis | 1 (1.0) | 2 (2.5) | 0.581 |
| Treatments for COVID‐19, n (%) | |||
| None | 16 (15.2) | 36 (44.4) | <0.001 |
| Hydroxychloroquine | 82 (78.1) | 1 (1.2) | <0.001 |
| Azithromycin | 42 (40) | 7 (8.6) | <0.001 |
| Lopinavir/ritonavir | 29 (27.6) | 0 (0) | <0.001 |
| Remdesivira | ND | 8 (9.9) | – |
| Interferon‐beta | 10 (9.5) | 0 (0) | <0.001 |
| Tocilizumab | 10 (9.5) | 8 (9.9) | 0.564 |
| Corticosteroidsa | ND | 34 (42) | – |
| Supplemental oxygen requirements | 30 (28.6) | 16 (19.8) | 0.113 |
| Mechanical ventilation | 6 (5.7) | 6 (7.4) | 0.430 |
| Non‐invasive ventilation | 3 (2.9) | 4 (4.9) | 0.359 |
| Palliative care | 12 (11.4) | 5 (6.2) | 0.165 |
| Recanalization therapies (if ischaemic stroke), n (%) | |||
| Any recanalization therapy | 30 (35.7) | 27 (45) | 0.171 |
| IVT | 13 (15.5) | 6 (10) | 0.338 |
| MT | 5 (6) | 15 (25) | 0.001 |
| IVT + MT | 12 (14.3) | 6 (10) | 0.443 |
| Median metrics in stroke management, min (IQR) | |||
| Stroke onset to hospital arrivalb | 130 (86; 176) | 154 (75.75; 434.5) | 0.190 |
| DTI time | 31.5 (19.75; 57.75) | 32 (18.5; 66.25) | 0.757 |
| DTN (if IVT) | 55 (27; 100) | 40 (19; 92) | 0.564 |
| DTP (if MT) | 110 (82; 165.75) | 73 (52.75; 137.25) | 0.121 |
| Outcomes on discharge (if alive) | |||
| In‐hospital mortality, n (%) | 44 (42.3) | 13 (16) | <0.001 |
| Death or dependency (mRS 3–6), n (%) | 75 (72.1) | 48 (60) | 0.084 |
| Median stay, days (IQR) | 12 (5; 21) | 12 (6; 20.5) | 0.197 |
| Cause of death | |||
| Related to COVID‐19 pneumonia | 28 (26.7) | 9 (11.1) | 0.009 |
| Related to stroke | 14 (13.3) | 2 (2.5) | 0.008 |
| Destination on discharge (if alive), n (%) | |||
| Home | 37 (35.6) | 45 (56.2) | 0.005 |
| Nursing home | 7 (6.7) | 3 (3.8) | 0.518 |
| Another hospital including rehabilitation facilities | 16 (15.4) | 19 (23.8) | 0.152 |
Abbreviations: AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; COVID‐19, coronavirus disease 2019; DTI, door‐to‐imaging; DTN, door‐to‐needle; DTP, door‐to‐puncture; ICU, intensive care unit; IQR, interquartile range; IVT, intravenous thrombolysis; mRS, modified Rankin Scale; MT, mechanical thrombectomy; NIHSS; National Institutes of Health Stroke Scale; TIA, transient ischaemic attack.
aNot included in the first wave database.
bData on 64 patients with known stroke onset date who arrived at the hospital by emergency medical services or their own transport (34 in the first wave and 30 in the second wave). Data on patients with stroke during the first pandemic wave have been reported previously [10].
Cerebral infarction
A total of 1269 patients were diagnosed with cerebral infarction: 406 in the first and 863 in the second wave, with confirmed COVID‐19 in 85 (20.9%) and 60 (7%) patients, respectively (p < 0.001). The demographics and comorbidities were similar in the two waves except for a higher proportion of patients with prior stroke in the second wave (19.6% vs. 14.6%; p = 0.019).
Cerebral infarction severity was lower in the second wave. The proportion of patients undergoing reperfusion therapy was similar, as were the time metrics. During the second wave, fewer patients developed in‐hospital complications (seizures, hemorrhagic transformation, COVID‐19‐related pneumonia, deep vein thrombosis and urinary tract infections). There were no differences in stroke etiology (Table S1).
Confirmed COVID‐19 diagnoses were associated with a greater risk of death or dependence at hospital discharge during both waves, even after adjusting for age, stroke severity and reperfusion treatment (odds ratio 1.87, 95% confidence interval 1.01–3.48, p = 0.011, for the first wave [10] and odds ratio 2.08, 95% confidence interval 1.08–3.98, p = 0.027, for the second wave).
Intracerebral hemorrhage
A total of 222 patients were diagnosed with intracerebral hemorrhage (77 and 145 in the first and second waves, respectively), 14 (18.2%) and 13 (9%) of whom had a confirmed COVID‐19 diagnosis (p = 0.018). Overall, the clinical profiles, time metrics and outcomes were similar (Table S2), except for a higher frequency of COVID‐19‐related pneumonia during the first wave (64.3% vs. 23.1%; p = 0.038). Patients with intracerebral hemorrhage and confirmed COVID‐19 had higher rates of in‐hospital death (50% vs. 30.8%; p = 0.267) and of death or dependence at discharge (100% vs. 92.3%; p = 0.481) during the first wave than during the second.
Organizational changes in stroke care provision
During the first COVID‐19 wave, there was a reduction in SU beds and neurology ward beds at four and seven hospitals, respectively. The SUs were reallocated to provide semicritical non‐stroke care in one hospital, and the neurological ward was moved elsewhere in three hospitals. However, the second wave had a lower impact on stroke care infrastructure, with only one hospital reporting a reduction in SU beds (Figure 4a). Moreover, neurologists and neurology residents were less frequently reallocated to COVID‐19 wards (Figure 4b). During the first wave, the availability of 24 h/7 days MT was implemented in all seven hospitals, whilst during the second wave it was not deemed necessary, and the rotatory shift between hospitals was maintained due to reduced EMS overload (Figure 4c).
FIGURE 4.

Organizational changes in stroke care provision. (a) Changes in infrastructures. (b) Changes in human resources. (c) Changes in stroke code pathways. (d) Changes in stroke rehabilitation provision. (e) Other activities (education and research)
However, the provision of rehabilitation therapies did not improve from the first to the second wave, with delays in starting physiotherapy in four hospitals and reductions in the number of in‐hospital rehabilitation‐dedicated beds. Nevertheless, none of the hospitals reported discharging disabled patients to home due to a lack of resources during the second wave, which was a limitation faced by two hospitals during the first wave (Figure 4d). In fact, a significant increase in the proportion of stroke patients discharged to other hospitals including rehabilitation facilities was found during the second wave, with no significant changes in the discharges to nursing homes (Table 1).
The COVID‐19 pandemic also affected the educational and research activities during the first wave, with a slight improvement during the second wave, although education for medical students remained restricted in all seven hospitals (Figure 4e). Fewer stroke physicians were on leave due to COVID‐19 compared with the first wave (Figure S1).
DISCUSSION
This was a multicenter cohort study aimed at describing the differences in clinical characteristics and outcomes of patients with acute stroke and COVID‐19 as well as in the impact of the COVID‐19 pandemic on acute stroke care provision between the first two waves. A decrease was found in not only concomitant COVID‐19 and stroke but also in‐hospital strokes, mainly in the COVID‐19 group, during the second wave. Furthermore, acute stroke patients with concomitant COVID‐19 experienced less severe strokes and milder COVID‐19 disease and were more frequently treated with MT alone whilst undergoing fewer drug therapies empirically targeted against COVID‐19.
The COVID‐19 pandemic changed the clinical profile of patients with acute stroke, with an increased prevalence of younger patients and more severe strokes attributed to large vessel occlusions and higher in‐hospital mortality compared with pre‐COVID controls [20]. Our study shows no changes in the demographic and risk factor profile between the first two waves, but lower stroke severity and in‐hospital mortality in the second wave. Similarly, the characteristics of COVID‐19 have changed throughout the pandemic. As previously reported, patients admitted during the second wave presented milder COVID‐19 symptoms, less inflammatory analytical profiles and a lower mortality rate, even for COVID‐19 pneumonia‐related death [21, 22]. These differences could be related to the increased awareness of patients who consulted before presenting severe symptoms, as well as to a more prepared and experienced health system. Also, there was a change in the therapeutic approach towards more targeted treatment with a more extensive use of corticosteroids, which helped improve outcomes for patients with COVID‐19 [23].
One important concern related to the COVID‐19 outbreak was its negative impact on acute stroke care, with reported reductions in ischaemic and hemorrhagic stroke hospitalizations, as well as in IVT and MT rates compared with historical controls [1, 2]. A prior report from the Madrid Stroke Network analyzing the first COVID‐19 pandemic wave showed a reduction in stroke admissions but a high rate of reperfusion therapies in patients with ischaemic stroke (43.3%), with no differences depending on COVID‐19 diagnoses [10]. This finding, together with the maintenance of door‐to‐imaging, door‐to‐needle and door‐to‐puncture times within the recommended range, suggest that the solid organized framework helped address the COVID‐19 pandemic without a major impact on acute stroke care [10]. In this new analysis, a number of the organizational changes implemented in our network during the first two waves of the COVID‐19 pandemic are described. During the second wave, it was possible to maintain better stroke care organization by creating COVID‐19 SU beds and avoiding the reduction in non‐COVID SU beds, as well as reallocating SU infrastructure and personnel to COVID‐19‐dedicated wards. These efforts might have contributed to maintaining stroke care quality and metrics. However, the provision of rehabilitation therapies has not improved from the first to second wave.
One of the challenges in providing acute stroke care was to ensure protection of stroke care workers against SARS‐CoV‐2 contagion, and specific recommendations from scientific societies and expert‐based consensus on this topic were released [13, 24, 25, 26, 27]. Earlier studies have shown that front‐line healthcare workers had a three‐fold higher risk of a positive COVID‐19 test compared with the general population, even after accounting for other risk factors [28]. This finding might have been associated with the scarcity and reuse of personal protective equipment during the first wave of the pandemic [28]. In our setting, a study from the Stroke Group of the Spanish Society of Neurology reported an 18% rate of medical leaves affecting staff neurologists and 23% of the neurology residents [29]. During the second wave, when protection measures were clearly improved, five of the seven hospitals participating in this study reported a reduction in the proportion of neurologists infected, and only one hospital showed a higher contagion rate compared with the first wave. The limitations of this analysis were that data were not collected on the serological prevalence or infection rates amongst other members of the stroke teams (stroke nurses, neurointerventionalists, rehabilitation physicians and neurology residents). It is not possible to specify whether the stroke neurologists were infected during stroke care, because of their redeployment to attend COVID‐19 patients, or due to transmission from coworkers before the universal use of masks [28, 30, 31].
Stroke education for medical students and neurology trainees in our network were negatively impacted by the COVID‐19 pandemic in line with other reports [29], and certain modifications to neurology residence training have been proposed to promote resident safety such as virtual education activities. Also, different approaches have been implemented to rapidly adapt to redeployment, service needs and trainee illness [32].
The number or the extent of the clinical trials or academic studies whose recruitment was delayed or stopped or those that could not be started because of the delayed regulatory or funding approval during the national lockdown was not specifically addressed. However, the negative impact on stroke research has been highlighted as collateral damage of the COVID‐19 pandemic [33]. Our results suggest a slight but encouraging improvement in research activities in our setting during the second wave.
Our study has several strengths. First, the study was based on a multicenter hospital registry covering all the hospitals with SU and endovascular facilities in the Madrid Health Region. Secondly, it included consecutive patients with acute stroke without selection based on type of stroke; therefore, ischaemic and hemorrhagic strokes were included. Thirdly, the outcomes of patients with COVID‐19 were compared with concurrent patients with acute stroke but without COVID‐19 who were treated using the same management protocols.
The study's main limitations were as follows. (i) There was a lack of information regarding stroke admissions to hospitals without SUs or those with SUs but without endovascular treatment facilities in the Madrid Health Region. This limitation reduces the external validity of our results, given that it is possible that some patients arrived at those hospitals on their own and without activating the stroke code. Therefore, the characteristics and impact on stroke care of the first and second COVID‐19 waves might be different in hospitals without those facilities. (ii) The time periods considered for the first and second waves differed (2 vs. 4 months) due to the different temporal profile of the two waves in our region. The strict lockdown might have contributed to a fast reduction in the total duration of the first wave, whilst no lockdown was imposed during the second wave. (iii) The underdiagnosis of COVID‐19 during the first wave due to the shortage of PCR tests, which were mainly restricted to patients with COVID‐19‐suggestive respiratory symptoms, could explain the paradox of the lower COVID‐19 incidence at the first wave compared with the second wave, in which PCR was performed for any patient arriving at the hospitals. A higher seroprevalence was detected during the first compared with the second wave in Madrid (12.6% vs. 7.7%) [34], and a national seroprevalence study highlighted the undertesting of COVID‐19 during the first wave of COVID‐19 in Spain [35]. (iv) It was not possible to evaluate the potential impact of vaccines against COVID‐19 on the stroke incidence or stroke severity since they were not yet available in Spain during either of the two COVID‐19 waves analyzed.
In conclusion, during the second COVID‐19 wave, fewer patients with stroke were diagnosed with COVID‐19, and those that were showed milder stroke and COVID‐19 severity. Despite a reduction in in‐hospital mortality of patients with COVID‐19 and stroke in the second wave, COVID‐19 remained significantly associated with poorer stroke outcomes. Learning from experience has helped us maintain a strong stroke care organization, avoiding a reduction in SU beds and reduced reallocation of the SU infrastructure and personnel to COVID‐19‐dedicated wards.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Blanca Fuentes: Conceptualization (lead); formal analysis (lead); investigation (equal); methodology (lead); writing—original draft (lead); writing—review and editing (equal). María Alonso de Leciñana: Conceptualization (lead); formal analysis (lead); investigation (equal); methodology (lead); writing—original draft (lead); writing—review and editing (equal). Ricardo Rigual: Investigation (equal); writing—review and editing (equal). Sebastián García‐Madrona: Investigation (equal); writing—review and editing. Fernando Díaz‐Otero: Investigation (equal); writing—review and editing (equal). Clara Aguirre: Investigation (equal); writing—review and editing (equal). Patricia Calleja: Investigation (equal); writing—review and editing (equal). José A. Egido: Investigation (equal); writing—review and editing (equal). Joaquín Carneado‐Ruiz: Investigation (equal); writing—review and editing (equal). Gerardo Ruiz‐Ares: Investigation (equal); writing—review and editing (equal). Jorge Rodríguez‐Pardo: Investigation (equal); writing—review and editing (equal). Angela Rodríguez‐López: Investigation (equal); writing—review and editing (equal). Álvaro Ximénez‐Carrillo: Investigation (equal); writing—review and editing (equal). Alicia De Felipe: Investigation (equal); writing—review and editing (equal). Fernando Ostos‐Moliz: Investigation (equal); writing—review and editing (equal). Guillermo González‐Ortega: Investigation (equal); writing—review and editing. Patricia Simal: Investigation (equal); writing—review and editing. Carlos I Gómez‐Escalonilla: Investigation (equal); writing—review and editing. Pablo Gomez‐Porro: Investigation (equal); writing—review and editing (equal). Borja Cabal‐Paz: Investigation (equal); writing—review and editing. Gemma Reig: Investigation (equal); writing—review and editing. Antonio Gil‐Nuñez: Investigation (equal); writing—review and editing. Jaime Masjuan: Investigation (equal); writing—review and editing. Exuperio Diez‐Tejedor: Conceptualization (lead); methodology (lead); writing—review and editing (equal).
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The support of Morote Traducciones for editing assistance is greatly appreciated. Supported by the INVICTUS‐Plus Spanish Network of the Carlos III Health Institute (ISCIII) (RD16/0019/0005).
Fuentes B, Alonso de Leciñana M, Rigual R, et al. Fewer COVID‐19‐associated strokes and reduced severity during the second COVID‐19 wave: The Madrid Stroke Network. Eur J Neurol. 2021;28:4078–4089. 10.1111/ene.15112
See commentary by A. H. Katsanos and G. Tsivgoulis on page 3881
Contributor Information
Blanca Fuentes, Email: blanca.fuentes@salud.madrid.org.
María Alonso de Leciñana, Email: malecinanacases@salud.madrid.org.
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Nogueira RG, Qureshi MM, Abdalkader M, et al. Global impact of COVID‐19 on stroke care and intravenous thrombolysis. Neurology. Published online March 25, 2021;93(23):e2824‐e2838. 10.1212/WNL.0000000000011885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nogueira RG, Abdalkader M, Qureshi MM, et al. Global impact of COVID‐19 on stroke care. Int J Stroke. 2021;16(5):573‐584. 10.1177/1747493021991652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aguiar de Sousa D, van der Worp HB. Stroke care in Europe during the COVID‐19 pandemic. Eur J Neurol. 2020;27(9):1793. 10.1111/ene.14401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qureshi AI, Abd‐Allah F, Al‐Senani F, et al. Management of acute ischemic stroke in patients with COVID‐19 infection: insights from an international panel. Am J Emerg Med. 2020;38(7):1548.e5‐1548.e7. 10.1016/j.ajem.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khosravani H, Rajendram P, Notario L, Chapman MG, Menon BK. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019 (COVID‐19) pandemic. Stroke. 2020;51(6):1891‐1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodríguez‐Pardo J, Fuentes B, Alonso de Leciñana M, et al. Acute stroke care during the COVID‐19 pandemic. Ictus Madrid Program recommendations. Neurologia. 2020;35(4):258‐263. 10.1016/j.nrl.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baracchini C, Pieroni A, Kneihsl M, et al. Practice recommendations for the neurovascular ultrasound investigations of acute stroke patients in the setting of COVID‐19 pandemic: an expert consensus from the European Society of Neurosonology and Cerebral Hemodynamics. Eur J Neurol. 2020;27(9):1776‐1780. 10.1111/ene.14334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lyden P. Temporary emergency guidance to US stroke centers during the coronavirus disease 2019 (COVID‐19) pandemic. Stroke. 2020;51(6):1910‐1912. 10.1161/STROKEAHA.120.030023 [DOI] [PubMed] [Google Scholar]
- 9. Richter D, Eyding J, Weber R, et al. A full year of the COVID‐19 pandemic with two infection waves and its impact on ischaemic stroke patient care in Germany. Eur J Neurol. 2021. 10.1111/ene.15057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fuentes B, Alonso de Leciñana M, García‐Madrona S, et al. Stroke acute management and outcomes during the COVID‐19 outbreak: a cohort study from the Madrid Stroke Network. Stroke. 2021;52(2):552‐562. 10.1161/STROKEAHA.120.031769 [DOI] [PubMed] [Google Scholar]
- 11. Ntaios G, Michel P, Georgiopoulos G, et al. Characteristics and outcomes in patients with COVID‐19 and acute ischemic stroke: the Global COVID‐19 Stroke Registry. Stroke. 2020;51(9):e254‐e258. 10.1161/STROKEAHA.120.031208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fuentes B, Alonso de Leciñana M, Calleja‐Castaño P, et al. Impact of the COVID‐19 pandemic on the organisation of stroke care. Madrid Stroke Care Plan. Neurologia. 2020;35(6):363‐371. 10.1016/j.nrl.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodríguez‐Pardo J, Fuentes B, Alonso de Leciñana M, et al. Acute stroke care during the COVID‐19 pandemic. Ictus Madrid Program recommendations. Neurología. 2020;35(4):258‐263. 10.1016/j.nrl.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alonso de Leciñana M, Fuentes B, Ximénez‐Carrillo A, et al. A collaborative system for endovascular treatment of acute ischaemic stroke: the Madrid Stroke Network experience. Eur J Neurol. 2016;23(2):297‐303. 10.1111/ene.12749 [DOI] [PubMed] [Google Scholar]
- 15. Consejería de Sanidad C de M . Casos positivos de Covid‐19 notificados por PCR. Published 2021. Accessed May 9, 2021. https://www.comunidad.madrid/sites/defahttps://www.comunidad.madrid/sites/default/files/doc/sanidad/210131_cam_covid19.pdf
- 16. Li Y, Yao L, Li J, et al. Stability issues of RT‐PCR testing of SARS‐CoV‐2 for hospitalized patients clinically diagnosed with COVID‐19. J Med Virol. 2020;92(7):903‐908. 10.1002/jmv.25786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in false‐negative rate of reverse transcriptase polymerase chain reaction‐based SARS‐CoV‐2 tests by time since exposure. Ann Intern Med. 2020;173(4):262–267. 10.7326/M20-1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y‐Y, Jin Y‐H, Ren X‐Q, et al. Updating the diagnostic criteria of COVID‐19 “suspected case” and “confirmed case” is necessary. Mil Med Res. 2020;7(1):17. 10.1186/s40779-020-00245-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2018;2019(95):103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Katsanos AH, Palaiodimou L, Zand R, et al. Changes in stroke hospital care during the COVID‐19 pandemic: a systematic review and meta‐analysis. Stroke. Published online August 4, 2021. 10.1161/STROKEAHA.121.034601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saito S, Asai Y, Matsunaga N, et al. First and second COVID‐19 waves in Japan: a comparison of disease severity and characteristics. J Infect. 2021;82(4):84‐123. 10.1016/j.jinf.2020.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iftimie S, Lopez‐Azcona AF, Vallverdu I, et al. First and second waves of coronavirus disease‐19: a comparative study in hospitalized patients in Reus, Spain. PLoS One. 2021;16(3):1‐13. 10.1371/journal.pone.0248029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. RECOVERY Collaborative Group , Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with COVID‐19. N Engl J Med. 2021;384(8):693‐704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qureshi AI, Abd‐Allah F, Al‐Senani F, et al. Management of acute ischemic stroke in patients with COVID‐19 infection: report of an international panel. Int J Stroke. 2020;15(5):540‐554. 10.1177/1747493020923234 [DOI] [PubMed] [Google Scholar]
- 25. Baracchini C, Pieroni A, Kneihsl M, et al. Practice recommendations for neurovascular ultrasound investigations of acute stroke patients in the setting of the COVID‐19 pandemic: an expert consensus from the European Society of Neurosonology and Cerebral Hemodynamics. Eur J Neurol. 2020;27(9):1776‐1780. 10.1111/ene.14334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khosravani H, Rajendram P, Notario L, Chapman MG, Menon BK. Protected code stroke. Stroke. 2020;51:4‐5. 10.1161/STROKEAHA.120.029838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leira EC, Russman AN, Biller J, et al. Preserving stroke care during the COVID‐19 pandemic: potential issues and solutions. Neurology. 2020;95(3):124‐133. 10.1212/WNL.0000000000009713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID‐19 among front‐line health‐care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5(9):e475‐e483. 10.1016/S2468-2667(20)30164-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alonso De Leciñana M, Castellanos M, Ayo‐Martín Ó, Morales A. Stroke care during the COVID‐19 outbreak in Spain: the experience of Spanish stroke units. Stroke Vasc Neurol. 2021;6(2):267‐273. 10.1136/svn-2020-000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chou R, Dana T, Buckley DI, Selph S, Fu R, Totten AM. Update alert 8: epidemiology of and risk factors for coronavirus infection in health care workers. Ann Intern Med. 2021;172(1):L21‐0143. 10.7326/L21-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baker JM, Nelson KN, Overton E, et al. Quantification of occupational and community risk factors for SARS‐CoV‐2 seropositivity among health care workers in a large U.S. health care system. Ann Intern Med. 2021;174(5):649‐654. 10.7326/m20-7145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muir RT, Gros P, Ure R, et al. Modification to neurology residency training. Neurol Clin Pract. 2021;11(2):e165‐e169. 10.1212/cpj.0000000000000894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Markus HS, Martins S. COVID‐19 and stroke—understanding the relationship and adapting services. A global World Stroke Organisation perspective. Int J Stroke. 2021;16(3):241‐247. 10.1177/17474930211005373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soriano V, Ganado‐Pinilla P, Sanchez‐Santos M, et al. Main differences between the first and second waves of COVID‐19 in Madrid, Spain. Int J Infect Dis. 2021;105:374‐376. 10.1016/j.ijid.2021.02.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pollán M, Pérez‐Gómez B, Pastor‐Barriuso R, et al. Prevalence of SARS‐CoV‐2 in Spain (ENE‐COVID): a nationwide, population‐based seroepidemiological study. Lancet. 2020;396(10250):535‐544. 10.1016/S0140-6736(20)31483-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon request.


