Summary
Patients who are severely affected by coronavirus disease 2019 (COVID‐19) may develop a delayed onset ‘cytokine storm’, which includes an increase in interleukin‐6 (IL‐6). This may be followed by a pro‐thrombotic state and increased D‐dimers. It was anticipated that tocilizumab (TCZ), an anti‐IL‐6 receptor monoclonal antibody, would mitigate inflammation and coagulation in patients with COVID‐19. However, clinical trials with TCZ have recorded an increase in D‐dimer levels. In contrast to TCZ, colchicine reduced D‐dimer levels in patients with COVID‐19. To understand how the two anti‐inflammatory agents have diverse effects on D‐dimer levels, we present data from two clinical trials that we performed. In the first trial, TCZ was administered (8 mg/kg) to patients who had a positive polymerase chain reaction test for COVID‐19. In the second trial, colchicine was given (0·5 mg twice a day). We found that TCZ significantly increased IL‐6, α‐Defensin (α‐Def), a pro‐thrombotic peptide, and D‐dimers. In contrast, treatment with colchicine reduced α‐Def and Di‐dimer levels. In vitro studies show that IL‐6 stimulated the release of α‐Def from human neutrophils but in contrast to colchicine, TCZ did not inhibit the stimulatory effect of IL‐6; raising the possibility that the increase in IL‐6 in patients with COVID‐19 treated with TCZ triggers the release of α‐Def, which promotes pro‐thrombotic events reflected in an increase in D‐dimer levels.
Keywords: thrombosis, inflammation, neutrophils
The clinical spectrum of coronavirus disease 2019 (COVID‐19) infection ranges from asymptomatic to fatal, in part related to inflammation and vascular complications. Patients who are severely affected by COVID‐19 may develop a delayed onset ‘cytokine storm’, which includes an increase in interleukin‐6 (IL‐6). This may be followed by a pro‐thrombotic state with an increase in D‐dimers, 1 development of disseminated microvascular thrombi, and pulmonary decompensation due in part to impaired vascular perfusion. 2 , 3
It had been anticipated that tocilizumab (TCZ), an anti‐IL‐6 receptor monoclonal antibody, would mitigate inflammation and coagulation in patients with COVID‐19 infection. However, clinical trials with TCZ have not provided the expected benefits. 4 , 5 Indeed, an increase in D‐dimers, a marker of fibrin turnover, has been recorded unexpectedly in TCZ‐treated patients in some trials, 6 , 7 , 8 , 9 , 10 together with a trend to a higher death rate secondary to thromboembolism in one study. 11
Although it has been suggested that the protective effect reported of TCZ in some studies might be corticosteroid‐dependent, 12 other studies showed an increase in D‐dimers in patients treated with TCZ whether or not corticosteroids were co‐administered. 10 The beneficial effect of colchicine, another anti‐inflammatory medication used to treat patients with COVID‐19 has also been inconsistent, 13 , 14 , 15 , 16 but in contrast to TCZ, treatment reduced D‐dimer levels. 14 , 17
We sought to understand how two anti‐inflammatory agents, TCZ and colchicine, differ in their effects on D‐dimer levels with the hope that these results might be used to improve efficacy in patients with COVID‐19, rheumatoid arthritis and other inflammatory conditions. In the present study, we present results of two clinical trials in addition to in vitro data to help to elucidate the mechanism underlying the seeming paradoxical effect of TCZ and the discrepancy between the effect of TCZ and colchicine.
Methods
Patients aged ≥18 years admitted to Hadassah Hospital with a positive PCR test for COVID‐19 were enrolled. The studies were approved by the Helsinki Research Ethics Commissions (#0204‐20, #0055‐20 and #0224‐20). In these randomised, controlled, open‐label clinical trials, patients were enrolled after written consent and assigned consecutively to the treatment or to the no treatment arm. All patients received the same standard care otherwise.
Tocilizumab clinical trial
Tocilizumab was administered to patients entering the intensive care unit with severe acute respiratory failure. A total of 17 patients [mean (SD) age 62·2 (10·9) years; 11 males and six females] allocated to TCZ received the drug as a single intravenous (IV) infusion over 60 min (8 mg/kg up to total dose of 800 mg) in addition to standard care.
Colchicine clinical trial
Colchicine was given to 16 patients [mean (SD) age 51·4 (6·5) years, nine males and seven females] admitted to the Department of Internal Medicine with ‘moderate’ symptoms as previously defined. 18 Patients in the treatment arm were given colchicine 1 mg twice a day on day 1 and 0·5 mg twice a day for a mean (SD) of 7 (4) days thereafter, in addition to standard care.
Measurement of plasma components and statistical analyses were performed as previously reported. 18 For more details, see 18 and the Supplementary Material.
Results and discussion
We previously reported that α‐Def promotes coagulation in vivo, an effect that was inhibited by colchicine, which inhibits its release from neutrophils. 19 We also reported that plasma levels of α‐Def are increased in patients with COVID‐19, which is associated with an increase in IL‐6 and D‐dimers. 18 Therefore, it would be expected that inhibiting signal transduction in neutrophils by blocking IL‐6 receptors (IL‐6R) would decrease α‐Def and D‐dimers.
However, in line with the data of others, 7 , 10 we observed that IL‐6 levels measured 24 h after an initial IV dose of TCZ increased significantly compared with pretreatment levels (Fig 1A), presumably as a consequence of inhibiting uptake of the cytokine by its receptors. The increase in IL‐6 was accompanied by an increase in D‐dimers (Fig 1B) and, in line with our previous data, 18 by an increase in plasma α‐Def (Fig 1C). Plasma levels of all three analytes declined by 2 days after TCZ administration (Fig 1A–C). The difference in our data from those reported by Nisio et al., 20 who reported a decrease in D‐dimers after TCZ treatment, may be due to differences in populations studied, stage of disease or treatment modalities.
Fig 1.
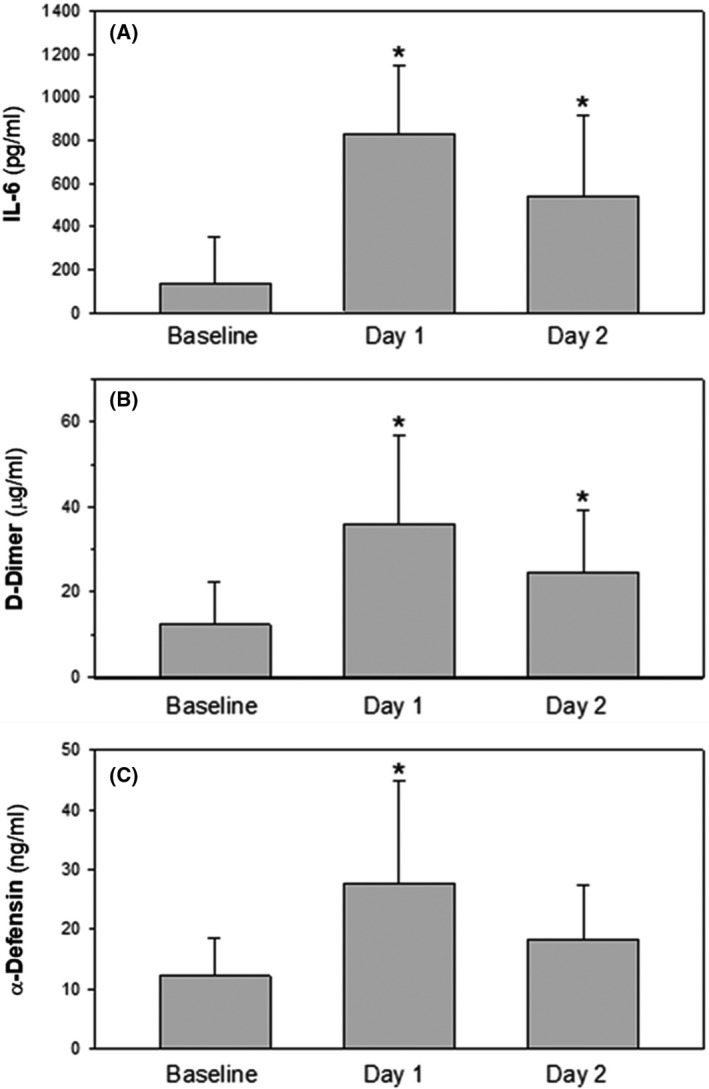
Plasma levels of interleukin‐6 (IL‐6) (A), D‐dimers (B) and α‐Defensins (C) in patients with COVID‐19 infection at baseline and 1 and 2 days after the administration of tocilizumab (8 mg/kg). Data are presented as mean ± SD (n = 17; *P < 0·001 vs. baseline, ANOVA with Newman Keuls test for post hoc comparisons).
The beneficial effect of colchicine, another anti‐inflammatory medication, has also been inconsistent in patients with COVID‐19· 13 , 14 , 15 , 16 However, in contrast to the increase in D‐dimers seen after TCZ, treatment with colchicine reduced D‐dimer levels. 14 , 17
To examine the relationship between α‐Def and D‐dimers in COVID‐19 infection in greater detail, we first asked if the decrease in D‐dimers seen in patients with COVID‐19 treated with colchicine is associated with a reduction α‐Def. To do so, we treated patients with COVID‐19 with oral colchicine (0·5 mg × 2 day). Figure 2A shows that, in contrast to TCZ, colchicine led to a decrease in α‐Def and D‐dimers after 1 day of treatment, consistent with a relationship between increased in α‐Def and pro‐thrombotic processes.
Fig 2.
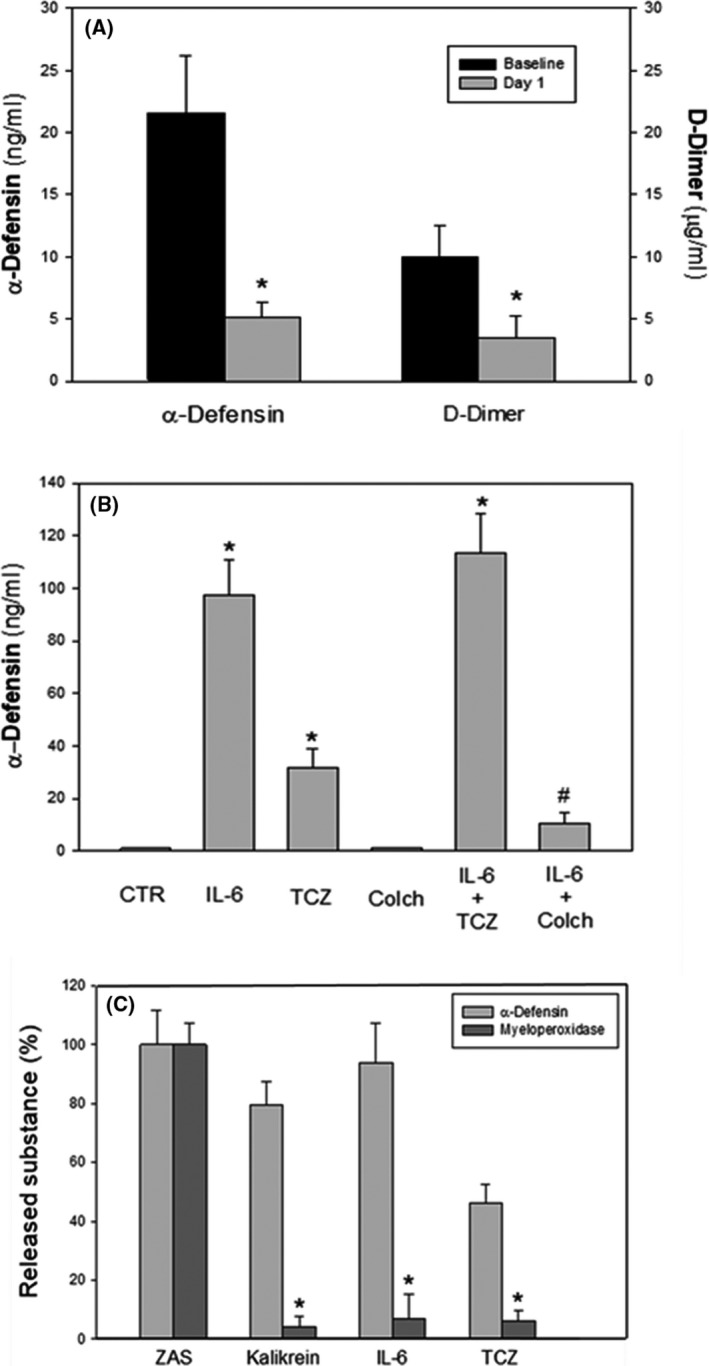
(A) Plasma levels of α‐Defensin (α‐Def) and D‐dimers in patients with COVID‐19 at baseline and 1 day following the administration of colchicine (colch; 0·5 mg twice a day). Data are presented as mean ± SD (n = 16; *P < 0·001 vs. baseline, paired t‐test). (B) The impact of tocilizumab (TCZ; 100 µg/ml 21 ) for 30 min prior to addition of interleukin‐6 (IL‐6) and colch (10 nmol/l) on the IL‐6‐mediated (100 ng/ml 21 ) release of α‐Def from human neutrophils in vitro. The result (mean ± SD) of three experiments, each done in triplicate, is shown (P < 0·0001). (C) Effects of IL‐6 and TCZ on the release of myeloperoxidase and α‐Def from neutrophils. The experiment was performed as in (B). TCZ (100 µg/ml) or IL‐6 was incubated with human neutrophils and the release of myeloperoxidase and α‐Def was measured. The effects of zymogen‐activated serum (ZAS) 25 and kallikrein (Kalikrein) 19 were used as controls for the release of myeloperoxidase and α‐Def or α‐Def alone respectively.
We then explored the difference between the effects of TCZ and colchicine on neutrophil activation on the release of α‐Def. As previously reported, 18 IL‐6 alone stimulated the release of pro‐thrombotic α‐Def peptides from human neutrophils (Fig 2B). Surprisingly, TCZ did not inhibit the stimulatory effect of IL‐6. Indeed, TCZ alone, i.e. in the absence of IL‐6, stimulated the release of α‐Def from neutrophils (Fig 2B). This raises the possibility that the increase in IL‐6 in patients with COVID‐19 treated with TCZ together with a direct effect of the antibody on neutrophil degranulation triggers the increase in α‐Def that promotes pro‐thrombotic events as reflected by an increase in D‐dimers. Activation of neutrophils by TCZ may also help to contribute to the transient neutropenia sometimes observed after treatment. 21 , 22
We then compared the effect of TCZ and IL‐6 on release of myeloperoxidase (MPO), a lysosomal protein released from azurophilic granules during neutrophil degranulation as a second marker of activation as compared with zymogen‐activated serum (ZAS), known to cause the release of α‐Def and MPO. 23 IL‐6, TCZ and ZAS each stimulated the release of α‐Def, but only ZAS stimulated the release of MPO (Fig 2C). The pattern of granule released by IL‐6 and TCZ is similar to that induced by kallikrein (Fig 2C). 19 Clearly, additional studies are needed to distinguish between the signalling pathways responsible for these different response patterns and their role under physiological and pathological conditions. The mechanism by which IL‐6 stimulates neutrophils in the presence of TCZ is also unclear and will require further study as well. In theory, TCZ may act as a mixed agonist‐antagonist of IL‐6R that does not completely inhibit IL‐6 binding, induces a cryptic site for IL‐6 binding to IL‐6R or IL6‐IL‐6R complexes may signal through another signal transduction pathway.
In contrast to TCZ and as previously reported, colchicine 18 inhibited IL‐6‐mediated release of α‐Def from neutrophils (Fig 2B ). These data support the hypothesis that an increase of α‐Def released from neutrophils promotes coagulation in patients with COVID‐19, evident by an increase in D‐dimer levels, 18 and that by inhibiting the release α‐Def from neutrophils (Fig 2B), colchicine attenuates pro‐thrombotic pathways with a consequent decline in D‐dimers (Fig 2A). This hypothesis is in line with previous data showing that colchicine decreases the coagulation tendency and lowers D‐dimers in patients with familial Mediterranean fever (FMF), 24 as well as in mice expressing α‐Def in their neutrophils. 19
In summary, our present data suggest that the unexpected incomplete benefit of TCZ in patients with COVID‐19 may be due to the finding that the antibody is only partially able to inhibit IL‐6‐mediated inflammatory activity 7 and fails to block the release of α‐Def and the subsequent acceleration of coagulation represented by increased D‐dimers. These results suggest that it may be beneficial to combine colchicine with TCZ to attenuate inflammation‐associated thrombosis, a question that could be addressed through a randomised trial of TCZ alone versus TCZ and colchicine. Furthermore, additional basic studies are required to understand the effect of IL‐6 on neutrophil activation, release of α‐Def and how is this process affected by TCZ.
Author contributions
Abd A.‐R. Higazi conceived the study; Abd A.‐R. Higazi and Suhair Abdeen designed the experiments; Abd A.‐R. Higazi, Suhair Abdeen, Rami Abu‐Fanne, Douglas B. Cines, Samuel N. Heyman and Khalil Bdeir analysed the data; Suhair Abdeen, Rami Abu‐Fanne, Emad Maraga and Mohamed Higazi performed and analysed the experiments; Abd A.‐R. Higazi, Samuel N. Heyman, Douglas B. Cines and Khalil Bdeir wrote the paper.
Conflict of interest
None of the authors has a relevant conflict of interest.
Supporting information
Supplementary Material: Methods: Clinical criteria, ex vivo and in vitro experiments and statistical analysis.
Acknowledgments
This research was supported by grants from the Israeli Science Foundation ISF: 930/04 and 3959/19 (Abd A.‐R. Higazi) and R01DK113142 (Douglas B. Cines and Khalil Bdeir).
References
- 1. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Connors J, Levy J. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zaim S, Chong J, Sankaranarayanan V, Harky A. COVID‐19 and multiorgan response. Curr Probl Cardiol. 2020;45:100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stone JH, Frigault MJ, Serling‐Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of tocilizumab in patients hospitalized with Covid‐19. N Engl J Med. 2020;383:2333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. Lancet. 2021;397:1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Griffin DO, Jensen A, Khan M, Chin J, Chin K, Saad J, et al. Pulmonary embolism and increased levels of d‐dimer in patients with coronavirus disease. Emerg Infect Dis. 2020;26:1941–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, et al. Tocilizumab for the treatment of severe COVID‐19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19:102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khiali S, Khani E, Entezari‐Maleki T. A comprehensive review of tocilizumab in COVID‐19 acute respiratory distress syndrome. J Clin Pharmacol. 2020;60:1131–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keske Ş, Tekin S, Sait B, İrkören P, Kapmaz M, Çimen C, et al. Appropriate use of tocilizumab in COVID‐19 infection. Int J Infect Dis. 2020;99:338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rubio‐Rivas M, Ronda M, Padulles A, Mitjavila F, Riera‐Mestre A, García‐Forero C, et al. Beneficial effect of corticosteroids in preventing mortality in patients receiving tocilizumab to treat severe COVID‐19 illness. Int J Infect Dis. 2020;101:290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan KH, Patel B, Podel B, Szablea ME, Shaaban HS, Guron G, et al. Tocilizumab and thromboembolism in COVID‐19: a retrospective hospital‐based cohort analysis. Cureus. 2021;13:e15208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rubin EJ, Longo DL, Baden LR. Interleukin‐6 receptor inhibition in Covid‐19 – cooling the inflammatory soup. N Engl J Med. 2021;384:1564–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tardif JC, Bouabdallaoui N, L’Allier PL, Gaudet D, Shah B, Pillinger MH, et al. Colchicine for community‐treated patients with COVID‐19 (COLCORONA): a phase 3, randomised, double‐blinded, adaptive, placebo‐controlled, multicentre trial. Lancet Respir Med. 2021;9:924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deftereos SG, Giannopoulos G, Vrachatis DA, Siasos GD, Giotaki SG, Gargalianos P, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO‐19 randomized clinical trial. JAMA Netw Open. 2020;3:e2013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scarsi M, Piantoni S, Colombo E, Airó P, Richini D, Miclini M, et al. Association between treatment with colchicine and improved survival in a single‐centre cohort of adult hospitalised patients with COVID‐19 pneumonia and acute respiratory distress syndrome. Ann Rheum Dis. 2020;79:1286–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. RECOVERY Collaboration Group . Colchicine in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. medRxiv 2021. (preprint). DOI: 10.1101/2021.05.18.21257267 [DOI] [PMC free article] [PubMed]
- 17. Kow CS, Hasan SS. Colchicine as an adjunct to heparin for prophylaxis of venous thromboembolism in patients with COVID‐19. Rheumatol Int. 2021;41:677–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abdeen S, Bdeir K, Abu‐Fanne R, Maraga E, Higazi M, Khurram N, et al. Alpha‐defensins: risk factor for thrombosis in COVID‐19 infection. Br J Haematol. 2021;194:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abu‐Fanne R, Stepanova V, Litvinov RI, Abdeen S, Bdeir K, Higazi M, et al. Neutrophil α‐defensins promote thrombosis in vivo by altering fibrin formation, structure, and stability. Blood. 2019;133:481–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di Nisio M, Potere N, Candeloro M, Spacone A, Pieramati L, Ferrandu G, et al. Interleukin‐6 receptor blockade with subcutaneous tocilizumab improves coagulation activity in patients with COVID‐19. Eur J Intern Med. 2021;83:34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wright HL, Cross AL, Edwards SW, Moots RJ. Effects of IL‐6 and IL‐6 blockade on neutrophil function in vitro and in vivo. Rheumatology (Oxford). 2014;53:1321–31. [DOI] [PubMed] [Google Scholar]
- 22. Lok LS, Farahi N, Juss JK, Loutsios C, Solanki CK, Peters AM, et al. Effects of tocilizumab on neutrophil function and kinetics. Eur J Clin Invest. 2017;47:736–45. [DOI] [PubMed] [Google Scholar]
- 23. Abu‐Fanne R, Maraga E, Abd‐Elrahman I, Hankin A, Blum G, Abdeen S, et al. α‐Defensins induce a post‐translational modification of Low Density Lipoprotein (LDL) that promotes atherosclerosis at normal levels of plasma cholesterol. J Biol Chem. 2016;291:2777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Demirel A, Celkan T, Kasapcopur O, Bilgen H, Ozkan A, Apak H, et al. Is Familial Mediterranean Fever a thrombotic disease or not? Eur J Pediatr. 2008;167:279–85. [DOI] [PubMed] [Google Scholar]
- 25. Higazi AA, Barghouti II, Ayesh SK, Mayer M, Matzner Y. Inhibition of neutrophil activation by fibrinogen. Inflammation. 1994;18:525–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material: Methods: Clinical criteria, ex vivo and in vitro experiments and statistical analysis.


