Abstract
Objectives
Myocardial injury during active coronavirus disease‐2019 (COVID‐19) infection is well described; however, its persistence during recovery is unclear. We assessed left ventricle (LV) global longitudinal strain (GLS) using speckle tracking echocardiography (STE) in COVID‐19 recovered patients and its correlation with various parameters.
Methods
A total of 134 subjects within 30–45 days post recovery from COVID‐19 infection and normal LV ejection fraction were enrolled. Routine blood investigations, inflammatory markers (on admission) and comprehensive echocardiography including STE were done for all.
Results
Of the 134 subjects, 121 (90.3%) were symptomatic during COVID‐19 illness and were categorized as mild: 61 (45.5%), moderate: 50 (37.3%) and severe: 10 (7.5%) COVID‐19 illness. Asymptomatic COVID‐19 infection was reported in 13 (9.7%) patients. Subclinical LV and right ventricle (RV) dysfunction were seen in 40 (29.9%) and 14 (10.5%) patients, respectively. Impaired LVGLS was reported in 1 (7.7%), 8 (13.1%), 22 (44%) and 9 (90%) subjects with asymptomatic, mild, moderate and severe disease, respectively. LVGLS was significantly lower in patients recovered from severe illness(mild: ‐21 ± 3.4%; moderate: ‐18.1 ± 6.9%; severe: ‐15.5 ± 3.1%; p < 0.0001). Subjects with reduced LVGLS had significantly higher interleukin‐6 (p < 0.0001), C‐reactive protein (p = 0.001), lactate dehydrogenase (p = 0.009), serum ferritin (p = 0.03), and troponin (p = 0.01) levels during index admission.
Conclusions
Subclinical LV dysfunction was seen in nearly a third of recovered COVID‐19 patients while 10.5% had RV dysfunction. Our study suggests a need for closer follow‐up among COVID‐19 recovered subjects to elucidate long‐term cardiovascular outcomes.
Keywords: COVID‐19, global longitudinal strain, speckle tracking echocardiography, subclinical left ventricle dysfunction
1. INTRODUCTION
The ongoing coronavirus disease‐2019 (COVID‐19) pandemic continues to cause considerable morbidity and mortality worldwide. 1 Cardiovascular manifestations in COVID‐19 include myocarditis, acute coronary syndrome, cardiac arrhythmias, heart failure, cardiogenic shock and venous thromboembolism. 2 , 3 Acute cardiac injury (defined by elevated cardiac troponin levels) has been reported in 8–28% of patients with COVID‐19 and is associated with worse clinical outcomes. 2 , 4 , 5 , 6 In addition, few studies have observed subclinical myocardial dysfunction in COVID‐19 patients. 7 , 8 However, there is paucity of data regarding myocardial dysfunction in COVID‐19 recovered patients.
Initial studies using cardiac magnetic resonance (CMR) imaging have shown relatively high prevalence of subclinical left ventricle (LV) dysfunction in recovered COVID‐19 patients. 9 , 10 , 11 Despite being the “gold standard”, CMR is often limited by its availability, longer imaging time and feasibility especially in light of ongoing pandemic. Hence, there is an urgent need for a systematic study to identify the prevalence of subclinical myocardial dysfunction using simple bedside tools. Global longitudinal strain (GLS) obtained by two‐dimensional speckle tracking echocardiography (STE) is a sensitive and validated method for detection of subclinical LV dysfunction compared with ejection fraction (EF). 12 In the present study, we assessed LVGLS in recovered COVID‐19 patients and its correlation with various laboratory parameters and inflammatory markers.
2. METHODS
This was a prospective single center study in a tertiary care center of North India from August 2020 to March 2021. A total of 527 consecutive subjects recently recovered (within 30–45 days) from COVID‐19 infection were screened. All these subjects were COVID‐19 positive in the past using reverse transcription‐polymerase chain reaction (RT‐PCR) swab test. Patients were considered recovered by the discharge criteria (normal temperature lasting longer than 3 days, resolved respiratory symptoms and two consecutive negative RT‐PCR test results separated by at least 24 h) and were isolated for a minimum of 14 days. Subjects with age greater than 85 years (n = 28), pre‐existing cardiovascular disease [coronary artery disease (n = 55), dilated (n = 16), restrictive (n = 1) and hypertrophic cardiomyopathies (n = 1)], uncontrolled hypertension [(stage 2 or more); n = 52], uncontrolled diabetes mellitus [(HbA1c ≥ 8); n = 70], prior cerebrovascular disease (n = 31), chronic liver (n = 15) or kidney disease [(eGFR < 30 ml/min/m2); n = 20], chronic obstructive pulmonary disease (n = 46) and poor echo window (n = 42) were excluded as these are known to impair LV strain analysis. 13 In addition, patients with impaired LV function (n = 16) on baseline transthoracic echocardiography (TTE) were also excluded. Post exclusion, 134 subjects were finally enrolled and underwent sequential testing (Figure 1, Central illustration). Baseline clinical and biochemical parameters including hemogram, liver and kidney function tests as well as inflammatory markers such as C‐reactive protein (CRP), serum ferritin, interleukin (IL)‐6, lactate dehydrogenase (LDH) and D‐dimer were obtained at the time of admission during COVID‐19 infection for all subjects. Data for cardiac troponins during index hospitalization were available for 69 (51.5%) COVID‐19 recovered patients.
FIGURE 1.
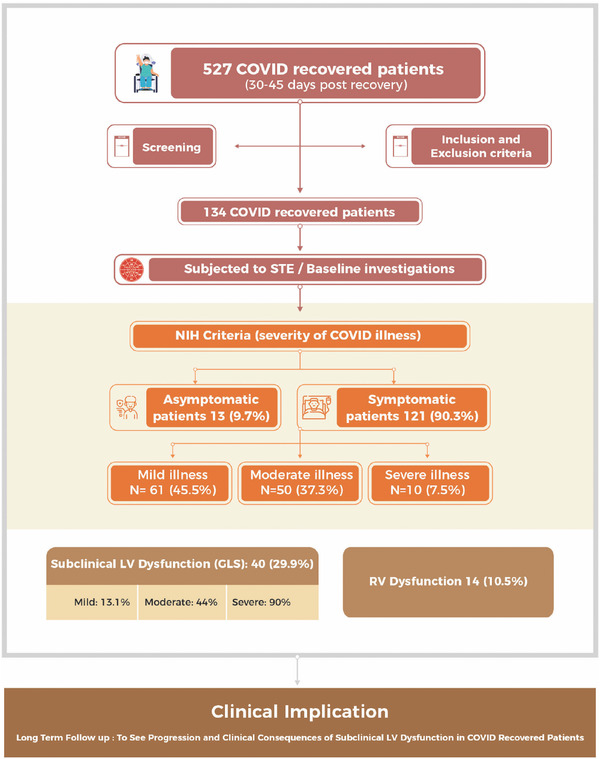
Central illustration of strain analysis of 134 COVID‐19 recovered subjects
A TTE (Philips EPIQ 7, USA) was performed and all baseline echocardiographic parameters such as LV end diastolic dimension (LVEDd), LV end systolic dimension (LVESd), left atrial (LA) size, LVEF (modified Simpson's method) and LV diastolic function were recorded as per the American Society of Echocardiography (ASE) guidelines. 14 , 15 , 16 LVGLS was determined using two dimensional‐STE. Three standard apical views (apical two chamber (A2C), apical three chamber (A3C), apical four chamber (A4C) views) were obtained at rest and for each of these views, well‐defined cardiac cycles were acquired and stored for offline analysis using the Automated Cardiac Motion Quantification (aCMQ) feature on the Qlab software (QLab Cardiac Analysis ver.10, Philips Healthcare Inc). The mean GLS was calculated by averaging the peak GLS values of the three apical views. A 17‐segment polar plot (Bulls’ eye) provided visual and quantitative representations of regional LV functions by plotting color‐coded values of peak‐systolic strain. 17 In addition, LVGLS was also computed for 20 age‐ and gender‐matched healthy individuals who served as controls. A LVGLS value less than that of the mean value of control population was considered abnormal. Right ventricular (RV) systolic function was also assessed using (a) Tricuspid annular plane systolic excursion (TAPSE), (b) Tissue Doppler systolic velocity of the tricuspid annulus (S’) and complimented by assessment of RV basal diameter. Echocardiogram and interpretation of strain imaging was done by two independent reviewers who were blinded to the study data. The study was approved by the Institutional ethics committee (F.1/IEC/MAMC/(82/10/ 2020/No.77) and a written informed consent was obtained from each patient prior to enrollment.
3. STATISTICAL ANALYSIS
Continuous data was expressed as mean ± standard deviation (SD) and categorical data was represented as proportions. Normality of distribution of continuous variables were assessed using the Kolmogorov‐Smirnov test. Comparison of means of continuous variables was done using Student's t‐test or Mann‐Whitney U test as appropriate while Fisher exact test or χ2 test was used for categorical variables. In addition, analysis of variance (ANOVA) or Kruskal Wallis with post‐hoc analysis was used to compare mean values of continuous variables between the three groups based on severity of COVID‐19. Correlation between inflammatory markers, cardiac troponins and LVGLS was done using Spearman correlation coefficient test. Multivariate logistic regression analysis was done to determine factors independently associated with reduced GLS. To investigate for inter‐observer variability for LVGLS, analysis of 20 random subjects was done by two independent investigators who were blinded to the clinical data. For intra‐observer variability, repeat offline LVGLS estimation was done ten days later in 20 randomly selected patients. The inter‐class correlation coefficients (ICCs) were calculated with point estimates and 95% confidence intervals (CIs) being reported. A two‐sided p value of < 0.05 was considered to be statistically significant. SPSS version 24.0 (IBM Corp, Armonk, NY, USA) and GraphPad Prism version 8.0.0 (GraphPad Software, San Diego, CA, USA) software were used for statistical analysis.
4. RESULTS
The baseline demographic characteristics of 134 subjects is depicted in Table 1. The mean age of the enrolled patients was 51.2 ± 12.4 years with 85 (63.4%) of them being males. Co‐morbidities such as hypertension and diabetes mellitus were reported in 37 (27.6%) and 23 (17.2%) subjects, respectively. A total of 121 (90.3%) subjects reported symptoms during COVID‐19 infection. Symptomatic patients were classified into mild [n = 61 (45.5%)], moderate [n = 50 (37.3%)] or severe [n = 10 (7.5%)] illness as per the National Institute of Health (NIH) severity criteria. 18 During the active COVID‐19 infection, fever, cough and dyspnea were the predominant symptoms observed in 95 (70.8%), 73 (54.4%) and 51 (38.1%) patients, respectively. During the post COVID‐19 recovery phase, 62 (46.2%) patients were symptomatic with palpitations, dyspnea and fatigue being the predominant symptoms reported in 29 (21.6%), 23 (17.2%), 18 (13.4%) subjects, respectively. The mean duration for follow‐up echocardiogram post COVID‐19 recovery was 36.4 ± 4.6 days.
TABLE 1.
Demographic and baseline clinical profile of study subjects and the controls
| Baseline parameters | Post COVID‐19 (n = 134) | Controls (n = 20) | p‐value |
|---|---|---|---|
| Age (years) | 51.2 ± 12.4 | 47.2 ± 10.4 | 0.21 |
| Male sex | 85 (63.4%) | 12 (60%) | 0.77 |
|
Comorbidities |
|||
| Hypertension | 37 (27.6%) | ‐ | |
| Diabetes mellitus | 23 (17.2%) | ‐ | |
| Smoking | 5 (3.7%) | ‐ | |
| Durations of symptoms (days) | 3.8 ± 1.6 | ‐ | |
| Duration of hospitalization (days) | 8.4 ± 7.4 | ‐ | |
| Heart Rate (per minute) | 81.4 ± 10.5 | 79.1 ± 2.7 | <0.0001 |
| Systolic blood pressure (mm Hg) | 132.8 ± 14.4 | 126.2 ± 2.7 | <0.0001 |
| Diastolic blood pressure (mm Hg) | 81.6 ± 7.5 | 78.7 ± 2.7 | 0.001 |
| Symptoms on admission | |||
| Fever | 95 (70.8%) | ||
|
Cough |
73 (54.4%) |
‐ |
|
| Dyspnea | 51 (38.1%) | ‐ | |
| Sore throat | 21 (15.7%) | ‐ | |
| Myalgias | 19 (14.2%) | ‐ | |
| Chest pain | 17 (12.7%) | ‐ | |
| Fatigue | 16 (11.9%) | ‐ | |
| Loss of smell | 13 (9.7%) | ‐ | |
| Palpitation | 9 (6.7%) | ‐ | |
| Loss of taste | 7 (5.2%) | ‐ | |
| Headache | 7 (5.2%) | ‐ | |
| Post COVID‐19 recovery symptoms | 62 (46.2%) | ‐ | |
| Palpitations | 29 (21.6%) | ‐ | |
| Dyspnea | 23 (17.2%) | ‐ | |
| Fatigue | 18 (13.4%) | ‐ | |
| Cough | 14 (10.4%) | ‐ | |
| Syncope | 3 (2.2%) | ‐ | |
| Pedal oedema | 3 (2.2%) | ‐ | |
| Fever | 1 (.7%) | ‐ | |
| Severity of COVID‐19 illness | |||
| Asymptomatic | 13 (9.7%) | ‐ | |
| Mild | 61 (45.5%) | ‐ | |
| Moderate | 50 (37.3%) | ‐ | |
| Severe | 10 (7.5%) | ‐ | |
| LV‐GLS (%) | −19.7 ± 4.6 | −19.2 ± 1.5 | 0.001 |
Abbreviation : LVGLS, left ventricle global longitudinal strain.
Echocardiographic profile of COVID‐19 recovered subjects is depicted in Supplementary Table S1. Mitral regurgitation was present in 12 (8.9%) subjects while tricuspid regurgitation was reported in 27 (20.1%) subjects. Diastolic dysfunction was reported in 38 (28.3%) subjects of whom 32 (23.8%) had grade I, 5 (3.7%) had grade II and 1 (.7%) had grade III diastolic dysfunction. Right ventricle systolic pressure (RVSP) was raised in 5 (3.7%) subjects while RV dysfunction (defined as TAPSE < 17 mm and RV S’ velocity < 9.5 cm/s) was observed in 14 (10.4%) subjects. The mean LVGLS for entire study cohort was ‐19.7 ± 4.6% while that of control population was ‐19.2 ± 1.5% with a significant difference between the two groups (p = 0.001). Impaired LVGLS was reported in 40 (29.9%) subjects.
4.1. Echocardiographic parameters based on severity of COVID‐19 illness
There was no significant difference in the prevalence of hypertension and diabetes mellitus between the three groups. Patients in severe COVID‐19 illness group had significantly higher levels of inflammatory markers such as CRP (p = 0.006) and IL‐6 (p = 0.002), total leucocyte count (p = 0.003) and lower hemoglobin levels (p = 0.004) as compared to those with mild disease. There was no significant difference with respect to conventional echocardiographic parameters (such as LVEF, LVEDd, LVESd, LA size) and diastolic dysfunction among the three groups. Of the 13 asymptomatic patients, reduced LVGLS was observed in only one (7.7%) patient. A significant difference was noted between mean LVGLS values between asymptomatic (‐21.8 ± 3.3%) and symptomatic (‐19.5 ± 4.6%) subjects (p = 0.03). Among symptomatic patients, there was a significant difference of mean LVGLS values among the three groups (mild: ‐21 ± 3.4%; moderate: ‐18.1 ± 6.9%; severe: ‐15.5 ± 3.1%; p < 0.0001) as depicted in Supplementary Table S2. The ICC for LVGLS measurement was .95 [95% CI: .84–.98] for inter‐observer agreement and .97 (95% CI: .94–.99) for intra‐observer agreement, indicating good inter‐observer and intra‐observer correlations. Representative bull's eye plot of LVGLS in mild (A), moderate (B) and severe (C) COVID‐19 subjects is illustrated in Figure 2.
FIGURE 2.
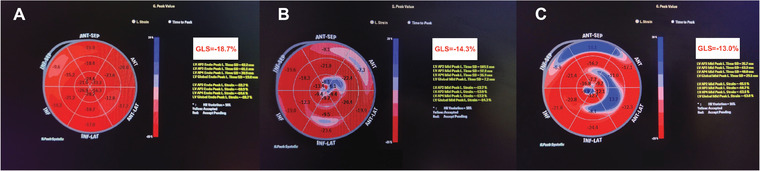
Bull's eye plot of left ventricular global longitudinal strain (LVGLS) values of subjects recovered from mild (A), moderate (B) and severe (C) COVID‐19 illness
4.2. Correlation of LVGLS with inflammatory markers
Levels of inflammatory markers during active COVID‐19 infection correlated significantly with LVGLS post COVID‐19 recovery (IL‐6 levels (r = .51; p < 0.0001), CRP levels (r = .39; p = 0.001), LDH (r = .27; p = 0.02) and serum ferritin levels (r = .25; p = 0.03). In addition, there was a significant correlation between cardiac troponins and LVGLS (r = .41; p < 0.0001) suggesting impaired LVGLS in patients with myocardial injury during index hospitalization for COVID‐19 (Figure 3).
FIGURE 3.
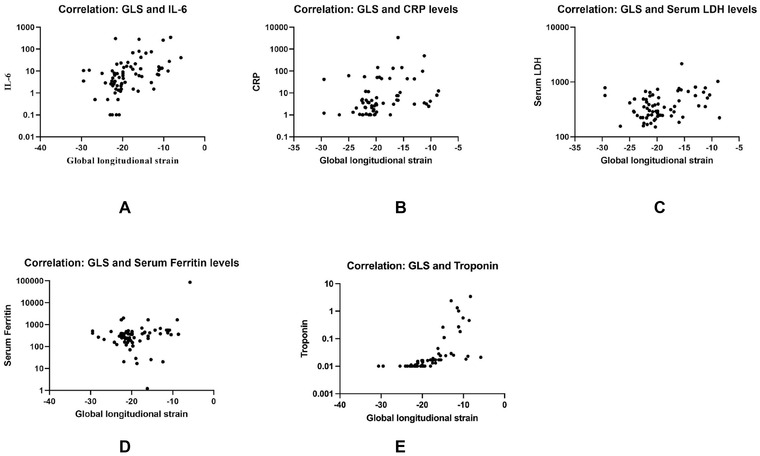
Scatter plot graph demonstrating the correlation between LVGLS and interleukin‐6 (IL‐6) (A), C‐reactive protein (CRP) (B), lactate dehydrogenase (LDH) (C), serum ferritin (D) and Troponin (E)
4.3. Predictors of reduced strain in COVID‐19 recovered subjects
A longer duration of hospital stay (greater in severe COVID‐19 infection) and male sex were the important predictors of reduced strain (Table 2). Age and co‐morbidities such as hypertension and diabetes mellitus had no impact on LVGLS in post COVID‐19 recovered subjects. Of note, subjects with reduced LVGLS had significantly higher levels of inflammatory markers such as IL‐6 (p < 0.0001) and CRP (p = 0.001) levels along with raised serum LDH (p = 0.009) and serum ferritin (p = 0.03) during active COVID‐19 infection (Table 2). Additionally, these subjects were more likely to have a lower TAPSE and a higher RV basal diameter. No significant difference was observed between the groups with respect to other echocardiographic parameters (LVEF, LVEDd, LVESd, LA size, RVSP and diastolic dysfunction). On Multivariate logistic regression, raised CRP (> 5 mg/L), raised IL‐6 (> 7 ρg/ml) and severity of COVID‐19 illness were the independent predictors of reduced LVGLS in COVID‐19 recovered subjects (Figure 4).
TABLE 2.
Comparison of demographic, biochemical, and echocardiographic parameters in subjects with and without reduced LVGLS
| Parameters | Group 1 (Normal LVGLS) (n = 94) | Group 2 (Reduced LVGLS) (n = 40) | p−value |
|---|---|---|---|
| Age | 52.2 ± 11.9 | 48.7 ± 13.2 | 0.13 |
| Sex (Male) | 54 (57.4%) | 31 (77.5%) | 0.03 |
| Comorbidities | |||
| Diabetes mellitus | 15 (15.9%) | 8 (20%) | 0.57 |
| Hypertension | 28 (29.7%) | 9 (22.5%) | 0.39 |
| Smoking | 4 (4.2%) | 1 (2.5%) | 0.62 |
| Asymptomatic | 12 (12.7%) | 1 (2.5%) | 0.18 |
| Duration of hospitalization (days) | 7.41 ± 6.36 | 10.7 ± 9.0 | 0.02 |
| Heart Rate (per minute) | 81.8 ± 9.7 | 80.6 ± 12.4 | 0.56 |
| Systolic blood pressure (mm Hg) | 133.7 ± 15.4 | 131 ± 11.9 | 0.34 |
| Diastolic blood pressure (mm Hg) | 81.5 ± 7.4 | 81.7 ± 7.7 | 0.88 |
|
Laboratory parameters |
|||
| Hemoglobin (gm%) | 12.5 ± 2.05 | 12.3 ± 1.6 | 0.61 |
| TLC (per mm3) | 10273.5 ± 14972.4 | 9278.4 ± 3047.4 | 0.69 |
| Platelet count (*10 5 /ml) | 2.46 ± 1.12 | 2.45 ± 1.11 | 0.96 |
| Serum creatinine (mg/dl) | .97 ± 1.19 | 1.01 ± .62 | 0.83 |
| D‐dimer (μg/L) | 460.8 ± 609.5 | 720.8 ± 1179.8 | 0.46 |
| Inflammatory markers | |||
| CRP (mg/L) | 12.43 ± 26.7 | 187.54 ± 693.9 | 0.001 |
| IL‐6 (ρg/ml) | 10.8 ± 44.0 | 48.2 ± 86.0 | < 0.0001 |
| LDH (U/L) | 352.8 ± 177.8 | 555.0 ± 404.1 | 0.009 |
| Ferritin (μg/L) | 335.6 ± 353.2 | 3712.5 ± 1572.2 | 0.03 |
| Troponin‐T (μg/L)* | .011±.002 | .344±.761 | 0.01 |
| Echocardiographic parameters | |||
| LVEF (%) | 60.3 ± 3.9 | 60.3 ± 6.1 | 0.96 |
| LVEDd (mm) | 46.7 ± 9.0 | 48.1 ± 3.9 | 0.64 |
| LVESd (mm) | 25.3 ± 6.1 | 27.1 ± 5.4 | 0.35 |
| LA diameter (mm) | 26.5 ± 4.8 | 27.6 ± 6.1 | 0.38 |
| RVSP (mm Hg) | 21.4 ± 5.9 | 30.7 ± 14.5 | 0.06 |
| RV basal diameter (mm) | 30.3 ± 2.3 | 31.7 ± 3.9 | 0.04 |
| TAPSE (mm) | 22.0 ± 2.3 | 20.7 ± 2.4 | 0.01 |
| RV S’ (cm/s) | 12.4 ± 2.1 | 12.2 ± 3.4 | 0.61 |
| LVGLS (%) | −21.1± 1.4 | −14.1±3.3 | < 0.0001 |
| Diastolic dysfunction | |||
| Grade I | 22 (23.4%) | 10 (25%) | 0.45 |
| Grade II | 3 (3.2%) | 2 (5%) | |
| Grade III | 0 | 1 (2.5%) |
Abbreviations : CRP, C reactive protein; IL, interleukin; LA, left atrium; LDH, lactate dehydrogenase; LVEDd, left ventricle end diastolic dimension; LVEF, left ventricle ejection fraction; LVESd, left ventricle end systolic dimension; LVGLS, left ventricle global longitudinal strain; RV, right ventricle; RV S’, right ventricle tricuspid annulus systolic velocity; RVSP, right ventricle systolic pressure; TAPSE, tricuspid annular plane systolic excursion; TLC, total leukocyte count.
Data available for 69 patients (36 patients in Group 1 and 33 patients in Group 2).
FIGURE 4.
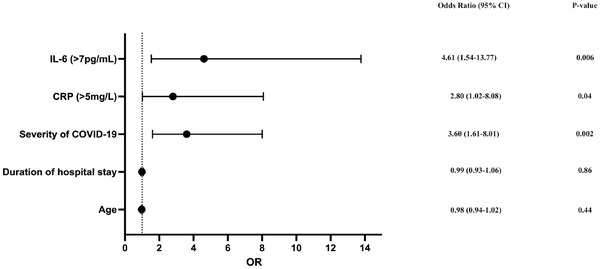
Forest plot showing the predictors of reduced LVGLS in multivariate logistic regression analysis. OR: Odds Ratio
5. DISCUSSION
The present study evaluated the presence of subclinical myocardial dysfunction using two‐dimensional strain analysis via STE in COVID‐19 recovered subjects. The major finding of our study was the presence of subclinical LV dysfunction in 40 (29.9%) patients who had recovered from COVID‐19. Impaired LV strain post COVID‐19 recovery was seen in 1 (7.7%), 8 (13.1%), 22 (44%) and 9 (90%) subjects, respectively, in asymptomatic, mild, moderate and severe COVID‐19 illness groups (Figure 1, Central illustration). It is noteworthy that even patients with mild to moderate disease had residual subclinical LV dysfunction during the recovery period. These findings are important as subclinical cardiac involvement is not well elucidated and is often overlooked in patients who have recovered from COVID‐19.
Cardiac involvement in COVID‐19 is often multifactorial with (a) systemic inflammatory response due to cytokine storm, (b) hypoxia induced oxygen supply‐demand mismatch, (c) micro‐or macrovascular thrombosis following inflammation and endothelial dysfunction, and (d) direct myocardial injury due to viral cytopathic effect being the postulated mechanisms. 3 In addition, there has been evidence of subclinical LV dysfunction in COVID‐19 recovered patients due to persistent cardiac inflammation in the form of peri‐myocarditis and early myocardial fibrosis. 10 , 11 LVGLS determination using STE is an objective and reproducible technique for evaluation of myocardial deformation. 19 LVGLS serves as a more sensitive and earlier predictor of LV systolic dysfunction as compared to LVEF. 20 Additionally, it has also been shown to predict subclinical myocardial dysfunction because of its ability to detect myocardial fibrosis early in the disease process. 19 Initial studies have shown the utility of LVGLS in predicting cardiac involvement patients with active COVID‐19 infection. 8 , 21 , 22 There is a limited evidence regarding the role of LVGLS in COVID‐19 recovered patients. In a small study from Turkey, LVGLS was impaired in 28 (37.8%) patients one month post discharge following COVID‐19 infection. 23 In our study, nearly one‐third of the patients (29.9%) had a reduced LVGLS following recovery from COVID‐19.
These findings have also been substantiated on CMR imaging wherein subclinical functional and myocardial tissue characteristic abnormalities have been detected in individuals post COVID‐19 infection. A small study of 26 COVID‐19 recovered patients reported myocardial edema in 14 (54%) patients and late gadolinium enhancement in 8 (31%) patients. 9 However, they included patients recovering from moderate or severe COVID‐19 illness. Another study of 100 recovered patients (majority with moderate and severe disease) found ongoing myocardial inflammation in 60 patients (60%). 10 This study included significant number of patients with various comorbidities like diabetes mellitus, hypertension, coronary artery disease that could have contributed to the ongoing inflammation. Though CMR imaging is the “gold standard” to detect subclinical myocardial involvement, its accessibility and affordability in low‐medium income countries is a major concern. Additionally, given the huge burden of COVID‐19 recovered patients, it is practically impossible to follow‐up all patients with CMR. In such situations, two‐dimensional strain imaging is a simple, cost‐effective and easily available tool to detect subclinical myocardial dysfunction in these patients.
In present study, mean LVGLS values (post recovery) among mild, moderate and severe COVID‐19 illness groups were ‐21 ± 3.4%, ‐18.1 ± 6.9% and ‐15.5 ± 3.1%, respectively, which implies that patient with severe COVID‐19 infection had a higher residual LV dysfunction as compared to those with mild/moderate disease. Similar patterns have been observed during active COVID‐19 infection where patients with severe disease had a marked reduction in LVGLS. 8 In our study, patients in the severe COVID‐19 group had significantly higher levels of inflammatory markers and lower hemoglobin levels. This was consistent with findings from previous studies as well as a metanalysis. 24 It was also seen that patients with higher levels of inflammatory markers during active COVID‐19 infection had a greater reduction in LVGLS post recovery from COVID‐19. Similar correlation has been demonstrated in a previous study between CRP levels and post COVID‐19 recovery LVGLS [r = .39, p < 0.001]. 23 In addition, a positive correlation was reported between myocardial injury in COVID‐19 and LVGLS in our study cohort suggesting reduced LVGLS in patients with myocardial injury during index hospitalization. Similar finding was reported in a small study among COVID‐19 recovered subjects. 23 Measurement of LVGLS and cardiac troponins during the course and following recovery from COVID‐19 infection complements each other. While cardiac troponins during the acute COVID‐19 infection detects myocardial inflammation and acute cardiac injury, LVGLS represents the clinical transformation of this myocardial injury into subclinical LV dysfunction over a period of time following recovery. Serial measurement of LVGLS and cardiac troponins following recovery from COVID‐19 infection has an incremental prognostic value and would be the ideal way to evaluate the progression of sub‐clinical LV dysfunction. Apart from subclinical LV dysfunction, RV dysfunction was documented in 14 (10.5%) subjects in our study. Patients with reduced LVGLS had significantly higher RV basal diameter and lower TAPSE. Previous studies have evaluated RV function in patients with active COVID‐19 infection and reported higher RV basal diameter or RV dilatation in approximately one third of patients. 8 , 25 , 26
6. CLINICAL IMPLICATIONS
The long‐term effects of COVID‐19 are still evolving and hence, the clinical significance of abnormal LVGLS in COVID‐19 recovered patients is still unclear. Use of LVGLS as a simple bedside tool would help in early identification of alterations in left ventricular mechanics following recovery from COVID‐19. This may translate into timely prediction of the impact of COVID‐19 infection on the cardiac function as well as the need for possible therapeutic interventions in order to prevent long term cardiovascular sequalae. Future studies in unselected COVID‐19 recovered patients over a period of time are needed to understand the natural progression of persistent myocardial damage and subclinical LV dysfunction. It will be of interest to see whether abnormal strain in recovered patients is an adverse prognostic marker.
7. LIMITATIONS
It was a single center study with a relatively small sample size. One of the inherent limitations of STE is the large number of clinical conditions and imaging quality which can impact the LVGLS. Hence, subjects with these confounding clinical conditions were excluded and STE was performed in a select subset of COVID‐19 recovered patients. One of the other limitations possibly could be a lack of baseline echocardiography during the active COVID‐19 infection due to the logistic issues in resource limited countries such as ours in the initial phases of the pandemic. Lack of data for cardiac troponins for some patients in our study was another limitation. Additionally, there was a lack of use of CMR imaging in our study. The COVID‐19 recovered patients in our study are being followed‐up longitudinally with STE at three monthly intervals for a period of one year to sequentially assess their strain pattern and determine the natural course of subclinical LV dysfunction following recovery from COVID‐19.
8. CONCLUSION
The present study provides important insights into the prevalence of subclinical myocardial dysfunction in recovered COVID‐19 subset. Nearly a third of patients recovering from COVID‐19 had subclinical LV dysfunction while 10.5% of them had RV dysfunction. The presence of such persistent myocardial dysfunction in recovered subjects suggests cardiac involvement as a possible lasting consequence of COVID‐19. This study reiterates the importance of STE as a practical imaging modality for detection of subclinical myocardial dysfunction especially amidst the huge burden of recovered patients. It also highlights the need for long term follow‐up of these subjects to unmask long‐term cardiovascular consequences of COVID‐19 infection.
CONFLICT OF INTEREST
Authors have no conflict of interest to declare.
Supporting information
Supplementary Information
Supplementary Information
ACKNOWLEDGMENT
None.
Mahajan S, Kunal S, Shah B, et al. Left ventricular global longitudinal strain in COVID‐19 recovered patients. Echocardiography. 2021;38:1722–1730. 10.1111/echo.15199
Sudhanshu Mahajan and Shekhar Kunal have equal contribution to the MS.
REFERENCES
- 1. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shi S, Qin Mu, Shen Bo, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5:802‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID‐19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chapman AR, Bularga A, Mills NL. High‐sensitivity cardiac troponin can be an ally in the fight against COVID‐19. Circulation. 2020;141:1733‐1735. [DOI] [PubMed] [Google Scholar]
- 5. Zhou F, Yu T, Du R, Fan G, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kunal S, Sharma SM, Sharma SK, et al. Cardiovascular complications and its impact on outcomes in COVID‐19. Indian Heart J. 2020;72:593‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baycan OF, Barman HA, Atici A, et al. Evaluation of biventricular function in patients with COVID‐19 using speckle tracking echocardiography. Int J Cardiovasc Imag. 2021;37:135‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang Lu, Zhao P, Tang D, et al. Cardiac involvement in patients recovered from COVID‐2019 identified using magnetic resonance imaging. J Am Coll Cardiol Img. 2020;13:2330‐2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:1265‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang H, Li R, Zhou Z, Jiang H, et al. Cardiac involvement in COVID‐19 patients: mid‐term follow up by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2021;23:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wibowo A, Pranata R, Astuti A, et al. Left and right ventricular longitudinal strains are associated with poor outcome in COVID‐19: a systematic review and meta‐analysis. J Intensive Care. 2021;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soufi Taleb Bendiab N, Meziane‐Tani A, Ouabdesselam S, et al. Factors associated with global longitudinal strain decline in hypertensive patients with normal left ventricular ejection fraction. Eur J Prev Cardiol. 2017;24:1463‐1472. [DOI] [PubMed] [Google Scholar]
- 14. Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. J Am Coll Cardiol Img. 2018;11:260‐274. [DOI] [PubMed] [Google Scholar]
- 15. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1‐39. [DOI] [PubMed] [Google Scholar]
- 16. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277‐314. [DOI] [PubMed] [Google Scholar]
- 17. Voigt J‐U, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28:183‐193. [DOI] [PubMed] [Google Scholar]
- 18. COVID‐19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID‐19) Treatment Guidelines. National Institutes of Health . Available at: https://www.covid19treatmentguidelines.nih.gov/ 2021. [PubMed] [Google Scholar]
- 19. Kostakou PM, Kostopoulos VS, Tryfou ES, et al. Subclinical left ventricular dysfunction and correlation with regional strain analysis in myocarditis with normal ejection fraction. A new diagnostic criterion. Int J Cardiol. 2018;259:116‐121. [DOI] [PubMed] [Google Scholar]
- 20. Collier P, Phelan D, Klein A. A test in context: myocardial strain measured by speckle‐tracking echocardiography. J Am Coll Cardiol. 2017;69:1043‐1056. [DOI] [PubMed] [Google Scholar]
- 21. Croft LB, Krishnamoorthy P, Ro R, et al. Abnormal left ventricular global longitudinal strain by speckle tracking echocardiography in COVID‐19 patients. Future Cardiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhatia HS, Bui QM, King K, et al. Subclinical left ventricular dysfunction in COVID‐19. Int J Cardiol Heart Vasc. 2021;34:100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Özer S, Candan L, Özyıldız AG, et al. Evaluation of left ventricular global functions with speckle tracking echocardiography in patients recovered from COVID‐19. Int J Cardiovasc Imaging. 2021:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID‐19–a systematic review. Life Sci. 2020;254:117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Y, Li He, Zhu S, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID‐19. J Am Coll Cardiol Img. 2020;13:2287‐2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Argulian E, Sud K, Vogel B, Narula J. Right ventricular dilation in hospitalized patients with COVID‐19 Infection. J Am Coll Cardiol Img. 2020;13:2459‐2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information


