Abstract
Background
The novel COVID‐19 vaccines have side effects that require efficient and close monitoring.
Aims of the Study
To examine whether the Pfizer‐BioNTech vaccine is associated with multiple cranial neuropathy.
Methods
We report the case of a 29‐year‐old male patient with no notable history who presented with left oculomotor, abducens, trigeminal and facial palsies 6 days after receiving the first dose of the Pfizer‐BioNTech COVID‐19 vaccine.
Results
Gadolinium‐enhanced MRI of the brain revealed enhancement in the left facial, trigeminal and oculomotor nerves, which persisted upon repeated examination. The cerebrospinal fluid analysis showed no sign of inflammation, both initially and after 1 month from the start of the patient's symptoms. Other causes were excluded by laboratory tests. The patient received high doses of corticosteroids, with improvement of symptoms.
Conclusions
In our case, the most probable etiology of the patient's multiple cranial neuropathy is the Pfizer‐BioNTech vaccine, which highlights the need for prolonged surveillance of COVID‐19 vaccine neurological complications.
Keywords: adverse effects, COVID‐19, cranial nerve palsies, vaccination
1. INTRODUCTION
The side effects of the COVID‐19 vaccines are permanently monitored and reported. In phase III trials of Pfizer‐BioNTech vaccine (PBV), four cases of Bell's palsy (occurring 3, 9, 37, and 48 days after the vaccination) were recorded among the inoculated individuals, resulting in an incidence of 0.0091%, similar to that in the general population, thus a direct causal relationship could not be established between PBV and Bell's palsy. 1
2. MATERIALS AND METHODS
We present the case of a 29‐year‐old Caucasian male, with no known history, who presented with Bell's palsy and diplopia. The patient's symptoms started 6 days after receiving the first dose of the PBV. He denied any allergic history. A signed Patient Consent has been obtained for this case report. We used the CARE reporting guidelines 2 for this case report.
3. RESULTS
Neurologic examination revealed multiple left‐sided cranial neuropathies (an incomplete oculomotor palsy without ptosis, partial abducens palsy, hypoesthesia in middle and lower trigeminal nerve distributions, as well as facial nerve palsy—House‐Brackmann grade III). Nasal swabs tested negative for SARS‐CoV‐2. There were no other focal signs and symptoms; the patient did not have signs of meningeal irritation or increased intracranial pressure. Gadolinium‐enhanced MRI of the brain revealed only diffuse gadolinium enhancement in the intracanalicular and labyrinthic portion of the left facial nerve (Figure 1A), as well as in the intracisternal course of the left trigeminal (Figure 1B) and oculomotor nerve. The MRI did not find any other meningeal or intracerebral involvement, apart from the intracranial nerve contrast.
FIGURE 1.
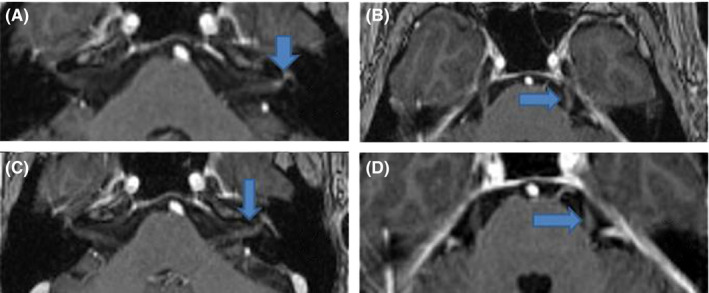
Images were acquired by use of T1‐weighted, contrast‐enhanced MPRAGE TRA ISO sequences, in the axial plane. (A) Gadolinium enhancement in the intracanalicular and labyrinthic segments of the left facial nerve (blue arrow). (B) Contrast enhancement in the intracisternal length of the trigeminal nerve (blue arrow). (C) The contrast enhancement of the facial nerve persists upon repeated examination a month later (blue arrow). (D) The contrast enhancement of the trigeminal nerve persists upon repeated examination a month later (blue arrow)
The involvement of multiple cranial nerves made other causes highly probable. Brain MRI excluded tumors, vascular causes or demyelinating diseases. There was no history of toxic exposures and the patient was immunocompetent. The screening for systemic inflammatory and systemic autoimmune diseases, and for granulomatosis were all negative. As for infectious causes, given that tuberculosis is endemic for our country, we have used Ziehl‐Neelsen staining to rule out this etiology.
Cerebrospinal fluid (CSF) biochemical analysis yielded no evidence of inflammation or infection—there was no pleocytosis and proteins and glucose were normal. CSF cytology revealed rare medium‐sized cells with basophilic cytoplasm on H&E staining; therefore, the lumbar puncture was repeated 1 month later, with cell block preparation and immunocytochemical examination, which excluded a malignant cause or an inflammatory reaction. The gadolinium‐enhanced MRI repeated after 1 month showed the persistence of the previously described gadolinium enhancement in the left facial (Figure 1C), trigeminal (Figure 1D), and oculomotor nerves. The patient received 1 g of methylprednisolone daily, for 5 days, with improvement of symptoms, but with persistence 1 month later of minimal left facial palsy. No other cranial nerve was involved at clinical follow‐up 1 month later and 2 months later.
4. DISCUSSION
Taking into account the CSF aspect on both lumbar punctures, together with the immunocytochemical study, the absence of the specific MRI enhancement, the lack of other signs and symptoms and the patient's response to corticosteroids, with no specific oncologic treatment for lymphomatous and carcinomatous meningitis, these etiologies were excluded. There is a high degree of probability that the cells with basophilic cytoplasm identified upon the first lumbar puncture were simply ependymal cells, a normal occurrence in the CSF. A highly improbable malignant nature of these cells was ruled out by the repeated lumbar puncture followed by detailed histopathological examination. 3
We did not have any reasons to suspect the patient might be suffering from fungal infection. The patient was immunocompetent. Furthermore, upon follow‐up it became evident that the patient responded to corticosteroids, which again ruled out a fungal etiology. For these reasons, a fungal culture was not deemed necessary.
According to US Vaccine Adverse Event Reporting System (VAERS) 4 cranial nerve palsies, either singular or multiple (aside from the most frequently involved facial nerve) have been reported after inoculation with other vaccines, both inactivated and live attenuated (e.g., the Haemophilus influenzae type B vaccine, the pneumococcal 7‐valent conjugate, the diphtheria, tetanus, whole‐cell pertussis vaccine, as well as the measles, mumps, and rubella vaccine).
Several cases are reported of peripheral facial palsy after immunization with PBV: a patient with two episodes of contralateral Bell's palsies arising shortly after inoculation with the first and second dose of the PBV 5 and other cases of unilateral facial palsy occurring 36 h after the second dose. 6 Another published case involved abducens nerve palsy 2 day after PBV. 7
There is no clearly defined mechanism linking cranial nerve palsies to vaccination. Immune‐mediated damage resulting in demyelination or localized nerve blood flow reduction was posited. Multiple cranial nerve palsies were associated with SARS COV2 infection. 8 The underlying pathophysiology behind COVID‐19‐related cranial nerve includes direct invasion of endothelial cells by the SARS COV2 and indirect injury through pro‐inflammatory attack of infected leukocytes. 7 However, to our knowledge, there are no other publications on the possibility of SARS COV2 vaccines causing multiple cranial neuropathy.
The particularity of this case consists in multiple cranial neuropathies 6 days after having received the first dose of the PBV. A direct causal relationship cannot be established at this point. However, given the onset of the patient's symptoms shortly after the immunization and the exclusion of all other potential causes, the most probable etiology of the cranial nerves impairment in this case was the PBV.
The strength of this study resides in the thorough work‐up performed which enabled the exclusion of other potential causes. However, as it is the first case of its kind (multiple cranial neuropathy after SARS‐CoV2 vaccination), its inherent and unavoidable limitation is that it comprises a single case. Hence, it is necessary that other similar cases be reported to gain a progressively fuller understanding of the potential for neurological damage of these newly developed vaccines.
While the efficacy and safety of these novel vaccines are important, the monitoring period for the appearance of post‐vaccinal complications is presently limited to a few months, hence neurological side effects require close observation.
CONFLICT OF INTEREST
The authors declare they have no conflicts of interests.
AUTHOR CONTRIBUTIONS
All authors contributed equally to this work and should therefore be considered first authors. All authors read and approved the final manuscript.
ETHICAL APPROVAL
Was in accordance if ethical guidelines. The study was approved by the institutional review board.
CONSENT TO PARTICIPATE
Written informed consent was obtained from the patient.
CONSENT FOR PUBLICATION
The participant has consented to the submission of the case report to the journal.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ane.13548.
ACKNOWLEDGMENT
None.
Manea MM, Dragoș D, Enache L, Sirbu AG, Tuta S. Multiple cranial nerve palsies following COVID‐19 vaccination—Case report. Acta Neurol Scand.2022;145:257–259. 10.1111/ane.13548
Funding information
No funding was received to assist with the preparation of this manuscript
The Clinical Commentary refers to ANE13593 (https://onlinelibrary.wiley.com/doi/10.1111/ane.13593)
DATA AVAILABILITY STATEMENT
n/a.
REFERENCES
- 1. Fda . Vaccines and Related Biological Products Advisory Committee December 10, 2020 Meeting Briefing Document‐ FDA. 2020.
- 2. Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. The CARE guidelines: consensus‐based clinical case reporting guideline development. Glob Adv Heal Med. 2013;2:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ependymal cells in cerebrospinal fluid: A traumatic occurrence. https://imagebank.hematology.org/image/60670/ependymal‐cells‐in‐cerebrospinal‐fluid‐a‐traumatic‐occurrence. Accessed October 15, 2021. [DOI] [PubMed]
- 4. Woo EJ, Winiecki SK, Ou AC. Motor palsies of cranial nerves (excluding VII) after vaccination: reports to the US vaccine adverse event reporting system. Hum Vaccin Immunother. 2014;10:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burrows A, Bartholomew T, Rudd J, Walker D. Sequential contralateral facial nerve palsies following COVID‐19 vaccination first and second doses. BMJ Case Rep. 2021;14(7):e243829. 10.1136/bcr-2021-243829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Repajic M, Lai XL, Xu P, Liu A. Bell’s Palsy after second dose of Pfizer COVID‐19 vaccination in a patient with history of recurrent Bell’s palsy. Brain Behav Immun Health. 2021;13:100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reyes‐Capo DP, Stevens SM, Cavuoto KM. Acute abducens nerve palsy following COVID‐19 vaccination. J Aapos. 2021:1–2. 10.1016/J.JAAPOS.2021.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gogia B, Gil Guevara A, Rai PK, Fang X. A case of COVID‐19 with multiple cranial neuropathies. Int J Neurosci. 2020:1‐3. 10.1080/00207454.2020.1869001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
n/a.


