Abstract
Aims
Tocilizumab has emerged as an important therapy in treating patients with coronavirus disease (COVID‐19). Our purpose was to evaluate the efficacy and safety of tocilizumab versus standard care/placebo in patients with COVID‐19.
Methods
We searched a variety of sources from 1 January 2020 to 5 May 2021. All randomized controlled trials that reported tocilizumab efficacy as a primary agent in COVID‐19 patients were considered. RCTs had to include mortality events, incidence of mechanical ventilation and serious adverse events. Two reviewers were independently responsible for data extraction. Assessment of bias and certainty of evidence was carried out using the Cochrane Risk of Bias Tool and GRADE methodology. RR for mortality events was evaluated using a fixed‐effects model.
Results
A total of 6837 patients were included from 10 RCTs, of which nine were peer‐reviewed. Pooled risk ratio (RR) for all‐cause mortality in patients with tocilizumab administration was RR = 0.88 (95% CI: 0.81–0.95, P = .0009). RR for incidence of mechanical ventilation at 28–30 days was 0.79 (95% CI: 0.71–0.88). Serious adverse events (SAE) with tocilizumab use were associated with lower RR (RR = 0.91, 95% CI: 0.76–1.09) but the certainty of evidence was downgraded to moderate due to serious risk of bias.
Conclusion
In COVID‐19 patients with moderate to critical COVID‐19, use of tocilizumab reduces all‐cause mortality and progression to mechanical ventilation. This efficacy was not associated with higher number of serious adverse events.
Keywords: COVID‐19, meta‐analysis, randomized clinical trial, tocilizumab
1. INTRODUCTION
The COVID‐19 pandemic is one of the biggest challenges to human health in recent years and decades. As of 20 April 2021 it has officially infected more than 155 million people, of whom more than 3 million have died. 1 Of course, official data are conservative, so the real number of infected and deceased is expected to be higher.
Different therapies are being studied to examine their efficacy in reducing the consequences of this serious disease. The first robust evidence for effective therapies in COVID‐19 has been produced by the RECOVERY trial, which has revealed modest but important benefits from corticosteroid therapy in patients with severe and critical forms of the disease. 2 Other therapies, like the antiviral remdesivir, though promising, still lack definitive evidence for efficacy. 3
The need for new therapies is therefore paramount to reduce the burden of COVID‐19. But, in order to find and recommend the appropriate agents, there is a need for high‐quality randomized clinical trials (RCT). In this respect, recent results from a number of RCTs regarding the use of tocilizumab have been encouraging. This means that we can evaluate with higher certainty the role of this agent in COVID‐19.
Tocilizumab is an interleukin 6 receptor (IL‐6R) inhibitor used in autoimmune inflammatory diseases, like rheumatoid arthritis. 4 Anti‐inflammatory agents are logically drugs of interest in COVID‐19 as severe and critical disease is characterized by overactive inflammatory signalling. 5 An important mediator of inflammatory response is IL‐6 with it actions through IL‐6R. 6 Previously published observational data summarized through meta‐analysis have shown that tocilizumab could reduce mortality and the need for mechanical ventilation. 7 Since then, a number of RCTs evaluating the role of tocilizumab in COVID‐19 have been published. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 This meta‐analysis is the first systematic overview which reveals the role of tocilizumab in COVID‐19 by combining results from 10 RCTs.
2. METHODS
This systematic review and meta‐analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) statement. 20 The review protocol for this meta‐analysis was not registered due to the dynamic nature of COVID‐19 research in the pandemic year.
2.1. Search strategy and RCT selection
Two independent investigators (Z.V.G. and M.R.) searched PubMed, Cochrane Central Register of Controlled Trials, ClinicalTrials.gov, EU Clinical Trials Register, MedRxiv, SSRN and Researchsquare databases from 1 January 2020 to 5 May 2021 using the following combination of keywords: “COVID 19”, “SARS COV 2” with “tocilizumab”, “IL‐6 receptor antagonist”, “IL‐6 receptor inhibitor” with “randomized trial”. No language restrictions were used. Additional searches were carried out by analysing references from retrieved papers and reviews to minimize the chance of omissions.
The inclusion criteria for this meta‐analysis were RCTs comparing the efficacy of tocilizumab in patients with COVID‐19. Included RCTs were eligible for analysis if tocilizumab efficacy was the primary objective of the study. Any randomized trial had to include mortality events or incidence of mechanical ventilation or incidence of adverse events in their results section. Exclusion criteria for this meta‐analysis were nonrandomized studies, combination therapy and presence of serious risk of bias.
2.2. Outcomes
The outcomes that were analysed included mortality events between tocilizumab group and placebo/standard care group, incidence of mechanical ventilation, number of deaths with/without mechanical ventilation at randomization, number of deaths with/without corticosteroid use, and number of serious adverse events.
2.3. Data extraction and quality assessment
Two independent investigators (R.O. and V.H.) were responsible for data extraction from each of the included trials. Data collected from the trials included first author name, trial design, country, study sponsor, timeline of the trial, number of subjects by groups, inclusion criteria, exclusion criteria, study strengths/limitations, mode of tocilizumab administration, age, proportion of patients in old age groups, time from symptom onset to randomization, concomitant corticosteroid use in both groups, proportion of subjects with mechanical ventilation at baseline, and mean or median values of inflammatory parameters in both groups.
Risk of bias 2 (RoB2) was assessed using the Cochrane Collaboration risk of bias for randomized trials. 21 This method uses five domains to categorize this risk. Two independent investigators were responsible for evaluating RoB (Z.V.G. and R.N.). Any disagreement was resolved by consulting a third reviewer (D.V.). The summary of this evaluation for each study is shown in Figure S1. Certainty of evidence for outcomes was analysed using GRADE methodology. 22
2.4. Data synthesis and analysis
RevMan 5.4.1 (The Cochrane Collaboration, 2020) was used to analyse data from the studies. We used a fixed‐effects model with inverse variance statistic (or Mantel–Haenszel statistic where appropriate) to pool RR for outcomes. Heterogeneity was assessed using Chi‐squared and I2 test. Results are presented as risk ratio (RR) with 95% confidence intervals (CI). P‐values are presented to contextualize data (significance set at P < .05).
2.5. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2021/22. 23
3. RESULTS
The initial searches yielded 2171 records from selected databases. After removing duplicates and studies that were not randomized trials and did not fulfil other inclusion criteria, 12 articles remained. Two randomized trials were omitted because they did not fulfil inclusion/exclusion criteria. 17 , 18 The flowchart of the search strategy and results can be seen in Figure S2.
3.1. Trial characteristics
The number of subjects between trials ranged from 123 to 4116, with a total of 6837 subjects. Three out of 10 trials were global, while seven trials were national multicentre studies. Most of the patients in all the trials suffered from low oxygen saturation. In nine out of 10 trials, median or mean C‐reactive protein (CRP) levels in the tocilizumab group were >100 mg/L. Differences between trials were observed in percentage of concomitant corticosteroid use and percentage of patients on mechanical ventilation.
Tocilizumab was administered intravenously as a single dose initially, but in eight of the 10 trials a second dose was allowed if the clinical status did not improve. Details of the trial characteristics can be seen in Table S1.
3.2. All‐cause mortality
All‐cause mortality was reported in all trials. Treatment with tocilizumab resulted in lower risk of death (RR = 0.88, 95% CI: 0.81–0.95, P = .0009) (Figure 1A). Since the RECOVERY trial had the most impact on the results, we measured RR without the inclusion of the RECOVERY trial. The pooled RR was similar but with wider 95% CI and loss of statistical significance (0.73–1.01, P = .07) (Figure 1B).
FIGURE 1.
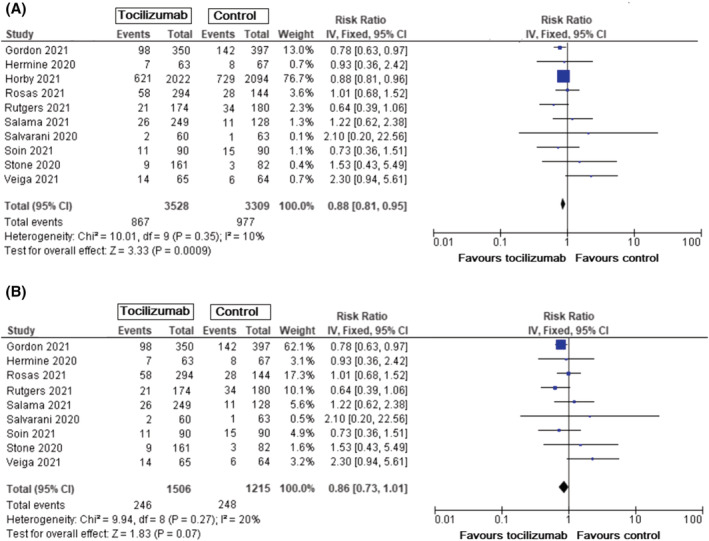
All‐cause mortality (follow‐up 28 days) in all patients with COVID‐19, tocilizumab vs standard care/placebo. A. Mortality events for all patients with COVID‐19. B. Mortality events in patients with COVID‐19 without inclusion of results from RECOVERY trial
All the trials reported concomitant corticosteroid use. According to the RECOVERY trial, tocilizumab efficacy is dependent on corticosteroid use. 14 Three studies from this meta‐analysis reported events with and without corticosteroid use. Pooled results from these trials showed that concomitant corticosteroid use with tocilizumab is associated with lower risk of death and progression to ventilation support (RR = 0.82, 95% CI: 0.74–0.90, P < .0001) (Figure S3A). On the other hand, lack of concomitant corticosteroid use did not show this association (RR = 1.09, 95% CI: 0.91–1.31, P = .36) (Figure S3B). We also compared RR between two groups of studies; studies that reported low percentage of concomitant corticosteroid use (<50%) and high percentage of concomitant corticosteroid use (>50%). In studies that reported high percentage of concomitant corticosteroid use, tocilizumab administration was associated with lower risk of death (RR = 0.87, 95% CI: 0.80–0.94, P = .0005) (Figure S4A). But, in studies with low percentage of concomitant corticosteroid use, tocilizumab administration was not associated with lower risk of death (RR = 1.05, 95% CI: 0.74–1.50, P = .78) (Figure S4B).
RR for death events was also analysed by assessing the presence or absence of mechanical ventilation at start of the randomization. In patients who were not on mechanical ventilation at start of randomization, tocilizumab use was associated with a lower risk of death (RR = 0.85, 95% CI: 0.78–0.93, P = .0005) (Figure 2A). RR was lower when results from the RECOVERY trial were omitted without loss of statistical significance (RR = 0.79, 95% CI: 0.65–0.97, P = .03) (Figure 2B). RR for death for patients with mechanical ventilation at baseline treated with tocilizumab was not significant (RR = 0.96, 95% CI: 0.83–1.10, P = .55) (Figure 2C).
FIGURE 2.
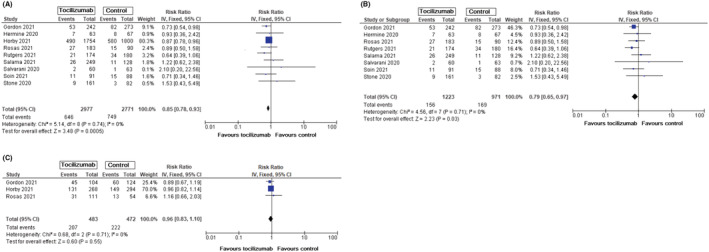
All‐cause mortality in patients with COVID‐19 without mechanical ventilation at baseline, tocilizumab vs standard care/placebo. A. Mortality events in patients with COVID‐19 without mechanical ventilation at baseline. B. Mortality events in patients with COVID‐19 without mechanical ventilation at baseline (without results from RECOVERY trial). C. Mortality events in patients with COVID‐19 with mechanical ventilation at baseline
3.3. Mechanical ventilation
Incidence of mechanical ventilation was evaluated in all studies. This risk was lower in all patients with tocilizumab use (RR = 0.79, 95% CI: 0.71–0.88, P < .0001) (Figure 3A). When results from the RECOVERY trial were omitted, results showed identical RR for incidence of mechanical ventilation with preserved statistical significance (RR = 0.78, 95% CI: 0.67–0.91, P = .002) (Figure 3B).
FIGURE 3.
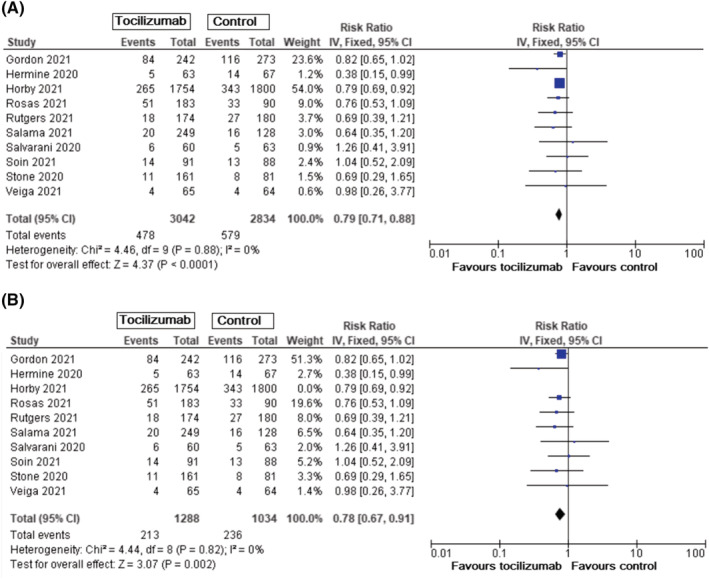
Incidence of mechanical ventilation in patients with COVID‐19, tocilizumab vs standard care/placebo. A. Incidence of mechanical ventilation in all patients with COVID‐19. B. Incidence of mechanical ventilation in patients with COVID‐19 without results from RECOVERY trial
3.4. Serious adverse events
Serious adverse events were somewhat lower in the tocilizumab group, though the results were nonsignificant (RR = 0.91, 95% CI: 0.76–1.09, P = .30) (Figure 4).
FIGURE 4.
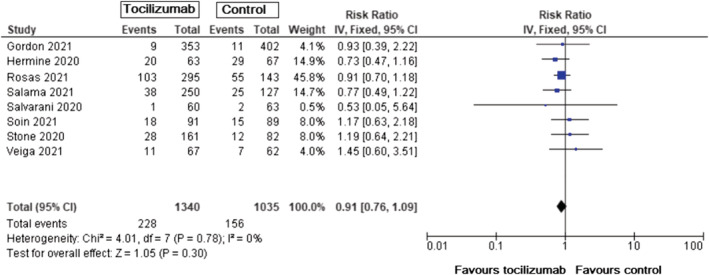
Incidence of serious adverse events in patients with COVID‐19, tocilizumab vs standard care/placebo
3.5. Sensitivity analysis
We used sensitivity analysis to address the robustness of the data. Results from this analysis were compatible with the main analysis (Table S2).
3.6. Absolute risk reduction and quality of reported outcomes
We calculated absolute risk reduction based on reported RR and baseline risk. GRADE methodology was used to categorize the quality of evidence for outcomes. Summarized results can be found in Table 1.
TABLE 1.
Certainty of evidence for major outcomes using GRADE methodology
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Tocilizumab | Standard care/placebo | Relative (95% CI) | Absolute (95% CI) | ||
| All‐cause mortality (follow up: 28–30 days) | ||||||||||||
| 10 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 867/3528 (24.6%) | 977/3309 (29.5%) | RR 0.88(0.81 to 0.95) | 35 fewer per 1000(from 56 fewer to 15 fewer) | ⊕⊕⊕⊕HIGH | CRITICAL |
| All‐cause mortality in patients without mechanical ventilation at baseline | ||||||||||||
| 9 | Randomized trials | Not serious a | Not serious | Not serious | Not serious | None | 646/2977 (21.7%) | 749/2771 (27%) | RR 0.85(0.78 to 0.93) | 41 fewer per 1000(from 59 fewer to 19 fewer) | ⊕⊕⊕⊕HIGH | CRITICAL |
| Incidence of mechanical ventilation | ||||||||||||
| 9 | Randomized trials | Not serious b | Not serious | Not serious | Not serious | None | 478/3042 (15.7%) | 579/2834 (20.4%) | RR 0.79(0.71 to 0.88) | 43 fewer per 1000(from 59 fewer to 25 fewer) | ⊕⊕⊕⊕HIGH | CRITICAL |
| Serious adverse events (follow up: 28 days) | ||||||||||||
| 8 | Randomized trials | Serious c | Not serious | Not serious | Not serious | None | 228/1340 (17.0%) | 156/1035 (15.1%) | RR 0.91(0.76 to 1.09) | 14 fewer per 1000(from 36 fewer to 14 more) | ⊕⊕⊕◯MODERATE | IMPORTANT |
CI: confidence interval; RR: risk ratio.
In Soin et al. study the outcome was measured by including 5/91 (5.5%) patients on tocilizumab group and 4/88 (4.5%) patients in standard care who were on mechanical ventilation. In Stone et al. study the outcome was measured by including 1/82 (1,2%) patients on mechanical ventilation in placebo group. We did not downgrade for risk of bias because the total number of patients who were on mechanical ventilation for this outcome was 5/2803 (0.18%) for tocilizumab group and 5/2591 (0.19%) for standard care/placebo group.
In Hermine et al. study incidence of mechanical ventilation was measured at Day 14 of follow up (compared to other studies in which this outcome was measured at 28 days). We did not downgrade for risk of bias because we do not think that this small trial would have significantly impacted the overall results.
No data were available for the biggest trial.
4. DISCUSSION
This is the first comprehensive meta‐analysis evaluating the efficacy of tocilizumab which includes 10 RCTs. A previous meta‐analysis from observational studies has shown that tocilizumab significantly reduces the risk of mortality, need for ventilation and risk of admission to ICU. 7 A recent meta‐analysis with eight RCTs showed a reduced risk of death in COVID‐19 patients treated with tocilizumab. 24 This meta‐analysis did not report outcomes such as incidence of mechanical ventilation as a single outcome, impact of concomitant corticosteroid use on tocilizumab efficacy and mortality events based on baseline presence/absence of mechanical ventilation.
Our study examined results from 10 RCTs that reported mortality events, incidence of mechanical ventilation and serious adverse events. Recent preliminary analyses with nine RCTs showed significantly lower RR in patients treated with tocilizumab. 14 , 25 But these analyses were not comprehensive; they reported only the efficacy of tocilizumab on mortality, but not on other outcomes. Also, there was no subgroup analysis regarding the effects of tocilizumab on COVID‐19 patients. Our meta‐analysis also showed that treatment with tocilizumab was associated with lower RR for mortality. These results were influenced by the biggest trial (RECOVERY trial). Exclusion of results from this trial did preserve RR but not the statistical significance of the results.
The results from our meta‐analysis have shown that mortality benefits from treatment with tocilizumab were applicable to certain group of patients. For example, concomitant corticosteroid use was associated with a statistically significantly lower RR for death. This benefit was not observed in patients with no corticosteroid use. It has to be mentioned that this analysis was done by obtaining results from only three studies, but, importantly, it included results from the biggest trial. 10 , 11 , 14 A study conducted by Gordon et al. did not report the number of events but it reported the odds ratio (OR) for tocilizumab‐corticosteroid combination; these results reveal that the combined administration of these agents has additive benefits for patients with COVID‐19. 16 Therefore, two of the biggest trials concluded that tocilizumab + corticosteroid administration has additive effects on COVID‐19 patients. To further analyse this association, we also decided to group studies based on reported concomitant corticosteroid use. Studies that reported a high percentage of concomitant corticosteroid use showed significantly lower RR for mortality. This was not observed in studies with low percentage of concomitant corticosteroid use.
The question that arises is why might tocilizumab and corticosteroids act in synergy in COVID‐19 patients? This could come down to the mode of action of these drugs. Corticosteroid effects as anti‐inflammatory agents are wide; they include complex interaction with IL‐6 expression which can be enhanced by systemic corticosteroid use. 26 On the other hand, tocilizumab effects on IL‐6 are more direct and are mediated by blocking IL‐6 receptor. Therefore, the administration of corticosteroid + tocilizumab can yield synergistic action by blocking different arms of inflammatory pathways. Still, the exact mode of this interaction remains unclear.
Another group that benefited from tocilizumab use in terms of mortality was the group of patients who did not need mechanical ventilation at the start of randomization. In this group, RR for mortality was lower in patients treated with tocilizumab compared to control. The reason for this association could mean that blocking the IL‐6 receptor in critical stages of the disease does not help the survival of the patients. On the other hand, as we have learned from the RECOVERY trial, corticosteroid use is beneficial in these patients. This could occur due to pleiotropic effects of corticosteroids in inflammation which might be important in this subgroup of patients. 27 Evidence suggests that pathophysiology of inflammatory response in COVID‐19 is distinct; levels of CRP and d‐dimer show progressive increase, while IL‐6 levels do not show progressive increase when comparing severe and critical form of COVID‐19. 5
Another important outcome that was influenced by tocilizumab use was the incidence of mechanical ventilation. Tocilizumab reduced this risk whether results from the biggest trial were included or not. Our results showed a somewhat better efficacy of tocilizumab for this outcome compared to results observed from NICE guidelines, which was done by analysing incidence of mechanical ventilation in only four studies (compared to our analysis with 10 studies). 28 Lower OR for incidence of ventilation with tocilizumab use was observed in previous meta‐analysis of observational studies but these results suffered from a high level of heterogeneity while our analysis with 10 RCTs showed clear benefits from tocilizumab use in this respect. 7 This suggests that tocilizumab use in COVID‐19 patients can reduce the risk for progression to mechanical ventilation. Since most of the trials included patients without mechanical ventilation at baseline, it was not possible to assess the efficacy of tocilizumab in successful removal of this. But the biggest trial did examine this issue and results showed that tocilizumab use in these patients was not associated with successful removal of mechanical ventilation. 14
Our analysis of the safety profile showed that tocilizumab administration was slightly associated with lower risk of serious adverse events but this result did not reach statistical significance. The final results did not include data from the RECOVERY trial (incomplete data for both groups), but this trial did report a very low number of serious adverse events attributed to tocilizumab use. In any case, our results were similar to those of a recent meta‐analysis with eight RCTs. 24
One must keep in mind that the results from this meta‐analysis are applicable to patient characteristics included in the trials. Most of these patients were male, suffered from lower than normal values of oxygen saturation but were mostly characterized by the absence of mechanical ventilation at baseline, and had high levels of CRP and ferritin levels (indicative of inflammation). IL‐6 levels were variable and not all studies reported IL‐6 levels. It is not clear if there are cut‐off values for inflammatory parameters which might affect tocilizumab benefits in patients with COVID‐19. Some of the RCTs included in this meta‐analysis did suggest this association. For example, a study by Veiga et al. reported lower OR for primary outcome for patients with CRP > 50 mg/L, though OR values, even in this case, did not favour tocilizumab use. 13 In a study by Salvarani et al., lower RR for primary outcome, which favoured tocilizumab use, was observed only for values of CRP > 150 mg/L. 15 In a study by Gordon et al., the biggest benefit for primary outcome was observed in patients with values >187 mg/L. 16 This is in line with a recent study which suggested that tocilizumab efficacy benefits mostly COVID‐19 patients with very high levels of CRP (>200 mg/L). 29 Therefore, data from our meta‐analysis suggest that tocilizumab benefits might be higher in patients with very high levels of inflammation (>150 mg/L). In our meta‐analysis, participants included in the tocilizumab group had high median or mean levels of CRP (>100 mg/L) but this did not reach the proposed >150 mg/L cut‐off value. Furthermore, the interquartile and absolute range of the values was wide and included patients with <100 mg/L or even <50 mg/L. Four out of 10 studies did include CRP levels as an inclusion criterion (from >50 mg/L to ≥100 mg/L), but in only one of them was this an obligatory criterion (RECOVERY trial). 8 , 13 , 14 , 15 Importantly, this was the biggest trial, and recruited the highest number of patients to date. One study used only ferritin levels as a marker of inflammation in their inclusion criteria. In any case, further studies should address this issue, which could reveal additional benefits of tocilizumab in this subgroup of patients.
The strength of this meta‐analysis is that it is based on results from the biggest number of RCTs up to date, which, in total, include nearly 7000 participants. Also, we analysed mortality events in a comprehensive manner, by addressing tocilizumab efficacy in different subgroups of patients.
Although patients included in this meta‐analysis are rather homogeneous, there were some important discrepancies which might influence final results. For example, the timeline of the trials is not identical; six out of 10 trials included patients enrolled in the period March–April 2020, which is the period when we observe the highest mortality rates in hospitalized patients. As data from May and June 2020 show, mortality from COVID‐19 dropped significantly, with improving standards of care being an important factor in this decline. 30
Gender as a probable confounding factor in determining tocilizumab efficacy has been evaluated in our included RCTs with different results. Four studies reported hazard ratios (HRs) or ORs for primary outcome; one study reported no significant differences in HR based on gender, while others reported higher but nonsignificant ORs for males and females respectively. 8 , 12 , 13 In the biggest trial, male gender was associated with more pronounced RR reduction for mortality though the result was not statistically significant. 14 With respect to age, five trials have evaluated the role of this factor in mortality with variable results (while using different age groups for comparison); two trials showed no differences between younger and older age groups, two trials suggested higher tocilizumab efficacy in older age groups, and one suggested higher tocilizumab efficacy in younger age groups. 8 , 9 , 12 , 13 , 14 It is not clear if these differences occurred due to variability of other confounding factors (like concomitant corticosteroid use, presence/lack of mechanical ventilation at baseline).
Another factor which might affect tocilizumab efficacy is time when tocilizumab was administered. Time from symptom onset to randomization across the trials ranged from 7–11 days in the tocilizumab group (8–10 days in the control group). Three studies evaluated the efficacy of early vs late tocilizumab administration but no significant associations were observed. 9 , 10 , 13
The variable levels of serum IL‐6 reported from the studies, and conflicting results from two studies which compared high and low levels of this cytokine, did not allow for any meaningful interpretation regarding the role of baseline levels of this cytokine in the efficacy of tocilizumab in COVID‐19 patients. 8 , 15 As we previously noted, cytokine storm is not a distinct feature of severe and critical COVID‐19, with IL‐6 levels increasing marginally and without showing significant changes between severe and critical forms of the disease, which is in contrast to other typical acute inflammatory syndromes. 5 On the other hand, acute‐phase reactants, such as CRP and d‐dimer, are progressively increased in severe and critical COVID‐19. 5
Finally, the RCTs included in this meta‐analysis differed subtly with respect to the mode of administration of tocilizumab; some used exclusively one IV dose of tocilizumab, while others allowed for a two‐dose approach. Two RCTs reported efficacy of tocilizumab with one vs two dose approaches but the results were conflicting. 9 , 10
5. CONCLUSIONS
The results from this meta‐analysis show that tocilizumab use lowers the risk of death and progression to mechanical ventilation in COVID‐19 patients with high levels of inflammation and low levels of oxygen saturation. The mortality benefit was most apparent in patients who also received systemic corticosteroids and in those with no need for mechanical ventilation at baseline. These benefits are important to reduce the high burden of COVID‐19 in patients.
ACKNOWLEDGEMENT
No funding was received for this manuscript.
COMPETING INTERESTS
The authors declare there are no conflicts of interest.
CONTRIBUTORS
All authors contributed to the conception and design of the study; Z.V.G. and M.R. conducted the literature search; R.O. and V.H. conducted the data extraction; Z.V.G., R.N. and D.V. were responsible for evaluating the risk of bias; D.V. and Z.V.G. conducted the data analysis and interpretation of results; all authors contributed in writing the first draft of the manuscript; all authors contributed in the manuscript revision and all authors read and approved the final version.
Supporting information
FIGURE S1 Risk of bias assessment for included RCTs according to five domains
FIGURE S2 Flowchart of search strategy
FIGURE S3 Death or death + mechanical ventilation in patients with COVID‐19 with and without concomitant corticosteroid use, tocilizumab vs standard care/placebo
FIGURE S4 All‐cause mortality in patients with COVID‐19 in studies with high vs low percentage of concomitant corticosteroid use, tocilizumab vs standard care/placebo
TABLE S1 Study and patient characteristics
TABLE S2 Sensitivity analysis
Data S1. Supporting information
Vela D, Vela‐Gaxha Z, Rexhepi M, Olloni R, Hyseni V, Nallbani R. Efficacy and safety of tocilizumab versus standard care/placebo in patients with COVID‐19; a systematic review and meta‐analysis of randomized clinical trials. Br J Clin Pharmacol. 2022;88(5):1955-1963. doi: 10.1111/bcp.15124
DATA AVAILABILITY STATEMENT
The data used in this study are available upon request from the corresponding author.
REFERENCES
- 1. Johns Hopkins Coronavirus Resource Center . COVID‐19 Map. https://coronavirus.jhu.edu/map.html. Accessed ???
- 2. RECOVERY Collaborative Group , Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2021;384(8):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lamontagne F, Agoritsas T, MacDonald H, et al. A living WHO guideline on drugs for covid‐19. BMJ. 2020;370:m3379. [DOI] [PubMed] [Google Scholar]
- 4. Shafran IH, Shafran IH, Alasti F, et al. Implication of baseline levels and early changes of C‐reactive protein for subsequent clinical outcomes of patients with rheumatoid arthritis treated with tocilizumab. Ann Rheum Dis. 2020;79(7):874‐882. [DOI] [PubMed] [Google Scholar]
- 5. Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID‐19: a rapid systematic review, meta‐analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8(12):1233‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pope JE, Choy EH. C‐reactive protein and implications in rheumatoid arthritis and associated comorbidities. Semin Arthritis Rheum. 2021;51(1):219‐229. [DOI] [PubMed] [Google Scholar]
- 7. Zhao M, Lu J, Tang Y, Dai Y, Zhou J, Wu Y. Tocilizumab for treating COVID‐19: a systemic review and meta‐analysis of retrospective studies. Eur J Clin Pharmacol. 2021;77(3):311‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stone JH, Frigault MJ, Serling‐Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid‐19. N Engl J Med. 2020;383(24):2333‐2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid‐19 pneumonia. N Engl J Med. 2021;384(1):20‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid‐19 pneumonia. N Engl J Med. 2021;384(16):1503‐1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hermine O, Mariette X, Tharaux PL, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID‐19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):32‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soin AS, Kumar K, Choudhary NS, et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID‐19‐associated cytokine release syndrome (COVINTOC): an open‐label, multicentre, randomised, controlled, phase 3 trial. Lancet Respir Med. 2021;9(5):511‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Veiga VC, Prats JAGG, Farias DLC, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021;372:n84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horby PW, Pessoa‐Amorim G, Peto L, et al. Tocilizumab in patients admitted to hospital with COVID‐19 (RECOVERY): randomised, controlled, open‐label, platform trial. Lancet. 2021;397(10285):1637‐1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID‐19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):24‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gordon AC, Mouncey PR, Al‐Beidh F, et al. Interleukin‐6 receptor antagonists in critically ill patients with Covid‐19. N Engl J Med. 2021;384(16):1491‐1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao H, Zhu Q, Zhang C, et al. Tocilizumab combined with favipiravir in the treatment of COVID‐19: a multicenter trial in a small sample size. Biomed Pharmacother. 2021;133:110825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang D, Fu B, Peng Z, et al. Tocilizumab in patients with moderate or severe COVID‐19: a randomized, controlled, open‐label, multicenter trial. Front Med. 2021;15(3):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rutgers A, Westerweel PE, van der Holt B, et al. Timely administration of tocilizumab improves survival of hospitalized COVID‐19 patients. 10.2139/ssrn.3834311 [DOI] [PMC free article] [PubMed]
- 20. PRISMA . http://www.prisma-statement.org/PRISMAStatement/
- 21. Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions, version 6.2. Cochrane Training; 2021. https://training.cochrane.org/handbook/current/chapter-08 [Google Scholar]
- 22. Siemieniuk R, Guyatt G. What is GRADE? Accessed November 9, 2021. https://bestpractice.bmj.com/info/us/toolkit/learn-ebm/what-is-grade/
- 23. Alexander SP, Fabbro D, Kelly E, et al. The Concise Guide to PHARMACOLOGY 2021/22: Catalytic receptors. Br J Pharmacol. 2021;178(S1):S264‐S312. [DOI] [PubMed] [Google Scholar]
- 24. Ghosn L, Chaimani A, Evrenoglou T, et al. Interleukin‐6 blocking agents for treating COVID‐19: a living systematic review. Cochrane Database Syst Rev. 2021;3(3):CD013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murthy S, Lee TC. IL‐6 blockade for COVID‐19: a global scientific call to arms. Lancet Respir Med. 2021;9(5):438‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eddy JL, Krukowski K, Janusek L, Mathews HL. Glucocorticoids regulate natural killer cell function epigenetically. Cell Immunol. 2014;290(1):120‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hardy RS, Raza K, Cooper MS. Therapeutic glucocorticoids: mechanisms of actions in rheumatic diseases. Nat Rev Rheumatol. 2020;16:133‐144. [DOI] [PubMed] [Google Scholar]
- 28. NICE . COVID‐19 rapid guideline: Managing COVID‐19. Accessed November 9, 2021. https://app.magicapp.org/#/guideline/L4Qb5n/section/LAJvRn
- 29. Cavalli G, Larcher A, Tomelleri A, et al. Interleukin‐1 and interleukin‐6 inhibition compared with standard management in patients with COVID‐19 and hyperinflammation: a cohort study. Lancet Rheumatol. 2021;3(4):e253‐e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Asch DA, Sheils NE, Islam MN, et al. Variation in US hospital mortality rates for patients admitted with COVID‐19 during the first 6 months of the pandemic. JAMA Intern Med. 2020;181(4):471‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Risk of bias assessment for included RCTs according to five domains
FIGURE S2 Flowchart of search strategy
FIGURE S3 Death or death + mechanical ventilation in patients with COVID‐19 with and without concomitant corticosteroid use, tocilizumab vs standard care/placebo
FIGURE S4 All‐cause mortality in patients with COVID‐19 in studies with high vs low percentage of concomitant corticosteroid use, tocilizumab vs standard care/placebo
TABLE S1 Study and patient characteristics
TABLE S2 Sensitivity analysis
Data S1. Supporting information
Data Availability Statement
The data used in this study are available upon request from the corresponding author.


